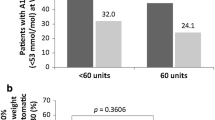
Abstract
Introduction
This study aimed to evaluate glycemic outcomes in subphenotypes of type 2 diabetes (T2D) with HbA1c > 7.0%, previously on basal insulin (pre-BI) alone (≥ 42 U/day) or on basal-bolus therapy (pre-BB), and who were switched to either basal insulin glargine 300 U/mL (IGlar-300) or 100 U/mL (IGlar-100), with or without pre-prandial insulin.
Methods
Participants from EDITION 2 (pre-BI, n = 785), and EDITION 1 (pre-BB, n = 792) trials were assigned retrospectively to subphenotypes of T2D: severe insulin deficient diabetes (SIDD), mild age-related diabetes (MARD), mild obesity diabetes (MOD), and severe insulin resistant diabetes (SIRD). Key efficacy and safety parameters were analyzed at baseline, and after 26 weeks, for IGlar-300 and IGlar-100 pooled groups according to subphenotypes. Outcomes were also compared with insulin-naïve subphenotypes on oral antihyperglycemic drugs (OADs) from the EDITION 3 trial (pre-OAD, n = 858).
Results
Pre-BI and pre-BB treated subphenotypes with SIDD had a higher mean HbA1c (8.9% and 9.1%) at baseline compared to those of MARD (7.7% and 7.8%) and MOD (8.1% and 8.2%) and after 26 weeks remained above target HbA1c (7.7% and 8.0%) despite mean glargine doses of 0.7 to 1.0 U/kg/day and pre-prandial insulin use in the pre-BB SIDD subgroup. Pre-BB treated individuals with MARD and MOD achieved lower HbA1c levels (6.9% and 7.2%) than the pre-BI groups (7.3% and 7.5%) despite similar mean FPG levels (123–130 mg/dL). Only 19–22% of participants with SIDD achieved HbA1c < 7.0% compared to 33–51% with MARD and MOD, respectively. Pre-BI and pre-BB treated subphenotypes experienced more hypoglycemia than pre-OAD treated subphenotypes.
Conclusion
Individuals with T2D assigned post hoc to the SIDD subphenotype achieved suboptimal glycemic control with glargine regimens including basal-bolus therapy, alerting clinicians to improve further diabetes treatment, particularly post-prandial glycemic control, in individuals with SIDD.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Why carry out the study? |
New subphenotypes of type 2 diabetes (T2D) have been proposed that characterize the clinical heterogeneity of T2D. |
Insulin-naïve subphenotypes of T2D respond similarly to basal insulin glargine 300 U/mL and 100 U/mL, but there is no data currently available for insulin pre-treated subphenotypes. |
What was learned from the study? |
The SIDD subphenotype benefits more from basal-bolus therapy than from basal insulin alone but glycemic control remains above recommended targets. |
Insulin glargine 300 U/mL may be more beneficial than glargine 100 U/mL to minimize hypoglycemia risk in SIDD and MOD, irrespective of pre-treatment regimen. |
Recognition and better management of individuals with SIDD in clinical practice are fundamental to achieving satisfactory glycemic control. |
The MARD subphenotype achieves good glycemic control with any glargine-based regimen. |
Introduction
Type 2 diabetes (T2D) is a very heterogeneous disease with different pathophysiological components and genetics [1,2,3]. By utilizing k-means clustering of clinical characteristics several subphenotypes of T2D have been described including mild age-related diabetes (MARD), mild obesity-Related diabetes (MOD), severe insulin-resistant diabetes (SIRD), and severe insulin-deficient diabetes (SIDD) [4, 5]. These T2D subphenotypes are characterized by variations in insulin deficiency, insulin resistance, body weight, and risk of development of diabetes-related microvascular complications [4, 6]. T2D subphenotypes may also respond differently to glucose-lowering treatments as such as sulfonylureas and pioglitazone [7, 8] and first- and second-generation basal insulin glargine, namely 300 U/mL (IGlar-300) and 100 U/mL (IGlar-100) [9, 10]. In two analyses of insulin-naïve T2D subphenotypes who were above target HbA1c (7.0%) on oral antihyperglycemic drugs (OADs), similar reductions in HbA1c and fasting plasma glucose (FPG) were reported both with IGlar-300 and IGlar-100 treatment at 6 months in the MARD, MOD, SIRD, and SIDD subphenotypes [9, 10]. Optimal glycemic control was achieved only in the MARD subphenotype, in contrast to the SIDD subphenotype, which failed to reach target HbA1c levels after initiation of basal insulin. When basal insulin was initiated within these insulin-naïve T2D subphenotypes the lowest hypoglycemia incidence was observed in SIRD [9, 10]. Recent analysis has suggested that hypoglycemia risk is lower with IGlar-300 than with IGlar-100 in the SIDD and MOD subphenotypes [10].
