Abstract
Introduction
Following pivotal trials, real-world evidence is important to assess the impact of new drugs in everyday clinical practice. The RESTORE-1 study aimed to compare effectiveness and safety of the second-generation basal insulins (2BI), i.e., insulin glargine 300 U/ml (Gla-300) vs. degludec 100 U/ml (IDeg-100), in type 1 diabetes (T1D).
Methods
Retrospective, non-inferiority, multicenter study, based on electronic medical records. All patients switching to Gla-300 or IDeg-100 from first-generation basal insulins (1BI) were 1:1 propensity score matched (PSM). Changes during 6 months in HbA1c (primary endpoint), fasting plasma glucose (FPG), body weight, and insulin doses were assessed using linear mixed models for repeated measures. Incidence rates (IR) of hypoglycemic events were assessed.
Results
Overall, 19 centers provided data on 585 patients in each PSM cohort. For both groups, statistically significant reductions in HbA1c from baseline to 6 months were documented: − 0.20%; (95% CI − 0.32; − 0.08) in the Gla-300 group and − 0.14%; (95% CI − 0.24; − 0.04) in the IDeg-100 group. The non-inferiority of Gla-300 vs. IDeg-100 was confirmed (non-inferiority margin of 0.30%; upper 95% CI at 6 months, 0.09%). No statistically significant between-group differences emerged in FPG and body weight. Dose changes of basal and short-acting insulin were small in both groups, but higher in the Gla-300 group than in the Deg-100 group (p < 0.006). Incidence rates (IR) of hypoglycemia (blood glucose ≤ 70 mg/dl and < 54 mg/dl) during the 6-month follow-up by treatment were slightly lower in the Gla-300 group than in the Deg-100 group [IR ratios 0.82 (95% CI 0.55; 1.22) and 0.83; (95% CI 0.38; 1.83), respectively]. Hypoglycemic events (blood glucose < 54 mg/dl) decreased at 6 months in both groups (p = 0.01 for Gla-300 and p < 0.001 for IDeg-100). There were no severe hypoglycemic events for Gla-300 and seven events for IDeg-100 (p = 0.02).
Conclusions
Switching from 1BI to 2BI in adults with T1D was associated with similar improvements in glycemic control and overall significant decrease in hypoglycemia, with no severe events with Gla-300. Effectiveness of both insulins was limited by under-titration.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Why carry out this study? |
Glargine 300 U/ml (Gla-300) and degludec 100 U/ml (IDeg-100) are second-generation basal insulins recently made available; in pivotal studies they provided similar efficacy and better safety in comparison with first-generation basal insulins |
As a result of the increasing relevance of real-world data to assess the impact of new therapeutic options, the RESTORE-1 study aimed to assess effectiveness and safety of switching to Gla-300 or IDeg-100 from first-generation basal insulins in adults with type 1 diabetes |
What was learned from the study? |
Switching from first-generation basal insulins to Gla-300 or IDeg-100 was associated with similar improvements in glycemic control, without weight gain |
Only minor changes in insulin doses were found, suggesting that effectiveness of both basal insulins could be improved by a more appropriate titration |
Trends of lower risk of hypoglycemia with Gla-300 vs. IDeg-100 emerged, deserving consideration for future research |
Digital Features
This article is published with digital features, including a summary slide, to facilitate understanding of the article. To view digital features for this article go to https://doi.org/10.6084/m9.figshare.13302359.
Introduction
Despite the substantial advances in basal insulin therapy and blood glucose monitoring over the last 90 years, hypoglycemia remains the most common complication of type 1 diabetes (T1D) [1] and generates as much anxiety in patients as the threat of advanced diabetes complications [2]. Hypoglycemia represents the principal limiting factor in achieving good glycemic control [3]. Currently in Italy, where high quality diabetes care is provided by a large network of diabetes clinics [4], optimal control (HbA1c < 7%) is reported only in one-quarter of men and one-fifth of women with T1D, and the percentage of subjects at target decreases with increasing disease duration. Furthermore, more than 80% of patients with T1D are treated with multiple daily injections of insulin, with a prevalent use of a basal-bolus regimen [5].
Recently, two second-generation basal insulin analogues (2BI) have been made available, providing similar or improved efficacy in comparison with first-generation basal insulins (1BI), with a better safety profile due to improved pharmacokinetic (PK) and pharmacodynamic (PD) properties. Specifically, both insulin glargine 300 U/ml (Gla-300), available in Italy since January 2017, and insulin degludec 100 U/ml (IDeg-100), available in Italy since 2014, show a more stable PK and PD profile vs. insulin glargine 100 U/ml (Gla-100), the most frequently used 1BI [6,7,8,9]. However, two head-to-head PK/PD insulin clamp comparisons between these two 2BI in T1D showed conflicting results [10, 11].
For both 2BI, phase 3 randomized clinical trials provided reassurance about a safe transition from 1BI. In the EDITION clinical program, Gla-300 was proven to be non-inferior to Gla-100 with respect to HbA1c reduction [12,13,14,15]. Of note, a significantly lower percentage of patients with T1D or type 2 diabetes (T2D) experienced confirmed and/or severe nocturnal hypoglycemic events on Gla-300 compared to Gla-100, particularly during the titration phase. The BEGIN clinical program showed comparable glycemic control with IDeg-100 vs. Gla-100 in the majority of trials [16,17,18,19,20,21], with lower rates of nocturnal hypoglycemia during the maintenance phase.
