Abstract
Introduction
To evaluate time in range metrics and HbA1c in people with type 2 diabetes (T2D) treated with ultra rapid lispro (URLi) using continuous glucose monitoring (CGM) for the first time in this population.
Methods
This was a Phase 3b, 12-week, single-treatment study in adults with T2D on basal-bolus multiple daily injection (MDI) therapy using basal insulin glargine U-100 along with a rapid-acting insulin analog. Following a 4-week baseline period, 176 participants were newly treated with prandial URLi. Participants used unblinded CGM (Freestyle Libre). Primary endpoint was time in range (TIR) (70–180 mg/dl) during the daytime period at Week 12 compared to baseline with gated secondary endpoints of HbA1c change from baseline and 24-h TIR (70–180 mg/dl).
Results
Improved glycemic control was observed at Week 12 versus baseline including mean daytime TIR (change from baseline [Δ] 3.8%; P = 0.007), HbA1c (Δ − 0.44%; P < 0.001), and 24-h TIR (Δ 3.3%; P = 0.016) with no significant difference in time below range (TBR). After 12 weeks, there was a statistically significant decrease in postprandial glucose incremental area under curve, overall, across all meals, within 1 h (P = 0.005) or 2 h (P < 0.001) after the start of a meal. Basal, bolus, and total insulin dose were intensified with increased bolus/total dose ratio at Week 12 (50.7%) versus baseline (44.5%; P < 0.001). There were no severe hypoglycemia events during the treatment period.
Conclusions
In people with T2D, URLi in an MDI regimen was efficacious with improved glycemic control including TIR, HbA1c, and postprandial glucose without increased hypoglycemia/TBR.
Clinical Trial registration number
NCT04605991.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Why carry out this study? |
Ultra rapid lispro (URLi) is an insulin lispro formulation developed to more closely match physiological insulin secretion and improve postprandial glucose control |
This study assessed time in range metrics from continuous glucose monitoring (CGM) and HbA1c in people with type 2 diabetes treated with URLi as part of multiple daily injection therapy. |
What was learned from the study? |
Treatment with URLi for 12 weeks resulted in improved glycemic control including time in range and HbA1c without increased hypoglycemia |
This study provided the first CGM data with URLi in people with type 2 diabetes, further confirming the efficacy and safety of URLi in this patient population |
Introduction
Overall glycemic control in people with diabetes is most commonly evaluated by mean HbA1c, which provides an average of glucose levels over a period of approximately 3 months [1]. While HbA1c is considered the gold standard for assessing long-term glycemic control and has been correlated to the risk of developing long-term diabetes complications [2], it does not measure short-term glucose fluctuations. Individuals may therefore experience differing levels of glycemic variability, hypoglycemia, and hyperglycemia for a given HbA1c level [3]. Continuous glucose monitoring (CGM) is increasingly used in the management of type 2 diabetes (T2D) and allows measurement of an individual’s daily glucose patterns, complementing HbA1c to provide a more complete picture of short- and long-term glycemic control [4]. An expert panel has published international consensus guidelines on the collection, interpretation, and reporting of CGM data [5]. Key metrics include time in range (TIR) (70–180 mg/dl), time in hypoglycemia/time below range (TBR) (< 54 mg/dl and < 70 mg/dl), and time in hyperglycemia/time above range (TAR) (> 180 and > 250 mg/dl) [5].
Despite advancements in diabetes therapy, elevated postprandial glucose (PPG) remains a challenge for many people with diabetes [6, 7]. Rapid-acting insulin analogs have been developed to target improvement in PPG control and improve glycemic outcomes [8]. Studies have shown that introducing a rapid-acting prandial insulin as part of a basal-bolus multiple daily injection (MDI) regimen is more effective at managing PPG than a basal-only regimen [9,10,11]. Nevertheless, rapid-acting insulins still may not have fast enough onset to match carbohydrate absorption resulting in inadequate control of PPG [12].
Ultra rapid lispro (URLi; Lyumjev®) is a formulation of insulin lispro with a faster onset and shorter duration of action compared to Lispro (Humalog®) and was developed to more closely match physiological insulin secretion and improve PPG control [12]. URLi has demonstrated non-inferiority to Lispro in HbA1c change from baseline after 26 weeks in people with type 1 diabetes (T1D) (PRONTO-T1D) and T2D (PRONTO-T2D) while providing superior PPG control when dosed at mealtime [13, 14]. The PRONTO-T1D study featured a blinded CGM substudy, which found that improvements in PPG control observed with mealtime URLi compared to Lispro were also associated with increased TIR (71–180 mg/dl) during the daytime period [15]. CGM was not included in the PRONTO-T2D study. The present PRONTO-TIR study provides the first CGM data in people with T2D treated with URLi.
The aim of this study was to evaluate TIR (70–180 mg/dl) from CGM with 12 weeks of URLi treatment in combination with insulin glargine U-100 in people with T2D on MDI therapy. Additionally, HbA1c change from baseline and other key CGM metrics were evaluated.