Little is currently known about glycemic achievements in people with long-standing T2D who are treated with basal insulin alone or with the addition of pre-prandial insulin when they are stratified according to these newly defined T2D subphenotypes. Therefore, the aim of this retrospective analysis was to examine glycemic outcomes in basal insulin (BI) pre-treated study participants withT2D derived from the randomized clinical trials EDITION 2 [11] and EDITION 1 [12], according to T2D subphenotypes. These study participants were pre-treated either with basal IGlar-100 or NPH insulin at a dose ≥ 42 U/day, either without (pre-BI; EDITION 2) or with additional thrice-daily pre-prandial insulin analogues (pre-BB; EDITION 1) prior to study entry and were randomly switched to either IGlar-300 or IGlar-100 with or without pre-prandial insulin, then treated for 26 weeks. Clinical outcomes for insulin pre-treated participants were compared further to those reported for insulin-naïve T2D subphenotypes from the EDITION 3 randomized trial who subsequently initiated glargine treatment [10, 13].
Methods
Insulin-naïve and basal insulin pre-treated participants from three EDITION T2D trials (ClinicalTrials.gov registrations NCT01676220, NCT01499095, NCT01499082) were selected for this post hoc analysis [10,11,12,13]. The EDITION study protocols were approved by ethics review boards at each site and written informed consent was collected from all participants. The trials were conducted in accordance with the principles of international ethics guidelines, including the Declaration of Helsinki, and applicable local laws and regulations. All participants had originally been enrolled solely according to clinical parameters and inclusion criteria across studies (see Table S1 in the electronic supplementary material for details). In all trials, IGlar-300 was studied as the investigational medicinal product and compared with IGlar-100 over a period of 26 weeks. A total of 2435 participants from EDITION 2 (pre-BI, n = 785), EDITION 1 (pre-BB, n = 792), and EDITION 3 trials (pre-OAD, n = 858) were identified and k-means clustering was performed to assign participants to the newly defined diabetes subphenotypes as described [4, 9] and briefly summarized in Table S2. Participants (n = 73) who were positive for glutamic acid decarboxylase-65 antibodies (GADA65) were excluded from analysis. The remaining participants with T2D (n = 2362) were assigned to one of the four T2D subphenotypes, using a sex-specific nearest centroid approach. Variables at baseline, which included age at onset of known diabetes, HbA1c, body mass index (BMI), and fasting C-peptide (FCP), were scaled and centered for each participant and then assigned to one of the four T2D subphenotypes to which they were most similar, as estimated by the smallest Euclidean distance to subphenotype centroids derived from ANDIS coordinates [4, 9]. FCP has been shown to be equivalent to HOMA2 parameters for the assignation of participants into the four T2D subphenotypes [6, 9, 14]. A detailed distribution of subphenotypes across the EDITION T2D trials is provided in Fig. S1.
All clinical outcomes (HbA1c, FPG, glycemic outcome achievement, 8-point self-monitored plasma glucose [SMPG], insulin dose, hypoglycemia, body weight) were assessed at baseline and over the 26-week study period following the introduction of either IGlar-300 or IGlar-100, administered once-daily before bedtime, and titrated once-weekly to aim for a fasting SMPG of 80–100 mg/dL (4.4–5.6 mmol/L). Because of similar glycemic outcomes with IGlar-300 and IGlar-100 in insulin-naïve T2D subphenotypes [10], insulin glargine treatment groups were pooled for analysis of outcome parameters, with the exceptions of insulin dose and frequency of hypoglycemia, which were also analyzed by insulin type. Hypoglycemia was determined according to an internationally agreed definition using a confirmed plasma glucose (PG) value of ≤ 70 mg/dL (≤ 3.9 mmol/L) for level 1 or < 54 mg/dL (< 3.0 mmol/L) for level 2 hypoglycemia [15]. Nocturnal hypoglycemia was any event recorded between 0000 and 0559 hours and severe hypoglycemia (level 3) was defined as an event associated with severe cognitive impairment requiring external assistance for recovery.
Statistical Analysis
Baseline variables and clinical outcomes are presented descriptively up to 26 weeks using mean (SD), median (range), or proportion (%). Categorical outcomes were analyzed using a logistic regression model adjusted for age, sex, race/ethnicity, and diabetes duration at study entry with fixed categorical covariates of study and diabetes subphenotypes providing odds ratios and corresponding 95% CI. Mapping and pooling of databases were performed using SAS software (version 9.4; SAS Institute, Cary, NC, USA).