Head-to-head comparisons between Gla-300 vs. IDeg-100 in T2D have shown that these 2BI provided similar glycemic control improvements with relatively low hypoglycemia risk. Hypoglycemia incidence and rates were comparable with both insulins during the full study period but lower with Gla-300 during the titration period [22].
It is recognized that following pivotal trials, real-world evidence (RWE) is particularly important to assess the use and the impact of new drugs in everyday clinical practice. In this respect, retrospective review of electronic medical records (EMRs) represents a new frontier in epidemiological and clinical research [23], offering the potential for low-cost, high-volume data on clinical effectiveness and safety.
RWE comparative data relating to the switch from 1BI to 2BI are available for T2D, all consistently documenting comparable improvements in glycemia and reduced risk of hypoglycemia without major differences between Gla-300 and IDeg-100 [24,25,26].
No comparison between these 2BI is available in mid- or long-term clinical studies in T1D, neither in experimental nor in real-life conditions. Thus, additional comparative clinical evidence is needed in patients switching from 1BI to 2BI, especially in T1D. Further information about the use of basal insulin and patient profiles relating to the switch would be useful to improve real-world effectiveness and appropriateness of use of these new therapeutic options.
Given these premises, the main aim of the REtrospective analysiS on pre-existing daTa On glaRgine-300 U/ml in typE 1 patients (RESTORE-1) study was to investigate the effectiveness and safety outcomes of patients switching to Gla-300 from 1BI compared to patients switching to IDeg-100 under real-life conditions.
Methods
The RESTORE-1 study was a retrospective, comparative, cohort, multicenter study, based on data anonymously extracted from EMRs. The study involved a network of diabetes centers located in different areas of Italy. MyStar Connect/Smart Digital Clinic™ (METEDA SRL, San Benedetto del Tronto, Italy) represented the EMR adopted in all participating centers. Both centers and patients were anonymous, and data were extracted and transferred via a standardized and validated secure procedure.
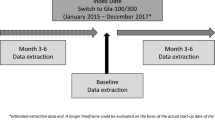
The following inclusion criteria were applied: male or female, aged 18 years or more, diagnosis of T1D, switching to either Gla-300 or IDeg-100 from 1BI (i.e., glargine-100, detemir, or NPH) prescribed for at least 6 months before switching, availability of clinical data in EMR for at least 6 months prior to (baseline) and 6 months after (follow-up) the switch date (i.e., baseline, T0), glycated hemoglobin A1c (HbA1c) levels measured less than 6 months before and more than 90 days after the index date, without any change in basal insulin prescription during the follow-up. Exclusion criteria were prescription of another basal insulin analogue after initiating Gla-300 or IDeg-100 and available follow-up shorter than 3 months.
The following characteristics were considered to describe the baseline patient profile: age, gender, diabetes duration, HbA1c, fasting plasma glucose (FPG), weight, BMI, lipid profile, blood pressure, renal function (albuminuria and estimated glomerular filtration rate), and glucose-lowering, antihypertensive, and lipid-lowering therapies (available in EMRs as ATC codes).
Main endpoints were the changes at 3 months (T3) and 6 months (T6) in HbA1c (primary endpoint), insulin doses, FPG, and body weight, proportion of patients with HbA1c < 7% and < 8% at each study visit in the two cohorts, hypoglycemic events (blood glucose [BG] ≤ 70 mg/dl [≤ 3.9 mmol/l] or < 54 mg/dl [< 3.0 mmol/l]) during 6 months [27]; severe hypoglycemic events during 6 months. Percentages of patients with at least one hypoglycemic event of BG ≤ 70 and < 54 mg/dl during 3 months before T0, T3, and T6, as well as incidence rates of BG ≤ 70 and < 54 mg/dl were evaluated in the subsample of the safety population having self-monitoring of blood glucose (SMBG) data downloaded in EMRs.
Changes in HbA1c at 6 months from the switch represented the primary endpoint.
Furthermore, diabetologists in each site filled in a specific questionnaire on the main reasons for switching patients from 1BI to 2BI. Specifically, for each cohort analyzed and for each site, the physician’s main reasons (i.e., better control; better adherence; preferred dosing; hypoglycemia concern; efficacy, etc.) for the switch to Gla-300 or IDeg-100 from other basal insulin were collected. The questionnaire addressed the general attitude of the diabetologists, and did not investigate the specific reason for the switch in each individual patient.
All procedures followed were in accordance with the ethical standards of the responsible committee on human experimentation (institutional and national) and with the Helsinki Declaration of 1975, as revised in 2008 [5]. The study protocol was approved by all local ethics committees of the participating centers. Informed consent was obtained from all patients for being included in the study.
Statistical Analysis
Sample size estimation was based on the following assumptions. From EMRs review, it was estimated that on average there were about 300 patients with T1D requiring multiple daily injections therapy per year per site, of whom about 45% were switching to 2BI (of whom at least 30% to Gla-300 and 60% to IDeg-100); therefore, the estimated number of patients was 1350 for those switching to Gla-300 (cohort 1) and 2025 patients for those switching to IDeg-100 (cohort 2).