Methods
Study Design
This was a Phase 3b, prospective, open-label, outpatient, multicenter, single-treatment-group study conducted in participants with T2D currently treated with a basal-bolus MDI regimen. The study consisted of a separate screening period, a 4-week baseline period, a 12-week treatment period, and a 2-week safety follow-up (Fig. 1). Participants used unblinded CGM (Freestyle Libre) for diabetes management and glucose data collection during the study.
The study was conducted at 31 sites in the USA in accordance with the Declaration of Helsinki, the International Conference on Harmonization Guidelines for Good Clinical Practice, and applicable local laws and regulations. The study protocol and all procedures were reviewed and approved by a central ethics review board (IntegReview Ethical Review Board and/or Advarra) for all study centers, as well as local ethics review boards, if required (details provided in Supplementary Table 1). All participants provided written informed consent before participating in the study. All authors gave approval for the final version of the manuscript to be published. The study was registered at ClinicalTrials.gov (NCT04605991).
Participants
Adults aged at least 18 years with T2D for at least 1 year, HbA1c 7.5–10.0%, and body mass index (BMI) ≤ 40.0 kg/m2 were eligible for participation if they had been treated with basal-bolus MDI insulin therapy for 90 days prior to screening that included insulin glargine U-100 as basal insulin along with prandial injection of a bolus insulin analog (lispro, aspart, or glulisine). Participants could be treated with up to three of the following oral antihyperglycemic medications (OAM): metformin, dipeptidyl peptidase-4 inhibitor, sodium glucose cotransporter 2 inhibitor, and oral glucagon-like peptide 1 (GLP-1) agonist. Participants could also be treated with an injectable GLP-1 receptor agonist. Participants had to be willing to use the CGM device supplied for glucose monitoring, diabetes management, and data collection.
Participants were excluded from the study if they had any episodes of severe hypoglycemia within the 6 months prior to screening; had an episode of diabetic ketoacidosis within 6 months prior to screening; had hypoglycemia unawareness, as judged by the investigator; or had excessive insulin resistance, defined as receiving a total daily dose of insulin greater than 2.0 U/kg at screening.
Interventions and Treatment
Participants continued their prestudy basal and prandial insulin therapy during the baseline period. At Week 0, participants started URLi treatment, initially transitioning unit-for-unit from their prestudy prandial insulin. URLi was injected immediately (0–2 min) prior to each meal. Starting at Week 0, all participants were treated with sponsor-provided glargine U-100 (Basaglar®).
During the treatment period, insulin doses were titrated to achieve protocol glucose targets in line with those recommended by the American Association of Clinical Endocrinologists (Supplementary Table 2) [16].
Basal insulin (glargine U-100) was titrated to a fasting blood glucose target of 100 mg/dl, and the dose could be adjusted by the investigator based on CGM and hypoglycemia data (Supplementary Table 3). Basal insulin dose was reviewed by the investigator at each visit during the 12-week treatment period or more frequently if required.
The study used two plans for determining prandial insulin dosing: a carbohydrate-counting plan where prandial insulin dose was based on the participant’s estimated carbohydrate content of the meal or a fixed-dose plan where the participant was prescribed a fixed dose or dose range of insulin for each meal, which could be individualized for each meal. The same prandial insulin dosing plan was maintained throughout the study. A correction factor could be implemented with either dosing plan. Prandial insulin was titrated to reach the protocol target glucose values (Supplementary Table 2) during the treatment period and dose could be adjusted based on the judgment of the investigator, for example, in response to hypoglycemia.
For participants using carbohydrate counting, the insulin to carbohydrate ratio and correction factor were reviewed and adjusted by the investigator, based on CGM and hypoglycemia data, during the treatment period.
With the fixed-dosing plan, participants utilized a self-titration prandial insulin algorithm based on review of CGM glucose values for the corresponding meal or bedtime from the previous day (Supplementary Table 4). The CGM glucose value from the premeal or bedtime from the previous day was used as the “adjustment value,” and change in dose was based on the following values: CGM glucose < 80 mg/dl, decrease by one unit; glucose 80–109 mg/dl, no change; CGM glucose > 109 mg/dl, increase by one unit (Supplementary Table 4). Prandial insulin doses and correction factor were reviewed by the investigator based on CGM and hypoglycemia data.
Continuous Glucose Monitoring
All participants used unblinded CGM (FreeStyle Libre 14 day flash glucose monitoring system or intermittently scanned CGM) for glucose data collection from the beginning of the baseline period through to the end of the treatment period. To ensure that only valid CGM days with sufficient data were included in analyses, the following criterion was used to determine a valid CGM day: minimum number of measures per day of at least 70% of the total measures that are supposed to be obtained for the 24-h period. For calculation of CGM outcome variables, daytime was defined as 06:00 to 23:59 and nighttime as 00:00 to 05:59.
Assessments
The primary endpoint was percentage of TIR (70–180 mg/dl) during the daytime period with 14 days of CGM use at Week 12 compared with baseline. Gated secondary endpoints were: (1) change from baseline to Week 12 in HbA1c and (2) percentage of TIR (70–180 mg/dl) during the 24-h period at Week 12 compared with baseline. Other prespecified secondary objectives were to evaluate daytime and 24-h TBR (< 54 mg/dl), TAR (> 180 mg/dl), and TAR (> 250 mg/dl); PPG incremental area under curve (iAUC); the bolus, basal, and total insulin doses and bolus/total insulin ratio; the incidence of severe hypoglycemia; and the Insulin Treatment Satisfaction Questionnaire (ITSQ) glycemic control domain scores.