Results
Reassignment of insulin pre-treated participants with T2D from the EDITION 2 and EDITION 1 trials revealed subphenotypes MOD (70–78%), SIDD (9–13%), MARD (8–13%), and SIRD (0.1–0.6%), with similar distributions in the IGlar-300 and IGlar-100 treatment groups (Fig. S1). Distributions of age at diagnosis, BMI, HbA1c, and FCP at baseline for each subphenotype from the EDITION trials are illustrated in Fig. 1 and baseline characteristics and demographics for the SIDD, MARD, MOD, and SIRD subphenotypes are summarized in Table 1. More insulin pre-treated people were assigned to MOD and fewer to the SIDD and MARD subphenotypes compared to insulin-naïve participants in the T2D EDITION 3 trial. Median age at onset of diabetes was highest in MARD (58 years) and SIRD (58–67 years), with SIDD, MARD, and MOD subphenotypes pre-treated with insulin having a longer median diabetes duration of up to 16 years compared to insulin-naïve subphenotypes (8–10 years) (Table 1, Fig. S2). As expected, MOD subphenotypes across trials had the highest mean BMI (37–38 kg/m2) and insulin pre-treated SIDD subphenotypes had the highest proportion (73–75%) of individuals with FCP levels ≤ 0.4 nmol/L. Mean HbA1c levels at baseline were consistently lower in insulin pre-treated MARD (7.7–7.8%; 61–62 mmol/mol), MOD (8.1–8.4%; 65–68 mmol/mol), and SIDD subphenotypes (8.9–9.1%; 74–76 mmol/mol) than in corresponding insulin-naïve subphenotypes (Table 1, Fig. S2).
Baseline cluster variables for pooled glargine treatment groups from EDITION T2D trials according to SIDD, MARD, MOD, and SIRD subphenotypes. ED 3 EDITION 3 (pre-OAD), ED 2 EDITION 2 (pre-BI ≥ 42 U/day), ED 1 EDITION 1 (pre-BB of BI ≥ 42 U/day plus thrice-daily pre-prandial insulin), BI basal insulin, BB basal-bolus, FCP, fasting C-peptide; boxes represent median with 25th and 75th percentiles, whiskers indicate minimum and maximum ranges; n.a., not analyzed (only 1 patient)
Glycemic Responses to Glargine Regimens in SIDD, MARD, and MOD Subphenotypes
HbA1c
Because of the very small number of participants (n = 32) assigned to the SIRD subphenotype across the trials, all treatment outcomes for these few participants are presented in the supplementary material only. The observed mean HbA1c for pooled glargine groups (IGlar-300 and IGlar-100) at baseline, 12 and 26 weeks and the change from baseline to 26 weeks as stratified by MARD, MOD, and SIDD subphenotypes are included in Fig. 2 (left panel) and summarized in Table S3. Despite the greatest mean HbA1c reduction from baseline to 26 weeks being observed in the pre-BI and pre-BB SIDD subphenotypes (− 1.2%, − 13 mmol/mol) compared to pre-BI and pre-BB MOD (− 0.7% to − 0.9%; − 8 to − 10 mmol/mol) and MARD (− 0.6% to − 0.7%; − 7 to − 8 mmol/mol), HbA1c levels remained unsatisfactory at 7.7–8.0% (61–63 mmol/mol) at the end of treatment in the pre-BI and pre-BB SIDD subphenotypes. Only 19–22% of individuals with SIDD achieved a target HbA1c < 7.0% (< 53 mmol/mol) at 26 weeks compared to 33–51% in the pre-BI and pre-BB MARD and MOD subphenotypes. In the pre-OAD MARD and MOD subphenotypes 56–62% reached the glycemic goal (Fig. 3). Compared to insulin-naïve (pre-OAD) T2D subphenotypes each insulin-pre-treated T2D subphenotype, when started at lower baseline levels, exhibited a smaller HbA1c reduction.
Observed mean HbA1c (left panel) and FPG (right panel) over 26 weeks and change from baseline to week 26 in pooled glargine treatment groups from EDITION T2D trials according to SIDD, MARD, and MO subphenotypes. ED 3 EDITION 3 (pre-OAD), ED 2 EDITION 2 (pre-BI ≥ 42 U/day), ED 1 EDITION 1 (pre-BB of BI ≥ 42 U/day plus thrice-daily pre-prandial insulin), OAD oral antihyperglycemic drug, BI basal insulin, BB basal-bolus
Achievements of HbA1c and FPG targets at 26 weeks in pooled glargine treatment groups from EDITION T2D trials according to SIDD, MARD, and MOD subphenotypes. ED 3 EDITION 3 (pre-OAD), ED 2 EDITION 2 (pre-BI ≥ 42 U/day), ED 1 EDITION 1 (pre-BB of BI ≥ 42 U/day plus thrice-daily pre-prandial insulin), OAD oral antihyperglycemic drug, BI basal insulin, BB basal-bolus
FPG and Eight-Point SMPG Profiles
Mean reductions in FPG from baseline to 26 weeks in the pre-BI and pre-BB subphenotypes ranged from − 24 to − 41 mg/dL (− 1.3 to − 2.3 mmol/L) in SIDD, from − 22 to − 28 mg/dL (− 1.2 to − 1.6 mmol/L) in MOD, and − 8 to − 13 mg/dL (– 0.4 to − 0.7 mmol/L) in MARD, respectively (Fig. 2 right panel, Table S4). The proportion of participants who achieved a FPG < 100 mg/dL (< 5.6 mmol/L) did not differ considerably between subphenotypes except for pre-BB MARD and MOD subphenotypes, in which the lowest proportions were observed (Fig. 3). With any of the glargine regimens (BI alone at ≥ 42 U/day with or without pre-prandial insulin) the MARD subphenotype attained the lowest FPG levels at 26 weeks (Table S4).