To allow an unbiased comparison between patients switching from other basal insulin to Gla-300 and those switching to IDeg-100, a propensity score matching (PSM) algorithm was applied on a 1:1 basis. Group sample sizes of 201 and 201 achieve 85% power to detect non-inferiority in HbA1c changes after 6 months using a one-sided, two-sample t test. The margin of non-inferiority was 0.3. The true difference between the means was assumed to be 0.0. Baseline standard deviation of HbA1c was assumed to be 1.0. The significance level (alpha) of the test was 0.025.
For PSM, a logistic regression model including age, sex, diabetes duration, and previous basal insulin type and dose as covariates was used to predict the probability to receive Gla-300. A 5:1 greedy matching algorithm was used to identify a unique matched control for each Gla-300 patient according to the propensity score. Adequacy of balance for the covariates in the matched sample was assessed via standardized mean differences between the two groups, considering differences less than 10% as good balance.
Patients’ characteristics according to the type of switch were compared using the unpaired t test or the Mann–Whitney U test in case of continuous variables and the chi-square test or the Fisher exact test for categorical variables, as appropriate.
Changes in HbA1c, FPG, and body weight were assessed using mixed models for repeated measurements. Results are expressed as estimated mean or estimated mean difference from T0 and 95% confidence interval (95% CI). Paired and unpaired t tests derived from linear mixed models for repeated measurements were applied for within-group and between-group comparisons, respectively.
As categorical secondary outcomes, the proportions of patients with HbA1c < 7.0% [53 mmol/mol] and < 8.0% [64 mmol/mol] at each visit were evaluated. Both within-group (McNemar test for change vs. baseline) and between-group (chi-square test) statistical comparisons were performed.
Incidence rates for hypoglycemic events were calculated and expressed as numbers of events per patient-month with their 95% CI. The proportions of patients experiencing at least one hypoglycemic event were compared within groups using the McNemar test and between groups using the chi-square test. Incidence of hypoglycemic events was compared within and between groups using Poisson regression models with correction for overdispersion.
The intention-to-treat (ITT) population included all patients in the EMR meeting the eligibility criteria. Baseline patient characteristics were assessed in the ITT population.
Primary analysis of the changes in HbA1c, FPG, body weight, and insulin doses was assessed in the ITT population after PSM (PS matched–ITT population).
The per protocol (PP) population included those patients from the PSM-ITT population with HbA1c values available at baseline and at 6 months (PSM-PP population). The PP analysis represented a secondary analysis.
For the evaluation of severe hypoglycemia, the safety population was represented by all PS matched patients (data derived from EMRs). For the evaluation of glycemic values ≤ 70 mg/dl and < 54 mg/dl the subsample of the safety population having at least one SMBG value available was considered.
Results
Study Population
Overall, 19 centers were involved, and provided the data relating to 2919 patients switching to Gla-300 or IDeg-100 from 1BI in the period between January 2017 and August 2019. Figure 1 shows the study flowchart.
Study flowchart. ITT population: All patients identified by the extraction software and meeting eligibility criteria. PSM-ITT population: ITT population after PSM used for the primary analysis. PSM-PP population: All patients from the PSM-ITT population with HbA1c value available at baseline and at 6 months used for secondary analysis
Before PSM, compared to patients switching to IDeg-100, patients initiating Gla-300 were younger (44.0 ± 16.4 vs. 46.4 ± 16.0 years, p = 0.001), had a shorter diabetes duration (18.1 ± 13.8 vs. 20.8 ± 15.6 years, p < 0.0001) and lower HbA1c levels (7.9 ± 1.2 vs. 8.1 ± 1.3%, p = 0.0009); they more frequently switched from Gla-100 (95.0% vs. 82.8%). Gender distribution (men 52.6% vs. 51.1%) and mean total daily insulin doses at baseline (45.3 ± 19.7 vs. 44.4 ± 18.8 U) were similar between groups (Table 1).
After PSM, each cohort was composed of 585 patients and the two groups were balanced for all the characteristics, except total cholesterol and albuminuria, although in clinical terms the differences documented were irrelevant (Tables 1, 2).
The mean duration of follow-up of patients on Gla-300 was shorter than that of the IDeg-100 group (4.7 ± 2.7 vs. 5.2 ± 2.5 months; p < 0.0001). The availability of patient data at each follow-up visit is reported in Table S1.
HbA1c
Results of longitudinal models are reported in Table 3 (within-group comparisons) and Table 4 (between-group comparisons).
For Gla-300 group, statistically significant reductions in HbA1c levels from baseline to 3 months (− 0.12%) and 6 months (− 0.20%) were documented. Similarly, for the IDeg-100 group, statistically significant reductions in HbA1c levels from baseline to 3 months (− 0.14%) and 6 months (− 0.14%) were found. No statistically significant between-group differences were found at 3 months (0.05%) and at 6 months (− 0.04%) (Fig. 2; Tables 3, 4). The non-inferiority of Gla-300 vs. IDeg-100 was confirmed (margin of non-inferiority of 0.30%; actual upper 95% CI at 6 months, 0.09%).
