Abstract
Introduction
To compare glycemic variability (GV) and time in range (TIR) in Chinese patients with type 2 diabetes (T2D) initiated on once-daily bedtime insulin glargine 300U/ml (Gla-300) versus neutral protamine Hagedorn (NPH) insulin using continuous glucose monitoring (CGM).
Methods
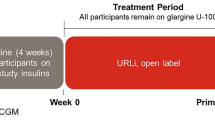
This was a 24-week, open-label exploratory study with 1:1 randomization comparing patient-adjusted titration of Gla-300 (n = 23) versus NPH (n = 23) at bedtime in insulin-naïve T2D patients on maximum oral glucose-lowering drugs. The starting dose was 0.2 U/kg/day and with self-titration of one unit per week to achieve a target fasting glucose of 4.4–6 mmol/l, without hypoglycemia. Participants had masked CGM at baseline, weeks 11 and 24. The primary outcome was between-treatment differences in CGM glucose standard deviation (SD) at week 24.
Results
HbA1c at week 24 were similar, with 21% of Gla-300 versus 4% of NPH-treated patients achieving HbA1c < 7% without confirmed hypoglycemia. There were no differences in anytime glucose SD at week 24 (LS mean difference − 0.08 mmol/l, 95% CI [− 0.42–0.26], p = 0.63). Anytime %TIRs (3.9–10.0 mmol/l) at week 24 were similar (p = 0.91). Nocturnal % time below range < 3.9 mmol/l was significantly lower in the Gla-300 group (least squares (LS) mean difference – 5.03% [− 9.92 to − 0.14], p = 0.04) with lower % coefficient of variation (LS mean difference − 4.5% [− 8.1 to − 0.8], p = 0.018). Diurnal TIR was higher in Gla-300 patients at week 11 but there were no differences at week 24.
Conclusions
Once-daily bedtime Gla-300 was associated with lower nocturnal GV, time below range and self-reported hypoglycemia in insulin-naïve Chinese T2D patients over a 24-week study period, as compared with NPH insulin.
Clinical Trial Registration
ClinicalTrials.gov Identifier: NCT03389490.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Why carry out this study? |
Insulin glargine 300U/ml (Gla-300) is a second-generation basal analogue with a more consistent pharmacokinetic profile and lower hypoglycemic risk than first-generation analogues and neutral protamine Hagedorn (NPH) insulin |
Gla-300 may be associated with lower glycemic variability (GV) but there have been few studies with continuous glucose monitoring (CGM) of insulin-naïve type 2 diabetes (T2D) patients |
What was learned from the study? |
Once-daily bedtime Gla-300 was associated with lower nocturnal GV, time below range and self-reported hypoglycemia in insulin-naïve Chinese T2D patients over a 24-week study period, as compared with NPH insulin |
Diurnal TIR was higher in Gla-300 patients at week 11 but there were no differences at week 24 |
Gla-300 was associated with lower self-reported anytime hypoglycemia and nocturnal hypoglycemia especially during the initial titration period |
Digital Features
This article is published with digital features, including a summary slide, to facilitate understanding of the article. To view digital features for this article go to https://doi.org/10.6084/m9.figshare.14135432.
Introduction
Insulin glargine 300 U/ml (Gla-300) is a second-generation basal insulin analogue with a lower risk of hypoglycemia compared with neutral protamine Hagedorn (NPH) and first-generation basal insulin analogues. It has a smoother and more consistent pharmacokinetic profile with an extended terminal half-life [1]. In phase 3 studies in insulin-naïve type 2 diabetes (T2D) patients, Gla-300 was associated with non-inferior glucose control compared with insulin glargine 100 U/ml (Gla-100) with a lower risk of nocturnal hypoglycemia [2]. These observations also extended to East Asians [3]. Use of Gla-300 resulted in similar glycemic control but lower risk of hypoglycemia when compared against another second-generation insulin analogue (insulin degludec) particularly in the initial titration period in insulin-naïve patients with T2D [4].