Eight-point SMPG profiles at baseline and 26 weeks according to differently pre-treated SIDD, MARD, and MOD subphenotypes showed that all pre-BB treated subphenotypes achieved flatter SMPG profiles with better post-prandial glycemic control at 26 weeks compared to pre-BI and pre-OAD treated subphenotypes (Fig. S3). Mean post-prandial reductions at breakfast, lunch, and dinner ranged similarly from − 20 to − 33 mg/dL (− 1.1 to − 1.8 mmol/L) in pre-BB SIDD, − 14 to − 32 mg/dL (− 0.8 to − 1.8 mmol/L) in pre-BB MARD, and − 20 to − 39 mg/dL (− 1.1 to − 2.2 mmol/L) in pre-BB MOD (Fig. 4, Table S5). The greatest post-prandial glucose reductions were observed in the pre-OAD treated SIDD (− 60 to − 92 mg/dL; − 3.3 to − 5.1 mmol/L) and pre-OAD treated MOD (− 40 to − 70 mg/dL; − 2.2 to − 3.9 mmol/L) subphenotypes. Lesser effects on SMPG reductions were observed across the differently pre-treated MARD subphenotypes. Notably, the SIDD subphenotypes remained at higher post-prandial glucose levels, not only in pre-OAD and pre-BI glargine regimens but also in the pre-BB glargine regimen when compared to MARD and MOD subphenotypes (Fig. 4).
Change of post-prandial glucose from baseline to 26 weeks in pooled glargine treatment groups according to SIDD, MARD, and MOD subphenotypes from the EDITION T2D trials. Mean glucose values are derived from self-monitored glucose measurements; bars represent mean glucose change from baseline to week 26; ED 3 EDITION 3 (pre-OAD), ED 2 EDITION 2 (pre-BI ≥ 42 U/day), ED 1 EDITION 1 (pre-BB of BI ≥ 42 U/day plus thrice-daily pre-prandial insulin), OAD oral antihyperglycemic drug, BI basal insulin, BB basal-bolus
Insulin Glargine 300 U/mL and 100 U/mL Dose in MARD, MOD, and SIDD Subphenotypes
The observed mean daily doses per body weight of IGlar-300 and IGlar-100 from study entry over 26 weeks are shown in Fig. 5 and summarized in Table S6. According to the study protocols the glargine starting doses differed considerably between pre-BI and pre-BB treated SIDD, MARD, and MOD subphenotypes (0.62–0.71 U/kg/day) and pre-OAD treated subphenotypes (0.19–0.20 U/kg/day). Subsequently, the mean insulin doses at 26 weeks reached 0.71–1.03 U/kg/day in insulin pre-treated and only 0.41–0.72 U/kg/day in insulin-naïve SIDD, MARD, and MOD subphenotypes. The lowest glargine dose increments of 0.24–0.34, 0.16–0.22, and 0.17–0.27 U/kg/day were recorded in pre-OAD, pre-BI, and pre-BB treated MARD subphenotypes at week 26, respectively. Except for the pre-BB treated SIDD subphenotypes, the final daily basal insulin dose was consistently higher for IGlar-300 than IGlar-100 at 26 weeks in all other subphenotypes, with the greatest differences in dose being observed in the leaner SIDD and MARD subphenotypes at 0.11 and 0.14 U/kg, respectively (Fig. 5, Table S6). In all pre-BB treated subphenotypes the average daily pre-prandial insulin dose was similar and remained stable at about 0.50 U/kg/day from baseline to week 26 (data not shown).