In both groups, about 20% of patients reached HbA1c < 7%, without relevant changes during 6 months. In the Gla-300 group, the proportion of patients achieving HbA1c < 8.0% varied from 58.0% at T0, to 62.4% at T3 (p = 0.05), and to 61.3% at T6 (p = 0.25), while in the IDeg-100 group, the proportions varied from 54.5% at T0, to 61.9% at T3 (p = 0.00001), and to 64.4% at T6 (p = 0.001). Between-group comparison at T6 was not statistically significant (p = 0.47).
FPG, Body Weight, and Insulin Dose
No statistically significant changes of FPG from baseline to 3 and 6 months were documented in the Gla-300 group, while statistically significant reductions of − 15.39 mg/dl at T3 and − 16.84 mg/dl at T6 were documented in the IDeg-100 group. Between-group estimated mean difference (Gla-300 vs. IDeg-100) was statistically significant at T3 (20.41 mg/dl; p = 0.004) but not at T6 (8.28 mg/dl; p = 0.34). At T6, in both groups estimated mean levels of FPG were higher than recommended values: 165.3 mg/dl in the Gla-300 group vs. 151.4 mg/dl in the IDeg-100 group (Tables 3, 4).
A slight, not statistically significant increase in body weight from baseline to 6 months was found in both groups, without statistically significant between-group difference.
As for insulin doses, small changes in both basal and short-insulin doses emerged in both groups (Table 3). Specifically, for basal insulin doses, at T6, the Gla-300 group was treated with 22.6 U/day and the change from baseline was of 1.58 U/day, corresponding to 0.32 U/kg (change from baseline 0.02 U/kg). In the IDeg-100 group basal insulin dose at T6 was 21.2 U/day and the change from baseline was of 0.70 U/day, corresponding to 0.30 U/kg (change from baseline 0.01 U/kg). Between-group differences (Gla-300 vs. IDeg-100) at T6 were 0.87 U (p < 0.006) for total daily basal insulin dose and 0.01 U (p < 0.003) for per kg basal insulin dose (Tables 3, 4).
As for short-acting insulin doses, a change from baseline of 0.01 U (p = 0.38) was documented in the Gla-300 group and − 1.81 U (p < 0.0001) in the IDeg-100 group.
Hypoglycemia
SMBG data downloaded in EMRs were available for 32.1% patients in the Gla-300 group and 26.0% patients in the IDeg-100 group. The balance of baseline characteristics between these subgroups was maintained (except marginal differences in systolic blood pressure and albuminuria) (Table S2). Large and comparable numbers of SMBG tests were available in the two groups at each study visit (Table 5).
Proportions of patients with at least one hypoglycemic event of BG ≤ 70 and < 54 mg/dl and related within- and between-group comparisons are shown in Fig. S1. Statistically significant between-group differences in favor of Gla-300 vs. IDeg-100 were documented in the proportions of patients with at least one episode of BG ≤ 70 mg/dl at T6 and BG < 54 mg/dl at all study visits.
Incidence rate of hypoglycemic events (BG ≤ 70 mg/dl and < 54 mg/dl) during the 6-month follow-up by treatment was slightly lower in the Gla-300 group than in the Deg-100 group, without reaching statistical significance (Table 5, top). Incidence rates of hypoglycemic events (BG ≤ 70 mg/dl and < 54 mg/dl) by treatment and study visit are shown in Table 5 (bottom). Hypoglycemic events (BG ≤ 70 mg/dl) significantly decreased by 6 months in the Gla-300 group vs. baseline, while they were unchanged in the IDeg-100 group (Table 5). In terms of between-group comparisons, a 24% lower likelihood to experience a hypoglycemic event of BG ≤ 70 mg/dl (IRR 0.76, 95% CI 0.60–0.96) was found in the Gla-300 group vs. the IDeg-100 group at T6.
Hypoglycemic events (BG < 54 mg/dl) decreased at 6 months in both groups (p = 0.01 for Gla-300 and p < 0.001 for IDeg-100). Furthermore, at T0 a statistically significant 30% lower likelihood to experience an event of BG < 54 mg/dl in the Gla-300 group vs. the IDeg-100 group was found (IRR 0.70, 95% CI 0.49–1.00), while no between-group differences in the risk of hypoglycemia emerged at T3 and T6 (Table 5, bottom).
As a result of the statistically significant lower risk of hypoglycemic event at T0 in Gla-300 vs. IDeg-100, a post hoc analysis was performed, using the frequency of BG < 54 mg/dl events in the 3 months before the switch as an additional PSM variable.
After the post hoc PSM (Table S3), all IRRs suggested a lower rate of hypoglycemia in the Gla-300 group vs. Deg-100 group, although statistical significance was never reached, likely because of the small sample size (Table S4).
There were zero and seven severe hypoglycemic events for the Gla-300 group and IDeg-100 group, respectively (p = 0.02), during the follow-up registered in EMRs in the safety population.
PSM-PP Population
The same analyses on all endpoints were repeated on the PSM-PP population, largely confirming the results obtained in the ITT population (Appendix 1 in the supplementary material).
Site Questionnaire
Overall, 18 site questionnaires were collected and 16 were suitable for statistical analysis. Hypoglycemia concern, less variability, and better control represented the first three reasons for switching to Gla-300. Flexibility of administration, hypoglycemia concern, and less variability represented the first three reasons for switching to IDeg-100 (Fig. S2).