Participant Satisfaction and Attitude Measures
Participants were asked to complete the ITSQ at baseline and Week 12. The ITSQ is a validated instrument containing 22 items to assess treatment satisfaction where items are measured on a 7-point Likert-type scale [17]. Scores were transformed to give an overall score as well as scores for domains of satisfaction, categorized as inconvenience of regimen, lifestyle flexibility, hypoglycemic control, glycemic control, and delivery system satisfaction.
Participants were asked to complete a single Study Mealtime Insulin Experience question specifically developed for this study at Week 12. This assessed the likelihood of incorporating the mealtime insulin used in this study (URLi) in their diabetes management routine. The question was rated on a 5-point scale ranging from “1 = very unlikely” to “5 = very likely.”
Participants were asked to complete the Diabetes-Specific Attitudes about Technology Use scale (DSAT) at baseline and Week 12. The DSAT is a 5-item scale where each item is rated on a 5-point Likert scale to indicate agreement with each statement, with higher scores indicating more positive attitudes about devices and technology [18]. Individual ratings were summed to obtain a total score.
Statistical Methods
Approximately 167 participants were planned to be assigned to URLi treatment such that 150 participants completed the study through the primary endpoint at Week 12. This sample size had approximately 80% power to detect an increase of 4.5% TIR (70–180 mg/dl) during the daytime period from baseline assuming no true difference in the TIR (70–180 mg/dl) between baseline and endpoint, a standard deviation (SD) of 19.5%, and a 10% dropout rate. Unless otherwise noted, all tests were conducted at a two-sided alpha level of 0.05, and two-sided confidence intervals (CIs) were calculated at 95%. A gatekeeper method was used to control the overall type 1 error for the primary and gated secondary objectives [19].
Efficacy analyses were conducted on the treated population consisting of all enrolled participants who received at least one dose of investigational product (IP) and included data collected prior to permanent discontinuation of IP. When change from baseline was included as a response variable of analysis models, participants were included in the analysis only if a baseline and a post-baseline measurement were available.
The primary efficacy comparison was based on a restricted-maximum-likelihood-based, mixed model repeated measurements (MMRM) analysis of change from baseline in TIR (70–180 mg/dl) during the daytime period. All observations at each scheduled postbaseline visit were included in the analysis. The model for analysis of the primary endpoint of change from baseline in TIR (70–180 mg/dl) during the daytime period included the fixed class effect of visit, the covariate of baseline, and the random effect of participant. An unstructured covariance structure was used to model the within-participant errors. Significance tests were based on the least squares means (LSMs) and Type III tests. SAS® PROC MIXED was used to perform the analysis.
If the change from baseline in daytime TIR (70–180 mg/dl) was found to be significant, gated secondary objectives were tested in sequence until the first null hypothesis in the sequence failed to be rejected at a two-sided 0.05 significance level.
For continuous longitudinal secondary and exploratory endpoints, an MMRM similar to that for the primary analysis was used. Continuous longitudinal secondary endpoints included duration and percent of TIR (70–180 mg/dl), TBR, and TAR as well as actual and change from baseline in basal, bolus, and total insulin dose and the basal/total dose ratio. Continuous nonlongitudinal endpoints, including iAUC0–1 h and iAUC0–2 h, were analyzed using an analysis of covariances (ANCOVA) model that included baseline as a covariate.
Safety analyses were conducted on all enrolled participants who received at least one dose of IP. Adverse events (AEs) from the first dose of IP through safety follow-up were listed and summarized by preferred term and/or system organ class using the Medical Dictionary for Drug Regulatory Activities (MedDRA). Severe hypoglycemia, defined as an episode requiring assistance due to neurological impairment as confirmed by the investigator, was reported as a serious adverse event (SAE).
Results
Participants
A total of 187 participants were enrolled in the study, 176 received at least one dose of study drug, and 154 completed treatment. Demographics and baseline characteristics are provided in Table 1. The mean (SD) age of participants was 62.8 (10.2) years, BMI was 32.0 (4.8) kg/m2, and duration of T2D was 19.2 (7.9) years. Most participants were treated with insulin lispro U-100 as their prestudy bolus insulin (63.1%). Most were using fixed dose for their prandial insulin dosing plan (85.8%) and 22.2% were using prior personal CGM. A total of 61.9% of participants were treated with at least one OAM, the most common being metformin, used by 54.0% of participants, and 24.4% of participants were treated with an injectable GLP-1 receptor agonist.
Primary Objective–Time in Range During the Daytime Period
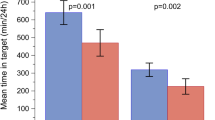
At Week 12, TIR (70–180 mg/dl) during the daytime period was 62.9% (679.7 min) (Fig. 2A). The primary objective was achieved: there was a statistically significant increase in daytime TIR (70–180 mg/dl) at Week 12 compared with baseline (LSM change from baseline 3.8%; P = 0.007).