Mean IGlar-300 and IGlar-100 dose over 26 weeks according to SIDD, MARD, and MOD subphenotypes from EDITION T2D trials. ED 3 EDITION 3 (pre-OAD), ED 2 EDITION 2 (pre-BI ≥ 42 U/day), ED 1 EDITION 1 (pre-BB of BI ≥ 42 U/day plus thrice-daily pre-prandial insulin), OAD oral antihyperglycemic drug, BI basal insulin, BB basal-bolus
Hypoglycemia with Glargine Regimens in MARD, MOD, and SIDD Subphenotypes
The cumulative incidences and event rates of level 1 and level 2 hypoglycemia at any time (during 24-h) and of nocturnal hypoglycemia for pooled glargine treatment groups were consistently higher in pre-BI and pre-BB treated subphenotypes than in pre-OAD treated subphenotypes over 26 weeks (Fig. S4). The proportion of participants experiencing at least one episode of non-severe hypoglycemia ranged from 72% to 87% (level 1 at any time of day), 30–47% (level 2 at any time of day), 32–51% (level 1 and nocturnal), and 11–17% (level 2 and nocturnal) in pre-BI and pre-BB treated subphenotypes. The incidence of level 3 (severe) hypoglycemia was very low (0 to 3%) across most subphenotypes, except for pre-BB MARD and pre-BB MOD subphenotypes in which a slightly higher incidence (6%) was observed (Fig. S4).
Cumulative hypoglycemia incidences analyzed separately by IGlar-300 and IGlar-100 treatment regimens and subphenotypes are shown in Fig. 6. A general trend of a numerically lower risk was observed in the SIDD and MOD subphenotypes with IGlar-300 for most of the hypoglycemia categories, particularly level 1. Incidences were markedly lower for level 1 (any time) hypoglycemia in pre-BB SIDD by 46% (OR 0.54, 95% CI 0.34–0.85) and pre-BI MOD by 34% (OR 0.66, 95% CI 0.45–0.98). Similarly, for nocturnal hypoglycemia level 1 incidence was lower in pre-OAD SIDD by 51% (OR 0.49, 95% CI 0.24–0.99), pre-OAD MOD by 41% (OR 0.59, 95% CI 0.36–0.97), pre-BI MOD by 48% (OR 0.52, 95% CI 0.36–0.76), and pre-BB MOD by 36% (OR 0.64, 95% CI 0.46–0.89). Level 2 (any time) hypoglycemia was markedly lower in pre-OAD SIDD by 69% (OR 0.31, 95% CI 0.13–0.77) and pre-BI MOD by 36% (OR 0.54, 95% CI 0.34–0.85) with IGlar-300.
Comparison of cumulative hypoglycemia incidences at 26 weeks between IGlar-300 and IGlar-100 treatment groups according to SIDD, MARD, and MOD subphenotypes from the EDITION T2D trials. ED 1 EDITION 3 (pre-OAD), ED 2 EDITION 2 (pre-BI ≥ 42 U/day), ED 1 EDITION 1 (pre-BB of BI ≥ 42 U/day plus thrice-daily pre-prandial insulin), OAD oral antihyperglycemic drug, BI basal insulin, BB basal-bolus, OR odds ratio, CI confidence interval, n.a. not applicable
Body Weight Change with Glargine Regimens in MARD, MOD, and SIDD Subphenotypes
Mean body weight at baseline and 26 weeks was consistently higher in pre-BI and pre-BB treated SIDD and MARD subphenotypes compared to corresponding pre-OAD subphenotypes (Table S7). Mean body weight increased in all T2D subphenotypes by 26 weeks and the effect of glargine regimens on body weight was greatest in the leaner SIDD subphenotypes (+ 1.2 to + 1.9 kg).
Discussion
This post hoc analysis has examined responses to different glucose-lowering regimens with basal insulin glargine (300 U/mL and 100 U/mL) for the first-time according to the newly proposed T2D subphenotypes that were derived from retrospective classification of 1577 participants from the EDITION 1 and 2 trials. These subphenotypes, which had diabetes of longer duration (12–16 years), were either pre-treated with IGlar-100 or NPH insulin (at mean doses between 56 and 72 U/day), with or without pre-prandial insulin. Outcomes were compared to insulin-naïve T2D subphenotypes which initiated glargine (300 U/mL or 100 U/mL) at mean starting doses between 15 and 21 U/day [10]. The considerable heterogeneity of T2D was again demonstrated by k-means clustering, not only in real-world populations [5] but also in these and other clinical trials [6]. Even though strict inclusion criteria had been applied in these trials, most of the insulin pre-treated participants with T2D were assigned to the MOD subphenotype (70–78%), followed by SIDD (9–13%) and MARD (8–13%). This distribution of subphenotypes differed slightly from the insulin-naïve EDITION 3 population [10] which consisted of more individuals with SIDD (22%) and MARD (18%) and had fewer participants with MOD (56%), probably because EDITION 3 participants had a lower mean BMI (33 kg/m2) than the insulin pre-treated participants (35–37 kg/m2). Classifications of insulin-naïve T2D study participants suggests that this population may consist of a higher proportion of the SIDD and SIRD subphenotypes than is observed in insulin pre-treated participants [6] though much lower FCP levels had been found in insulin pre-treated participants. It is unclear whether this observation was a consequence of slightly different inclusion criteria having been applied between studies of insulin-naïve and pre-treated participants or whether other factors have influenced the inclusion of originally unclassified individuals with SIDD and SIRD.