Discussion
This study adds important insights into the impact of switching to Gla-300 or IDeg-100 from 1BI in patients with T1D.
In the PS matched–ITT population, the reduction in HbA1c levels from baseline to 6 months was statistically significant, but of marginal clinical relevance both in the Gla-300 and IDeg-100 groups. Furthermore, a large proportion of patients maintained HbA1c levels > 8% and > 7% after 6 months, despite the slight reduction of mean HbA1c levels. This finding suggests a persistent fear of intensifying the basal insulin therapy, given that the starting HbA1c levels were not particularly elevated. This is also documented by the main reason reported for the switch in these patients that was to avoid hypoglycemic events, which also is the main barrier to proper insulin titration.
The need for further therapy intensification is also underlined by the moderate reduction in FPG levels, which remained well above the FPG target recommended by the American Diabetes Association (ADA)/European Association for the Study of Diabetes (EASD) guidelines and the Italian standards of care (80–130 mg/dl) [28, 29]. Basal insulin doses were only slightly increased, with a higher increase in the Gla-300 group which was in line with evidence from randomized clinical trials and summary of product characteristics [22, 30]. This slight dose titration was not associated with an increase in body weight, which is one of the critical aspects in insulin therapy [31, 32].
Another interesting aspect is the different use of the short-acting insulin: there was no dose modification in the Gla-300 group, while a significant reduction in the IDeg-100 group was documented. This is consistent with other reports in basal-bolus patients treated with IDeg-100 in T1D [33].
Despite the improved PK/PD profile of 2BI and lower risk of hypoglycemia, therapeutic inertia [34, 35] still remains a barrier towards full attainment of glycemic control. It should be considered that Gla-300 was introduced into the market in January 2017. Therefore, its suboptimal titration could be at least partially attributable to the lack of familiarity with the new insulin. Despite these problems, the switch to Gla-300 was as effective, and probably safer, as compared to IDeg-100, which was available in Italy since 2015.
The recent OneCARE real-world retrospective study based on the use of continuous glucose monitoring (CGM) in adults with T1D has shown that the effectiveness of Gla-300, when looking at the full day time in range (TIR) 70–180 mg/dl, was similar to that of IDeg-100. These results reflect those found in adults with T2D [22]. However, TIR results (70–140 and 70–180 mg/dl) favored Gla-300 in the nighttime, as did TIR > 180 mg/dl [36].
As for safety the reduction in the proportions of patients with at least one hypoglycemic event and in the incidence rates of hypoglycemia (BG ≤ 70 mg/dl and < 54 mg/dl) during 6 months in both groups documents that the switch from 1BI to a 2BI is safe. A recent meta-analysis in T1D demonstrated similar glycemic control with lower risk of severe hypoglycemia of Gla-300 versus Gla‐100, particularly during the titration period [37].
However, data showed a higher risk of hypoglycemia at baseline in patients switching to IDeg-100. Since IDeg-100 was available in Italy since 2015, it is plausible that patients showing a high risk of hypoglycemia had already been switched to a 2BI before Gla-300 was made available. The post hoc analysis, including the baseline risk of hypoglycemia as an additional PSM variable, confirmed the results of the primary analysis suggesting potential benefits of Gla-300 vs. IDeg-100 on hypoglycemic risk, although further studies possibly supported by CGM could better clarify this emerging picture. In this regard, the OneCARE study did not show a statistically significant difference between groups in time spent in hypoglycemic ranges (both < 70 and < 54 mg/dl); however, prospective, randomized studies are warranted in this setting [36, 38].
The number of severe hypoglycemic events (seven in the IDeg-100 group vs. none in the Gla-300 group) also suggest a benefit of Gla-300 vs. IDeg-100 on the risk of hypoglycemia. However, it has to be taken into account that the safety population was not PS-matched for the baseline hypoglycemic risk.
Finally, from a methodological standpoint, this study confirms the importance of the secondary use of pre-existing data for clinical research purposes. In this respect, the Italian network of diabetes centers adopting the same EMRs system represents a unique opportunity to conduct large, real-world effectiveness studies.
The study has strengths and limitations. Among the strengths, this is the first RWE comparative study available on the mid-term effectiveness and safety of 2BI in T1D. Furthermore, results are reasonably generalizable owing to the large sample of patients with T1D routinely cared for by centers located in different areas of Italy.
The main limitation of this retrospective analysis was the lack of information on SMBG tests for a large proportion of patients, although the performed analysis is robust owing to the large number of SMBG tests analyzed. The downloading of SMBG values from glucose meters into EMRs was not a common practice in participating centers, suggesting the need to promote the systematic revision of SMBG data through EMRs. Impact on hypoglycemia, both in the short and medium to long term after the switch from previous basal insulins, potentially represents the main clinical benefit of Gla-300 in clinical practice, particularly in this type of patient. Baseline risk of hypoglycemia was not included in the primary analysis to avoid a substantial reduction in the sample size; however, the post hoc analysis addressing the between-group different baseline risk of hypoglycemia largely confirmed overall results. In addition, between-group comparison is also limited by the substantial under-titration of both basal and short-acting insulin, precluding the possibility of a head-to-head comparison between the two basal insulins when optimally used. However, data reflect real life, and clinical inertia is a crucial aspect deserving further consideration. Finally, recent ADA guidelines [39] have modified the threshold of hypoglycemia to BG < 70 mg/ml, while it was BG ≤ 70 mg/dl when the study was designed. However, changing the definition did not impact on overall results.