Key CGM parameters for a daytime period (06:00–23:59) and b 24-h period. Data are LSM ± SE, *P < 0.05, **P < 0.01. Δ change from baseline, LSM least squares mean, n number of participants with valid CGM data per prespecified criteria, SE standard error, TAR time above range, TBR time below range, TIR time in range
Gated Secondary Objectives
HbA1c
Mean HbA1c improved during the 4-week baseline period from 8.57 to 8.20%. After 12 weeks of treatment with URLi, there was further improvement in mean HbA1c to 7.76%. The first gated secondary objective was achieved: there was a significant change from baseline in HbA1c at Week 12 (LSM change from baseline –0.44%; P < 0.001) (Fig. 3).
Twenty-four-hour Time in Range
At Week 12, TIR (70–180 mg/dl) during the 24-h period was 64.6% (929.8 min) (Fig. 2B). The second gated secondary objective was achieved: there was a significant increase in 24-h TIR (70–180 mg/dl) at Week 12 compared to baseline (LSM change from baseline 3.3%; P = 0.016).
Other Efficacy Results
CGM-Derived Metrics
At Week 12 compared to baseline, there was no significant difference in daytime TBR (< 54 mg/dl) (0.9% vs. 0.9%) or TBR (< 70 mg/d)l (3.6% vs. 3.2%) (Fig. 2A). Similar results were observed for the 24-h period: there was no significant difference in 24-h TBR (< 54 mg/dl) (1.2% vs. 1.2%) or TBR (< 70 mg/dl) (3.9% vs. 3.9%) at Week 12 compared to baseline (Fig. 2B).
There was significantly lower daytime TAR (> 180 mg/dl) at Week 12 compared to baseline (33.5% vs. 38.0%; LSM change from baseline − 4.3%; P = 0.005) and TAR (> 250 mg/dl) (8.6% vs. 10.5%; LSM change from baseline − 1.56%; P = 0.046) (Fig. 2A). There was significantly lower 24-h TAR (> 180 mg/dl) at Week 12 compared to baseline (31.6% vs. 35.3%; LSM change from baseline − 3.4%; P = 0.026) but no significant difference in 24-h TAR (> 250 mg/dl) (Fig. 2B).
Continuous Glucose Monitoring Consensus Targets
The proportion of participants achieving key CGM-based glycemic targets as recommended by the International Consensus on Time in Range is shown in Supplementary Table 5 [5]. The proportion of participants achieving the recommended CGM target of > 70% TIR (70–180 mg/dl) significantly increased at Week 12 compared to baseline (48.12% vs. 28.10%; P < 0.001), as did the proportion with < 5% TAR (> 250 mg/dl) (53.38% vs. 38.56%; P = 0.002). The proportion of participants with < 1% TBR (< 54 mg/dl) was similar (71.43% vs. 66.67%; P = 0.407).
Hourly Ambulatory Glucose Profiles, Mean Sensor Glucose, and Glucose Variability
Figure 4A illustrates hourly mean ambulatory glucose profiles (AGPs) over 24 h. The median and percentile hourly AGPs are provided in Supplementary Fig. 1. Glucose levels were generally lower at Week 12 compared to baseline. Mean sensor glucose for the 24-h period was 158.5 mg/dl at Week 12 versus 163.4 mg/dl at baseline (LSM change from baseline − 4.6 mg/dl; P = 0.050).
To evaluate glucose variability, actual and change from baseline within-day, between-day, and overall glucose variability (%CV) were analyzed. At Week 12 compared to baseline, there was a statistically significant decrease in between-day glucose variability (LSM change from baseline − 0.9%; P = 0.028) and overall glucose variability (LSM change from baseline − 1.2%; P = 0.003) while within-day variability was similar (Supplementary Table 6).
Postprandial Glucose
Figure 4B illustrates mean AGPs for the period 0–4 h after breakfast, lunch, and dinner, and across all meals. The median and percentile postmeal AGPs are provided in Supplementary Fig. 2. Lower PPG was observed at Week 12 compared to baseline.
Postprandial iAUC was calculated for each meal and across all meals. There was a statistically significant decrease in postprandial iAUC within 1 h after the start of a meal (iAUC0–1 h) at dinner (P = 0.035) and overall across meals (P = 0.005) at Week 12 compared to baseline (Fig. 5). There was a statistically significant decrease in postprandial iAUC within 2 h after the start of a meal (iAUC0–2 h) at breakfast (P = 0.035), dinner (P = 0.035), and overall across meals (P < 0.001) at Week 12 compared to baseline (Fig. 5).
Subgroup Analyses
Daytime TIR (70–180 mg/dl) was assessed by subgroups of participants with (n = 35) and without (n = 118) prior personal CGM use. The group with prior personal CGM use had a higher baseline daytime TIR (70–180 mg/dl) (63.9%) than the group with no prior CGM use (57.2%). Daytime TIR improved in both groups. There was a significant increase in the daytime time in range (70–180 mg/dl) at Week 12 for the subgroup with no prior CGM (LSM change from baseline 3.4%; P = 0.027). The subgroup with prior CGM use also had improved TIR at Week 12 but the change did not meet statistical significance (LSM change from baseline 5.0%; P = 0.133).