The present post hoc analysis has revealed that basal insulin treatment with glargine leads to similar FPG levels at 26 weeks in the SIDD, MARD, and MOD subphenotypes (Fig. 2), thus indicating appropriate basal insulin titration, but also illustrated the difficulty in optimizing HbA1c control in the SIDD subphenotype when basal insulin is either initiated after OAD failure (pre-OAD), continued (pre-BI), or when pre-prandial insulin is added (pre-BB). The problem remains the post-prandial glucose, which was higher in individuals with SIDD than in those with MARD and MOD, probably because of the greater endogenous insulin deficiency as indicated by the lower FCP levels in SIDD (Table 1). These outcomes suggest that basal insulin alone, despite optimal titration which lowers FPG close to target, cannot optimize post-prandial glucose levels when endogenous insulin secretion is severely compromised as in SIDD. This calls for the timely addition of pre-prandial insulin treatment in SIDD after initiation of basal insulin. Furthermore, even in a pre-BB regimen where pre-prandial insulin was applied, the treatment of post-prandial hyperglycemia needs to be improved either by further titration of pre-prandial insulin and/or by adding a glucagon-like peptide 1 receptor agonist.
In view of the small number of individuals with SIRD that were classified among the insulin pre-treated participants of the EDITION 1 and 2 trials, treatment responses in both the pre-BB and pre-BI treated SIRD subphenotype remain uncertain. However, in the present analysis, glycemic outcomes in the pre-OAD and pre-BI treated SIRD subphenotypes (Tables S3 and S4) suggest that the response in this population was good and satisfactory, similar to that observed in the insulin-naïve or pre-BI and pre-BB treated MARD and MOD subphenotypes. The findings also demonstrated consistency with outcomes observed in pre-OAD treated individuals with SIRD from pooled glargine trials [6].
As expected, the pre-BI and pre-BB treated subphenotypes, both of which had received higher glargine doses, experienced more frequent hypoglycemia events than the pre-OAD treated subphenotypes which initiated glargine and remained on maintenance doses that were 30–50% lower. The present analysis suggests that the SIDD and MOD subphenotypes may benefit more from IGlar-300 than from IGlar-100 basal regimens as shown by the lower hypoglycemia risk, which could be attributed to the different PK/PD profiles of the two glargine formulations. In contrast, the MARD subphenotype, which achieves the best HbA1c control with all glargine regimens, even at lower daily doses, experienced hypoglycemia of similar magnitude irrespective of the glargine type used in any regimen. Individuals with MARD, who are older and present with a milder form of T2D, might benefit more from a slower and more careful titration of basal insulin, and/or by simplification of the insulin regimen by switching from a basal-bolus regimen to a once-daily basal insulin regimen to minimize the risk of hypoglycemia at an equivalent level of glycemic control [16]. In addition, greater consideration of FCP in clinical practice as a biomarker of hypoglycemia risk in T2D subphenotypes who require basal insulin therapy [17] could further assist clinicians when deciding to select the most effective and safest glucose-lowering treatment based on the needs of the individual T2D subphenotypes.
In summary, data-driven k-means clustering of pre-treated people with T2D into one of the four subphenotypes identifies individuals with different responses to basal insulin treatment whilst revealing differences in insulin dose requirements, risk of hypoglycemia, and other clinical features (Table 2). Other purpose-specific models with simple quantitative measures (e.g., age at diagnosis, BMI, HbA1c, estimated glomerular filtration rate) have also been proposed to predict the development of complications or treatment responses to glucose-lowering drugs in T2D subphenotypes [7]. However, there is ongoing debate on the benefits of the different methodologies to provide a holistic view on T2D that warrants more exploration [18, 19].
Strengths and Limitations
This post hoc outcome analysis has strengths and limitations. The present analysis has compared treatment outcomes in study participants withT2D when assigned to newly proposed T2D subphenotypes, and who have received different basal insulin glargine regimens with or without pre-prandial insulin. However, as most of the participants across the trials had only the SIDD, MARD, and MOD subphenotypes, the generalizability of findings are mainly restricted to these subphenotypes. It should also be noted that T2D subphenotypes have been determined retrospectively and the study-defined insulin glargine regimens were not targeted to the different needs of the heterogeneous diabetes subpopulations.
A further limiting factor is inherent in the nature of a post hoc analysis, which can only be of exploratory value, such that any findings should be considered solely as hypothesis-generating and would need to be confirmed by larger prospective studies involving adequate sample sizes to demonstrate potential differences in outcomes between different basal insulin treatment regimens.