Conclusion
In this first comparative real-world study with PS matched cohorts of adult patients with T1D, switching from a first-generation basal insulin to Gla-300 or IDeg-100 was associated with similar improvements in glycemic control and overall significant decrease in hypoglycemia, with no severe hypoglycemic events with Gla-300. Trends of lower risk of hypoglycemia with Gla-300 vs. IDeg-100 were found, deserving consideration for future research.
References
Pramming S, Thorsteinsson B, Bendtson I, Binder C. Symptomatic hypoglycaemia in type 1 diabetic patients. Diabet Med. 1991;8:217–22.
Cryer PE, Davis SN, Shamoon H. Hypoglycemia in diabetes. Diabetes Care. 2003;2013(26):1902–12.
Kovatchev B, Cobelli C. Glucose variability: timing, risk analysis, and relationship to hypoglycemia in diabetes. Diabetes Care. 2016;39:502–10.
Rossi MC, Candido R, Ceriello A, et al. Trends over 8 years in quality of diabetes care: results of the AMD annals continuous quality improvement initiative. Acta Diabetol. 2015;52:557–71.
Manicardi V, Russo G, Napoli A, et al. Gender-disparities in adults with type 1 diabetes: more than a quality of care issue. A cross-sectional observational study from the AMD annals initiative. PLoS One. 2016;11:e0162960.
Becker RHA, Dahmen R, Bergmann K, Lehmann A, Jax T, Heise T. New insulin glargine 300 U/mL provides a more even activity profile and prolonged glycemic control at steady state compared with insulin glargine 100 U/mL. Diabetes Care. 2015;38:637–43.
Becker RHA, Nowotny I, Teichert L, Bergmann K, Kapitza C. Low within- and between-day variability in exposure to new insulin glargine 300 U/ml. Diabetes Obes Metab. 2015;17:261–7.
Bergenstal RM, Bailey TS, Rodbard D, et al. Comparison of insulin glargine 300 units/mL and 100 units/mL in adults with type 1 diabetes: continuous glucose monitoring profiles and variability using morning or evening injections. Diabetes Care. 2017;40:554–60.
Heise T, Hermanski L, Nosek L, et al. Insulin degludec: four times lower pharmacodynamic variability than insulin glargine under steady-state conditions in type 1 diabetes. Diabetes Obes Metab. 2012;14:859–64.
Bailey TS, Pettus J, Roussel R, et al. Morning administration of 0.4 U/kg/day insulin glargine 300 U/mL provides less fluctuating 24-hour pharmacodynamics and more even pharmacokinetic profiles compared with insulin degludec 100 U/mL in type 1 diabetes. Diabetes Metab. 2018;44:15–21.
Heise T, Hövelmann U, Nosek L, Hermanski L, Bøttcher SG, Haahr H. Comparison of the pharmacokinetic and pharmacodynamic profiles of insulin degludec and insulin glargine. Expert Opin Drug Metab Toxicol. 2015;11:1193–201.
Riddle M, Bolli GB, Ziemen M, Muehlen-Bartmer I, Bizet F, Home PD. New insulin glargine 300 units/mL versus glargine 100 units/mL in people with type 2 diabetes using basal and mealtime insulin: glucose control and hypoglycemia in a 6-month randomized controlled trial (edition I). Diabetes Care. 2014;37:2755–62.
Yki-Järvinen H, Bergenstal R, Ziemen M, et al. New insulin glargine 300 units/mL versus glargine 100 units/mL in people with type 2 diabetes using oral agents and basal insulin: glucose control and hypoglycemia in a 6-month randomized controlled trial (edition 2). Diabetes Care. 2014;37:3235–43.
Bolli GB, Riddle MC, Bergenstal RM, et al. New insulin glargine 300 U/ml compared with glargine 100 U/ml in insulin-naïve people with type 2 diabetes on oral glucose-lowering drugs: a randomized controlled trial (edition 3). Diabetes Obes Metab. 2015;17:386–94.
Home PD, Bergenstal RM, Bolli GB, et al. New insulin glargine 300 units/mL versus glargine 100 units/mL in people with type 1 diabetes: a randomized, phase 3a, open-label clinical trial (edition 4). Diabetes Care. 2015;38:2217–25.
Meneghini L, Atkin SL, Gough SC, et al. The efficacy and safety of insulin degludec given in variable once-daily dosing intervals compared with insulin glargine and insulin degludec dosed at the same time daily. Diabetes Care. 2013;36:858–64.
Mathieu C, Hollander P, Miranda-Palma B, et al. Efficacy and safety of insulin degludec in a flexible dosing regimen vs insulin glargine in patients with type 1 diabetes (BEGIN: Flex T1): a 26-week randomized, treatto-target trial with a 26-week extension. J Clin Endocrinol Metab. 2013;98:1154–62.
Heller S, Buse J, Fisher M, et al. Insulin degludec, an ultralongacting basal insulin, versus insulin glargine in basal-bolus treatment with mealtime insulin as part in type 1 diabetes (BEGIN Basal-Bolus Type 1): a phase 3, randomised, open-label, treat-to-target non-inferiority trial. Lancet. 2012;379:1489–97.