Daytime TIR (70–180 mg/dl) was also assessed by subgroups of participants using carbohydrate counting (n = 25) and fixed-dose (n = 128) plan for bolus insulin dosing. The group using carbohydrate counting showed an improvement in TIR (70–180 mg/dl) from 59.8% at baseline to 71.4% at Week 12 (LSM change from baseline 12.7%; P < 0.001). For the group using a fixed-dose plan, there was a numerical increase in TIR (70–180 mg/dl) from baseline (58.6%) to Week 12 (61.5%); however, the change did not meet statistical significance (LSM change from baseline 2.3%; P = 0.111).
Insulin Dose
At Week 12, there was a statistically significant increase in basal, bolus, and total insulin doses compared to baseline (Fig. 6), and the basal/total insulin ratio was significantly higher at Week 12 versus baseline (50.7% vs. 44.5%; P < 0.001).
Insulin Treatment Satisfaction Questionnaire
A summary and analysis of the ITSQ and domains are provided in Supplementary Table 7. There was a statistically significant increase in transformed score at Week 12 compared to baseline for the prespecified secondary objective of ITSQ glycemic control domain (LSM change from baseline 16.9; P < 0.001). At Week 12 compared to baseline, there was also a statistically significant increase in mean total ITSQ score (LSM change from baseline 7.4; P < 0.001) and in the mean transformed scores in the insulin delivery device satisfaction (P < 0.001), inconvenience of regimen (P < 0.001), and lifestyle flexibility domains (P = 0.038). The mean transformed score for the hypoglycemic control domain was similar at Week 12 (73.4) versus baseline (70.8).
Participants’ Willingness to Incorporate URLi into Diabetes Management Routine
Analysis of the Study Mealtime Insulin Experience question at Week 12 found that most participants were “very likely” to incorporate URLi into their diabetes management routine (64.1%), with 86% responding with “likely” or “very likely” (Supplementary Table 8).
Diabetes-Specific Attitudes About Technology Use
There was no statistically significant change in DSAT total score at Week 12 compared to baseline.
Safety
No severe hypoglycemia events were reported during the treatment period. There were no deaths in the study. From the first dose of IP through safety follow-up, there were three SAEs and three AEs that led to discontinuation from the study. The AEs that led to study discontinuation were COVID-19, hepatic cirrhosis, and iron deficiency anemia and were not considered by the investigator to be related to study treatment. A total of 46 participants (26.1%) reported at least one treatment-emergent adverse event (TEAE) (Table 2). Five participants experienced injection site reaction (ISR) TEAEs (Table 2). All ISRs were mild and recovered/resolved during the study. There were no discontinuations due to ISRs.
There were no clinically significant changes in vital signs from baseline to Week 12. There was a 0.7-kg increase in weight and a 0.3-kg/m2 increase in BMI from baseline to Week 12.
Discussion
This Phase 3b, single-treatment study assessed treatment with URLi for 12 weeks in combination with basal insulin glargine U-100 in people with T2D using MDI therapy. Twelve weeks of URLi treatment provided improved TIR (70–180 mg/dl) during the daytime and 24-h periods compared to baseline as well as improved HbA1c, without evidence of increased TBR or hypoglycemia.
This study is the first to report CGM data with URLi in people with T2D, providing additional information on daily glucose variations that cannot be captured by HbA1c alone. International consensus guidelines include CGM metrics as a key measure of glycemic control [5, 20], and lower TIR has been shown to be associated with increased long-term diabetes complications in people with T2D, including diabetic retinopathy [21] and all-cause and cardiovascular disease mortality [22]. In a survey of people with T1D or T2D, TIR was identified by one of the highest proportion of people as having a “big impact” on daily life, second only to food choices [23], demonstrating the importance of TIR to people living with diabetes.
The PRONTO-T1D trial included a CGM substudy [14, 15], which found that URLi, in an MDI regimen, administered at the start of a meal improved daytime TIR (71–180 mg/dl) and PPG control compared to Lispro [15]. The PRONTO-T2D study, however, did not include a CGM substudy. Therefore, evaluation of TIR metrics was not available to people with T2D using URLi prior to the present trial. In the PRONTO-T2D study, it was demonstrated that URLi, in an MDI regimen, was non-inferior for HbA1c and superior for PPG control compared to Lispro in participants with T2D on MDI therapy [13]. In an analysis of the PRONTO-T2D trial by age subgroups < 65 years and ≥ 65 years, treatment with URLi resulted in mean HbA1c < 7% after 26 weeks and significantly lowered PPG excursions compared to Lispro in both age groups [24]. The present study also overall included an older population with mean (SD) age of 62.8 (10.2) years and ~ 45% of participants over age 65 years of age. The findings of the present study reflect those of prior reports on URLi treatment for people with T2D and build on previous results by providing CGM data in the T2D population for the first time to our knowledge.