Conclusions
The present observations provide additional insights into the efficacy and safety of different formulations of basal insulin glargine (300 U/mL and 100 U/mL) according to the newly proposed T2D subphenotypes [4]. The analysis reveals heterogeneous patterns of glycemic achievement after 26 weeks of treatment in the SIDD, MARD, and MOD subphenotypes. Irrespective of the glargine regimen that was introduced, the SIDD subphenotype had suboptimal glycemic control, even with intensified basal-bolus insulin therapy. A low percentage of individuals with SIDD (< 33%) achieved either the HbA1c (< 7.0%; 53 mmol/mol) or FPG targets (< 100 mg/dL; 5.6 mmol/L) in the EDITION T2D trials, despite strict titration algorithms of glargine being applied in these trials. This emphasizes the need for optimizing the dose of pre-prandial insulin in the SIDD subphenotype, especially during the later part of the day. Improving the phenotypic criteria, e.g., routine testing of FCP and GAD65 antibodies for early identification of the presence of (severe) insulin deficiency and/or the presence of late autoimmune diabetes in adults (LADA), may help clinicians to select and initiate the most effective insulin regimen in a timely manner to optimize glycemic control in this vulnerable subphenotype. In contrast, the glycemic outcomes observed in the pre-OAD and pre-BB MARD and MOD subphenotypes suggest that they represent a more insulin-responsive T2D subpopulation, both when initiated with basal insulin alone and when intensified by the addition of pre-prandial insulin. The findings also suggest that, in general, T2D individuals with SIDD and MOD may derive greater benefit from the use of the second-generation basal insulin IGlar-300 to minimize their hypoglycemia risk.
This post hoc characterization of T2D study participants provides further evidence in support of the heterogeneity of T2D present in clinical trials. Similar inclusion criteria applied in the EDITION and other T2D trials could not prevent the incidental distribution of subphenotypes across trials [6] and any conclusions from unclassified study results do not reflect the individual treatment responses according to subphenotypes. The planning and conductance of future diabetes clinical trials should acknowledge the heterogeneity of T2D [1], by allocating T2D participants into these different subphenotypes when investigating the most suitable glucose-lowering drugs and regimens in order to optimize outcomes.
Data Availability
All data generated or analyzed during this study are included as supplementary information files.
References
Venkat Narayan KM, Jagannathan R, Ridderstråle M. Managing type 2 diabetes needs a paradigm change. Lancet Diabetes Endocrinol. 2023;11(8):534–6.
Tuomi T, Santoro N, Caprio S, Cai M, Weng J, Groop L. The many faces of diabetes: a disease with increasing heterogeneity. Lancet. 2014;383:1084–94.
Faerch K, Hulmán A, Solomon TPJ. Heterogeneity of pre-diabetes and type 2 diabetes: implications for prediction, prevention, and treatment responsiveness. Curr Diabetes Rev. 2016;12(1):30–41.
Ahlqvist E, Storm P, Käräjämäki A, et al. Novel subgroups of adult-onset diabetes and their association with outcomes: a data-driven cluster analysis of six variables. Lancet Diabetes Endocrinol. 2018;6:361–9.
Misra S, Wagner R, Ozkan B, et al. Precision subclassification of type 2 diabetes: a systematic review. Commun Med. 2023;3:138.
Landgraf W, Bigot G, Hess S, et al. Distribution and characteristics of newly-defined subgroups of type 2 diabetes in randomised clinical trials: post hoc cluster assignment analysis of over 12,000 study participants. Diabetes Res Clin Pract. 2022;190:110012.
Dennis JM, Shields BM, Henley WE, Jones AG, Hattersley AT. Disease progression and treatment response in data-driven subgroups of type 2 diabetes compared with models based on simple clinical features: an analysis using clinical trial data. Lancet Diabetes Endocrinol. 2019;7(6):442–51.
Abdul-Ghani T, Puckett C, Migahid O, et al. T2DM subgroups and response to glucose-lowering therapy: results from EDICT and Qatar Study. Diabetes Obes Metab. 2022;24:1810–8.
Landgraf W, Bigot G, Frier BM, Bolli GB, Owens DR. Response to insulin glargine 100 U/mL treatment in newly-defined subgroups of type 2 diabetes: post hoc pooled analysis of insulin-naïve participants from nine randomised clinical trials. Prim Care Diabetes. 2023;17:379–85.
Landgraf W, Owens DR, Frier BM, Bolli GB. Treatment responses to basal insulin glargine 300 U/mL and glargine 100 U/mL in newly-defined subphenotypes of type 2 diabetes: a post hoc analysis of the EDITION 3 randomised clinical trial. Diabetes Obes Metab. 2024;26:503–11.