Zinman B, Philis-Tsimikas A, Cariou B, et al. Insulin degludec versus insulin glargine in insulin-naive patients with type 2 diabetes: a 1-year, randomized, treat-to-tar-get trial (BEGIN Once Long). Diabetes Care. 2012;35:2464–71.
Garber AJ, King AB, Del Prato S, et al. Insulin degludec, an ultra-longacting basal insulin, versus insulin glargine in basal-bolus treatment with mealtime insulin aspart in type 2 diabetes (BEGIN Basal-Bolus Type 2): a phase 3, randomised, open-label, treat-to-target non-inferiority trial. Lancet. 2012;379:1498–507.
Gough SC, Bhargava A, Jain R, et al. Low-volume insulin degludec 200 units/mL once daily improves glycemic control similar to insulin glargine with low risk of hypo-glycemia in insulin-naive patients with type 2 diabetes: a 26-week, randomised, controlled, multinational, treat-to-target trial: the BEGIN LOW VOLUME trial. Diabetes Care. 2013;36:2536–42.
Rosenstock J, Cheng A, Ritzel R, et al. More similarities than differences testing insulin glargine 300 units/mL versus insulin degludec 100 units/mL in insulin-naive type 2 diabetes: the randomized head-to-head BRIGHT trial. Diabetes Care. 2018;41:2147–54.
Berger ML, Sox H, Willke RJ, et al. Good practices for real-world data studies of treatment and/or comparative effectiveness: recommendations from the joint ISPOR-ISPE special task force on real-world evidence in health care decision making. Value Health. 2017;20:1003–8.
Zhou FL, Ye F, Berhanu P, Gupta VE, et al. Real-world evidence on clinical and economic outcomes of switching to insulin glargine 300 units/mL vs other basal insulins in patients with type 2 diabetes on basal insulin. Diabetes Obes Metab. 2018;20:1293–7.
Blonde L, Zhou FL, Bosnyak Z, et al. Real-world evidence demonstrates comparable clinical outcomes of switching from insulin glargine 100 U/mL (Gla-100) to insulin glargine 300 U/mL (Gla-300) vs insulin degludec (IDeg) in patients with type 2 diabetes (T2D). Poster presented at WCIRDC; November 30–December 2 2017; Los Angeles.
Meneghini L, Zhou FL, Bosnyak Z, Berria R, Jimenez J, Bailey T. Hypoglycemia risk associated with basal insulin use in type 2 diabetes (T2DM): the lightning study. Poster presented at WCIRDC; November 30–December 2 2017; Los Angeles.
Siegmund T, Tentolouris N, Knudsen ST, et al. A European, multicentre, retrospective, non-interventional study (EU-TREAT) of the effectiveness of insulin degludec after switching basal insulin in a population with type 1 or type 2 diabetes. Diabetes Obes Metab. 2018;20:689–97.
International Hypoglycaemia Study Group. Glucose concentrations of less than 3.0 mmol/L (54 mg/dL) should be reported in clinical trials: a joint position statement of the American Diabetes Association and the European Association for the Study of Diabetes. Diabetes Care. 2017;40:155–7.
AMD-SID. Standard italiani per la cura del diabete mellito 2018. [Italian]. https://aemmedi.it/wp-content/uploads/2018/06/AMD-Standard-unico-protetto.pdf. Accessed 28 July 2020.
Summary of product characteristics. https://www.ema.europa.eu/en/documents/product-information/toujeo-epar-product-information_en.pdf. Accessed 17 Nov 2020.
Hodish I. Insulin therapy, weight gain and prognosis. Diabetes Obes Metab. 2018;20:2085–92.
Cahn A, Miccoli R, Dardano A, Del Prato S. New forms of insulin and insulin therapies for the treatment of type 2 diabetes. Lancet Diabetes Endocrinol. 2015;3:638–52.
Ponzani P, Berra C, Di Lelio A, et al. Switching patients with type 1 diabetes to insulin degludec from other basal insulins: real-world data of effectiveness and safety. Diabetes Ther. 2020;11:97–105.
Russell-Jones D, Pouwer F, Khunti K. Identification of barriers to insulin therapy and approaches to overcoming them. Diabetes Obes Metab. 2018;20:488–96.
Khunti K, Giorgino F, Berard L, Mauricio D, Harris SB. The importance of the initial period of basal insulin titration in people with diabetes. Diabetes Obes Metab. 2020;22:722.
Conget I, Delgado E, Mangas MA, et al. Effectiveness and safety of Gla-300 vs IDeg-100 evaluated with continuous glucose monitoring profile in adults with type 1 diabetes in routine clinical practice in Spain: OneCARE study. Diabetologia 2020;63(Suppl 1):S1–S485. (Abs 670). https://doi.org/10.1007/s00125-020-05221-5. EASD 2020 21–25 September - Virtual Meeting (P670). https://www.easd.org/virtualmeeting2020/#!contentsessions/3332.