The primary objective of this study was achieved with significantly improved daytime TIR (70–180 mg/dl) at Week 12 compared to baseline. Gated secondary objectives of HbA1c change from baseline and 24-h TIR (70–180 mg/dl) were also met. Importantly, improved TIR was achieved without increasing TBR (< 54 or < 70 mg/dl), which was similar at Week 12 compared to baseline. Additionally, there were no severe hypoglycemia events during the study, demonstrating that URLi treatment improved glycemic control without evidence of increased hypoglycemia/TBR.
TAR was decreased following URLi treatment especially during the daytime, which is the period when prandial insulins are typically used and so when the ultra-rapid acting nature of URLi might be expected to have the biggest impact. While TAR (> 180 mg/dl) was significantly lower at Week 12 versus baseline during the 24-h period, both TAR (> 180 mg/dl) and TAR (> 250 mg/dl) were significantly improved during the daytime.
The proportion of participants achieving the consensus guideline recommend [20] CGM target of > 70% TIR (70–180 mg/dl) significantly increased at Week 12 compared to baseline as did the proportion with < 5% TAR (> 250 mg/dl). There was no significant difference in the proportion of participants with < 1% glucose values in TBR (< 54 mg/dl).
Time in range metrics were reflected in the hourly mean AGPs. Mean glucose levels were lower at Week 12 compared to baseline for most of the day. Overall, mean sensor glucose for the 24-h period numerically decreased from baseline to Week 12 (change from baseline − 4.6 mg/dl; P = 0.050). Within-day and overall glucose variability (%CV) was significantly lower at Week 12 compared to baseline. Glucose variability measures were below the consensus guidelines target %CV of ≤ 36% [20], which is considered the threshold to distinguish stable and unstable glycemia in people with diabetes [20, 25].
Treatment with URLi also demonstrated improved PPG control compared to baseline as characterized by mean AGPs after meals and reflected in iAUC within 1 h or 2 h after the start of a meal. Following URLi treatment, iAUC0–1 h and iAUC0–2 h were significantly lower at dinner and overall across all meals while iAUC0–2 h was also significantly improved at breakfast. These results are in line with previous studies where URLi has consistently shown improved PPG control, including in PRONTO-T1D [14] and its CGM substudy [15] and in PRONTO-T2D [13].
Insulin dose was intensified with significantly higher basal, bolus, and total insulin doses at Week 12 versus baseline. There was also a statistically significant increase in the bolus/total insulin ratio from 44.5% at baseline to 50.7% at Week 12, demonstrating that the bolus insulin dose, in particular, was intensified during the study. There was a small increase in weight during the study (0.7 kg) associated with the intensification of insulin therapy and improvement in glycemic control. In the PRONTO-T2D study there was an increase in body weight of 1.4 kg with URLi and 1.6 kg with Lispro following 26 weeks of treatment with no significant difference between treatment groups [13].
The improved glycemic control with URLi was reflected in evaluations of participant satisfaction. The ITSQ, a 22-item patient-reported validated instrument that assesses treatment satisfaction for people with diabetes on insulin therapy, found that satisfaction with insulin treatment was improved following 12 weeks of URLi treatment compared to baseline including the total ITSQ score and the prespecified secondary objective of the glycemic control ITSQ domain that assessed participants’ satisfaction with the glycemic control they experienced. When asked about their mealtime insulin experience, 86% of participants indicated they were “likely” or “very likely” to incorporate URLi into their diabetes management. There were no statistically significant differences in the DSAT total score at Week 12 versus baseline.
There were no new or unexpected safety findings during the study. There were three discontinuations due to an AE; however, none were considered by the investigator to be related to study treatment. The incidence of ISR TEAEs was low (2.8%) and consistent with that previously reported for URLi during the PRONTO-T2D trial (~ 2.7%) [13]. All ISR TEAEs reported were of mild severity, recovered/resolved during the study, and did not result in discontinuation from the study.
Strengths of the study included that CGM data were collected throughout the whole treatment period and real-time CGM data were used to inform insulin dosing decisions. This enabled participants and investigators to best utilize the data available to them to titrate insulin doses and to improve participants’ glycemic control. This study allowed the use of up to three OAMs as well as an injectable GLP-1 receptor agonist, which is more reflective of the real-world T2D patient population compared to studies that limit the use of these therapies. While only people from the USA were enrolled in this study, participant diversity was relatively high, although there were still population groups that were underrepresented. The analyses evaluated change from baseline in each participant, as no comparator was included in this study; thus, direct comparison to other therapies is not available in this study. However, the Phase 3 PRONTO-T2D study comprehensively evaluated efficacy and safety of URLi compared with Lispro in 673 patients with T2D and demonstrated non-inferiority of URLi to Lispro for HbA1c and superiority for 1- and 2-h PPG excursions. The current study had a relatively short 12-week treatment period; however, this is considered a sufficient amount of time to see a clinical impact. In previous studies including PRONTO-T2D, the majority of HbA1c reduction was seen in the initial 12 weeks of treatment [13]. It would be of interest to follow patients with T2D for a longer period of time to evaluate potential continued improvement in glycemic control and CGM parameters as well as to assess longer term maintenance of treatment effects.