Yki-Järvinen H, Bergenstal R, Ziemen M, et al. New insulin glargine 300 units/mL versus glargine 100 units/mL in people with type 2 diabetes using oral agents and basal insulin: glucose control and hypoglycemia in a 6-month randomized controlled trial (EDITION 2). Diabetes Care. 2014;37(12):3235–43.
Riddle MC, Bolli GB, Ziemen M, et al. New insulin glargine 300 units/mL versus glargine 100 units/mL in people with type 2 diabetes using basal and mealtime insulin: glucose control and hypoglycemia in a 6-month randomized controlled trial (EDITION 1). Diabetes Care. 2014;37(10):2755–62.
Bolli GB, Riddle MC, Bergenstal RM, et al. New insulin glargine 300 U/ml compared with glargine 100 U/ml in insulin-naïve people with type 2 diabetes on oral glucose-lowering drugs: a randomized controlled trial (EDITION 3). Diabetes Obes Metab. 2015;17:386–94.
Pigeyre M, Hess S, Gomez MF, et al. Validation of the classification for type 2 diabetes into five subgroups: a report from the ORIGIN trial. Diabetologia. 2022;65:206–15.
International Hypoglycaemia Study Group. Glucose concentrations of less than 3.0 mmol/L (54 mg/dL) should be reported in clinical trials: a joint position statement of the American Diabetes Association and the European Association for the Study of Diabetes. Diabetes Care. 2017;40:155–7.
Munshi MN, Slyne C, Segal AR, et al. Simplification of insulin regimen in older adults and risk of hypoglycemia. JAMA Intern Med. 2016;176:1023–6.
Landgraf W, Owens DR, Frier BM, Zhang M, Bolli GB. Fasting C-peptide, a biomarker for hypoglycaemia risk in insulin-naïve people with type 2 diabetes initiating basal insulin glargine 100 U/mL. Diabetes Obes Metab. 2020;22(3):315–23.
Ahlqvist E, Tuomi T, Groop L. Clusters provide a better holistic view of type 2 diabetes than simple clinical features. Lancet Diabetes Endocrinol. 2019;7(9):668–9.
Dennis JM, Shields BM, Henley WE, Jones AG, Hattersley AT. Clusters provide a better holistic view of type 2 diabetes than simple clinical features—Authors’ reply. Lancet Diabetes Endocrinol. 2019;7(9):669.
Acknowledgements
Medical Writing, Editorial, and Other Assistance
The authors would like to thank Gregory Bigot and Lydie Melas-Melt, IVIDATA Life Sciences, Paris for their contributions to the data analysis and interpretation for this study. No medical writing assistance or editorial assistance in the preparation of this article was utilized.
Authorship
All named authors meet the International Committee of Medical Journal Editors (ICMJE) criteria for authorship for this article, take responsibility for the accuracy and integrity of the data presented in this manuscript, and have given their approval for this version to be published.
Funding
No funding or sponsorship was received for this research. The underlying EDITION studies, and Rapid Service Fee were funded by Sanofi, Paris, France.
Author information
Authors and Affiliations
Contributions
Wolfgang Landgraf: Conceptualization, Data curation, Project administration, Resources, Formal analysis, Methodology, Supervision, Validation, Visualization, Writing—original draft, Writing—review & editing. David R. Owens: Validation, Writing—original draft, Writing—review & editing. Brian M. Frier: Validation, Writing—original draft, Writing—review & editing. Geremia B. Bolli: Validation, Writing—original draft, Writing—review & editing.
Corresponding author
Ethics declarations
Conflict of Interest
Wolfgang Landgraf is an employee of Sanofi, Germany, and Sanofi shareholder. Brian M. Frier has served on an advisory panel for Zucara Therapeutics. David R. Owens has received honoraria for lecturing and consulting from Boehringer Ingelheim, Eli Lilly, Roche Diagnostics, Sanofi, and Takeda. Geremia B. Bolli is a consultant for Eli Lilly and Sanofi, has received research support from Sanofi and is on the speakers’ bureau for Eli Lilly, Menarini, and Sanofi.
Ethical Approval
This article is based on previously conducted studies and does not contain any new studies with human participants or animals performed by any of the authors. The underlying EDITION studies received ethics approval for all study sites, were conducted in accordance with the Declaration of Helsinki, and written informed consent was collected from all participants.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License, which permits any non-commercial use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc/4.0/.
About this article
Cite this article
Landgraf, W., Owens, D.R., Frier, B.M. et al. Responses to Basal Insulin Glargine (300 U/mL and 100 U/mL) with or Without Pre-prandial Insulin in Pre-treated Subphenotypes of Type 2 Diabetes: Insights from a Post Hoc Analysis. Diabetes Ther 15, 1769–1784 (2024). https://doi.org/10.1007/s13300-024-01608-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13300-024-01608-4