Danne T, Matsuhisa M, Sussebach C, et al. Lower risk for severe hypoglycaemia with insulin glargine 300 U/mL vs glargine 100 U/mL in participants with type 1 diabetes: a meta-analysis of 6-month phase 3 clinical trials. Diabetes Obes Metab. 2020. https://doi.org/10.1111/dom.14109.
Comparison of glucose values and variability between TOUJEO and TRESIBA during continuous glucose monitoring in type 1 diabetes patients (inRange). ClinicalTrials.gov Identifier: NCT04075513 https://www.clinicaltrials.gov/ct2/show/NCT04075513?term=LPS14947&rank=1.
American Diabetes Association. Standards of medical care in diabetes-2020. Diabetes Care. 2020;43(Suppl 1):S66-76.
Acknowledgements
Funding
The study was funded by Sanofi S.r.l., Milan, Italy. Sanofi also funded the journal’s Rapid Service Fee.
Additional Assistance
Coresearch (Pescara, Italy) was the Clinical Research Organization involved in the data management (Giuseppe Prosperini, Michele Sacco), statistical analysis (Giuseppe Lucisano, Antonio Nicolucci), and medical writing (Maria Chiara Rossi, Antonio Nicolucci) of the study. Meteda (San Benedetto, Italy) developed the software for the data extraction. OPIS (Milano, Italy) was the Clinical Research Organization involved in regulatory activities of the study.
Authorship
All named authors meet the International Committee of Medical Journal Editors (ICMJE) criteria for authorship for this article, take responsibility for the integrity of the work as a whole, and have given their approval for this version to be published.
RESTORE-1 Study Group
Participating centers (in alphabetical order by town): Rabini Rosa Anna, Galetta Marianna, ASUR Marche Area Vasta 5, Ascoli; Giorgino Francesco, Laviola Luigi, Nalla Montedoro, AO Universitaria Consorziale Policlinico di Bari, Bari; Frittitta Lucia, Tumminia Andrea, ARNAS Garibaldi, PO Garibaldi Nesima, Catania; Di Blasi Vincenzo, Ospedale Cava De’ Tirreni, Cava De' Tirreni; Gregori Giovanna, Azienda Usl 1—Ospedale Civile di Carrara, Massa Carrara; Di Benedetto Antonino, AOU Policlinico G. Martino, Messina; Orsi Emanuela, Radaelli Francesca, IRCCS Policlinico Ospedale Maggiore, Milano; Fiorina Paolo, Lunati Elena Maria, Teresa Letizia, Ospedale Fatebenefratelli e Oftalmico, Milano; Bosi Emanuele, Scavini Marina, Andrea Laurenzi, IRCCS Ospedale San Raffaele, Milano; Cavalot Franco, Barale Cristina, AOU San Luigi Gonzaga, Orbassano; Porcellati Francesca, Cioli Patrizia, Ospedale S. Maria della Misericordia, Perugia; Del Sindaco Paola, Di Loreto Chiara, Servizio Diabetologia del Perugino—USL Umbria 1, Perugia; Consoli Agostino, Ginestra Federica, Ospedale Civile di Pescara, Pescara; Del Prato Stefano, Garofolo Monia, AOU Pisana Ospedale Cisanello, Pisa; D'Angelo Paola, Carletti Silvia, Ospedale S. Pertini, Roma; Rabini Rosa Anna, Galetta Marianna, ASUR Marche Area Vasta 5, San Benedetto del Tronto; Oleandri Salvatore, Mainolfi Alessandra Rita, ASL CN1 Ospedale SS. Annunziata, Savigliano; Broglio Fabio, Beccuti Guglielmo, AOU San Giovanni Battista Le Molinette, Torino; Arnaldi Claudia, Struttura Territoriale ASL Viterbo, Viterbo.
Disclosures
Luigi Laviola has received consultation fees and speaker’s fees and from Abbott, AstraZeneca, Boehringer Ingelheim, Lilly, Medtronic, Mundipharma, NovoNordisk, Roche, Sanofi. Francesca Porcellati has received honoraria for lectures and consultations from AstraZeneca, Sanofi, Eli Lilly, Mundipharma and Medtronic. Daniela Bruttomesso has received a speaker honorarium from Sanofi, Novo Nordisk, Lilly. Monica Larosa is an employee of Sanofi. Antonio Nicolucci and Maria Chiara Rossi received funding for research from Sanofi, NovoNordisk, Alfasigma, Artsana, AstraZeneca, Johnson&Johnson, Medtronic, Shionogi, SOBI, Meteda and Theras.
Compliance with Ethics Guidelines
All procedures followed were in accordance with the ethical standards of the responsible committee on human experimentation (institutional and national) and with the Helsinki Declaration of 1975, as revised in 2008 [5]. The study protocol was approved by all local ethics committees of the participating centers. Informed consent was obtained from all patients for being included in the study.
Data Availability
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
Author information
Authors and Affiliations
Consortia
Corresponding author
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License, which permits any non-commercial use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc/4.0/.
About this article
Cite this article
Laviola, L., Porcellati, F., Bruttomesso, D. et al. Comparative Effectiveness of Switching From First-Generation Basal Insulin to Glargine 300 U/ml or Degludec 100 U/ml in Type 1 Diabetes: The RESTORE-1 Study. Diabetes Ther 12, 509–525 (2021). https://doi.org/10.1007/s13300-020-00982-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13300-020-00982-z