Conclusions
This Phase 3b study provided the first CGM data with URLi in people with T2D and demonstrated that 12 weeks of URLi treatment provided improved glycemic control, including time in range, HbA1c, and CGM glucose profiles, compared to baseline without evidence of increased hypoglycemia/time below range. Furthermore, URLi treatment provided improved postprandial glucose control. There were no new or unexpected safety findings during the study.
References
Saudek CD, Derr RL, Kalyani RR. Assessing glycemia in diabetes using self-monitoring blood glucose and hemoglobin A1c. JAMA. 2006;295(14):1688–97.
Diabetes C, Complications Trial Research G, Nathan DM, Genuth S, Lachin J, Cleary P, et al. The effect of intensive treatment of diabetes on the development and progression of long-term complications in insulin-dependent diabetes mellitus. N Engl J Med. 1993;329(14):977–86.
Beck RW, Connor CG, Mullen DM, Wesley DM, Bergenstal RM. The fallacy of average: how using HbA1c alone to assess glycemic control can be misleading. Diabetes Care. 2017;40(8):994–9.
Beck RW, Bergenstal RM, Riddlesworth TD, Kollman C, Li Z, Brown AS, et al. Validation of time in range as an outcome measure for diabetes clinical trials. Diabetes Care. 2019;42(3):400.
Battelino T, Danne T, Bergenstal RM, Amiel SA, Beck R, Biester T, et al. Clinical targets for continuous glucose monitoring data interpretation: recommendations from the international consensus on time in range. Diabetes Care. 2019;42(8):1593–603.
Brod M, Nikolajsen A, Weatherall J, Pfeiffer KM. The economic burden of post-prandial hyperglycemia (PPH) among people with type 1 and type 2 diabetes in three countries. Diabetes Ther. 2016;7(1):75–90.
Pfeiffer KM, Sandberg A, Nikolajsen A, Brod M. Postprandial glucose and healthcare resource use: a cross-sectional survey of adults with diabetes treated with basal-bolus insulin. J Med Econ. 2018;21(1):66–73.
Hirsch IB. Insulin analogues. N Engl J Med. 2005;352(2):174–83.
Raccah D, Bretzel RG, Owens D, Riddle M. When basal insulin therapy in type 2 diabetes mellitus is not enough—what next? Diabetes Metab Res Rev. 2007;23(4):257–64.
Liebl A, Prager R, Binz K, Kaiser M, Bergenstal R, Gallwitz B, et al. Comparison of insulin analogue regimens in people with type 2 diabetes mellitus in the PREFER Study: a randomized controlled trial. Diabetes Obes Metab. 2009;11(1):45–52.
Rosenstock J, Ahmann AJ, Colon G, Scism-Bacon J, Jiang H, Martin S. Advancing insulin therapy in type 2 diabetes previously treated with glargine plus oral agents: prandial premixed (insulin lispro protamine suspension/lispro) versus basal/bolus (glargine/lispro) therapy. Diabetes Care. 2008;31(1):20–5.
Heise T, Piras de Oliveira C, Juneja R, Ribeiro A, Chigutsa F, Blevins T. What is the value of faster acting prandial insulin? Focus on ultra rapid lispro. Diabetes Obes Metab. 2022;24(9):1689–701.
Blevins T, Zhang Q, Frias JP, Jinnouchi H, Chang AM. Randomized double-blind clinical trial comparing ultra rapid lispro with lispro in a basal-bolus regimen in patients with type 2 diabetes: PRONTO-T2D. Diabetes Care. 2020;43(12):2991–8.
Klaff L, Cao D, Dellva MA, Tobian J, Miura J, Dahl D, et al. Ultra rapid lispro improves postprandial glucose control compared with lispro in patients with type 1 diabetes: results from the 26-week PRONTO-T1D study. Diabetes Obes Metab. 2020;22(10):1799–807.
Malecki MT, Cao D, Liu R, Hardy T, Bode B, Bergenstal RM, et al. Ultra-rapid lispro improves postprandial glucose control and time in range in type 1 diabetes compared to lispro: PRONTO-T1D continuous glucose monitoring substudy. Diabetes Technol Ther. 2020;22(11):853–60.
Bailey TS, Grunberger G, Bode BW, Handelsman Y, Hirsch IB, Jovanovič L, et al. American association of clinical endocrinologists and American college of endocrinology 2016 outpatient glucose monitoring consensus statement. Endocr Pract. 2016;22(2):231–61.
Anderson RT, Skovlund SE, Marrero D, Levine DW, Meadows K, Brod M, et al. Development and validation of the insulin treatment satisfaction questionnaire. Clin Ther. 2004;26(4):565–78.
Tanenbaum ML, Hanes SJ, Miller KM, Naranjo D, Bensen R, Hood KK. Diabetes device use in adults with type 1 diabetes: barriers to uptake and potential intervention targets. Diabetes Care. 2016;40(2):181–7.
Westfall PH, Krishen A. Optimally weighted, fixed sequence and gatekeeper multiple testing procedures. J Stat Plan Inference. 2001;99(1):25–40.
Danne T, Nimri R, Battelino T, Bergenstal RM, Close KL, DeVries JH, et al. International consensus on use of continuous glucose monitoring. Diabetes Care. 2017;40(12):1631.
Lu J, Ma X, Zhou J, Zhang L, Mo Y, Ying L, et al. Association of time in range, as assessed by continuous glucose monitoring, with diabetic retinopathy in type 2 diabetes. Diabetes Care. 2018;41(11):2370.
Lu J, Wang C, Shen Y, Chen L, Zhang L, Cai J, et al. Time in range in relation to all-cause and cardiovascular mortality in patients with type 2 diabetes: a prospective cohort study. Diabetes Care. 2020;44(2):549–55.
Runge AS, Kennedy L, Brown AS, Dove AE, Levine BJ, Koontz SP, et al. Does time-in-range matter? Perspectives from people with diabetes on the success of current therapies and the drivers of improved outcomes. Clin Diabetes. 2018;36(2):112–9.
Zhang Q, Chigutsa F, Chang AM. Efficacy and safety of ultra-rapid lispro in younger and older patients with type 2 diabetes: randomized double-blind PRONTO-T2D study. Diabetes Ther. 2022;13(8):1547–57.
Monnier L, Colette C, Wojtusciszyn A, Dejager S, Renard E, Molinari N, et al. Toward defining the threshold between low and high glucose variability in diabetes. Diabetes Care. 2016;40(7):832–8.
Acknowledgements
The authors thank the study participants and the investigators and study coordinators who provided care for them.
Funding
This study and the journal Rapid Service Fee were funded by Eli Lilly and Company.
Author contributions
Annette M Chang was responsible for medical oversight during the trial and contributed to the study conception and design. Timothy S Bailey and Bruce W Bode were involved in the acquisition of data for the study. Qianqian Wang contributed to the study design and statistical analyses. All authors contributed to interpretation of results. Alastair W Knights contributed to the drafting of the manuscript and all authors were involved in critical review and revision of the manuscript. All authors gave final approval for the final version to be published.
Prior presentation
A portion of the data from this study was presented at the Association of Diabetes Care & Education Specialists, 2022 Annual Conference, held August 12–15, 2022 in Baltimore, MD, and at the Advanced Technologies & Treatments for Diabetes Conference, held February 22–25, 2023, in Berlin, Germany.
Disclosures
Timothy S Bailey reports research support from Abbott Diabetes, Abbott Rapid Diagnostics, Biolinq, Capillary Biomedical, Dexcom, Eli Lilly and Company, Kowa, LifePlus, Livongo, Mannkind, Medtronic, Novo Nordisk, PKvitality, REMD, Sanofi, Sanvita, Senseonics, Viacyte, vTv Therapeutics, Zealand Pharma; consulting honoraria from Abbott, CeQur, Lifescan, Mannkind, Medtronic, Novo, Sanofi; and speaker honoraria from Mannkind, Medtronic, Sanofi. Bruce W Bode is an employee of Atlanta Diabetes Associates. Atlanta Diabetes Associates has received research grants and support from Boehringer Ingelheim, Dexcom, Diasome, Eli Lilly and Company, Insulet, Janssen, Lexicon, Mannkind, Medtronic, National Institutes of Health (NIH), Nova Biomedical, Novo Nordisk, Provention Bio, Sanofi, Senseonics, REMD Biotherapeutics, vTv Therapeutics LLC, and Xeris. Qianqian Wang, Alastair W Knights, and Annette M Chang are employees and shareholders of Eli Lilly and Company.
Compliance with ethics guidelines
The study was conducted in accordance with the Declaration of Helsinki, the International Conference on Harmonization Guidelines for Good Clinical Practice, and applicable local laws and regulations. The study protocol and all procedures were reviewed and approved by a central ethics review board (IntegReview Ethical Review Board and/or Advarra) for all study centers, as well as local ethics review boards, if required (details provided in Supplementary Table 1). All participants provided written informed consent before participating in the study. All authors gave approval for the final version of the manuscript to be published.
Data availability
Eli Lilly and Company provides access to all individual participant data collected during the trial, after anonymization, with the exception of pharmacokinetic or genetic data. Data are available to request 6 months after the indication studied has been approved in the United States and European Union and after primary publication acceptance, whichever is later. No expiration date of data requests is currently set once data are made available. Access is provided after a proposal has been approved by an independent review committee identified for this purpose and after receipt of a signed data sharing agreement. Data and documents, including the study protocol, statistical analysis plan, clinical study report, blank or annotated case report forms, will be provided in a secure data sharing environment. For details on submitting a request, see the instructions provided at www.vivli.org.
Author information
Authors and Affiliations
Corresponding author
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License, which permits any non-commercial use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc/4.0/.
About this article
Cite this article
Bailey, T.S., Bode, B.W., Wang, Q. et al. Increased Time in Range with Ultra Rapid Lispro Treatment in Participants with Type 2 Diabetes: PRONTO-Time in Range. Diabetes Ther 14, 883–897 (2023). https://doi.org/10.1007/s13300-023-01400-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13300-023-01400-w