Gla-300 with its smoother pharmacokinetic (PK) profile may be associated with lower glycemic variability (GV). In a study involving 50 type 1 diabetes patients using continuous glucose monitoring (CGM), Gla-300 was associated with smoother average 24-h glucose profiles, lower glycemic excursions and less nocturnal hypoglycemia compared with Gla-100 [5]. However, few studies to date have evaluated GV and CGM profiles, including diurnal differences in T2D patients newly initiated on Gla-300. We hypothesized that Gla-300 administered once daily at bedtime would be associated with lower GV and hypoglycemic risk, with non-inferior glycemic control as determined by time-in-target (TIR) ranges and HbA1c compared with neutral protamine Hagedorn (NPH) insulin in T2D patients on maximal oral glucose-lowering drugs (OGLDs) newly initiated on insulin with self-titration. In this light, reduced hypoglycemia would encourage greater confidence in self-titration in the initiation period [6]. This exploratory study aimed to evaluate GV and TIR in Chinese T2D patients initiated on once-daily bedtime Gla-300 or NPH insulin.
Methods
Trial Design
This was a 24-week, single-center, open-label, parallel-group exploratory trial with 1:1 randomization comparing self-titration of Gla-300 versus NPH insulin on GV in insulin-naïve T2D patients. The study was conducted at the Prince of Wales Hospital, Hong Kong Special Administrative Region, between January 2018 and December 2019. The frequency of study visits was in line with usual practice following insulin initiation in the local setting. We selected NPH as a comparator since this remains the first-choice insulin in the publicly funded care setting in the absence of risk factors for hypoglycemia including advanced chronic kidney disease, history of severe hypoglycemia or significant comorbidities. The study protocol was approved by the Joint New Territories East Cluster and Chinese University of Hong Kong Clinical Research Ethics Committee. The study was conducted according to the Good Clinical Practice guidelines and Declaration of Helsinki. All participants provided written informed consent (ClinicalTrials.gov Identifier: NCT03389490).
Participants
Insulin-naïve T2D individuals were included if they were aged between 18 and 75 years, treated with three or fewer OGLDs, had a body mass index (BMI) < 40 kg/m2 and were capable and willing to perform regular self-monitored blood glucose at home (SMBG). Patients with HbA1c level > 7.0 and < 11% and fasting plasma glucose (FPG) > 7.5 and < 15 mmol/l at screening were included. Key exclusion criteria included known hypoglycemia unawareness or recurrent severe hypoglycemia, concomitant medications known to interfere with glucose metabolism, a change in dose of non-insulin GLDs or initiation of new OGLDs in the 8 weeks prior to screening, acute concurrent illness during the 3-month period prior to study, hepatic disease, end-stage kidney disease, pregnant or breastfeeding women and those who were unable to comply with follow-up visits.
Randomization
Eligible patients were randomized to Gla-300 insulin (Sanofi-Aventis, Paris France) or NPH insulin (either Humulin N, Eli Lilly, Indianapolis, IN, USA, or Protaphane HM, Novo Nordisk A/S, Bagsvaerd, Denmark) in a 1:1 ratio. Assignment to treatment group was by a computer-generated random sequence by personnel not involved in the study.
Interventions
Each patient was randomized to either Gla-300 or NPH insulin to be administered once daily between 9 p.m. and 12 midnight at approximately the same time every day. The starting doses of Gla-300 and NPH were both 0.2U/kg for comparison. Insulin was self-titrated weekly based on an average of three fasting SMBG readings per week, aiming for a mean fasting SMBG target of 4.4–6.0 mmol/l. Insulin was increased by 1U per week if the mean value was greater than the pre-set target. Patients were asked to contact the study team for adjustment of insulin doses if the average SMBG was ≥ 3.3 and < 4.4 mmol/l. The study team would decrease insulin by ≥ 2 U (according to investigator discretion) if SMBG < 3.3 mmol/l or severe or multiple symptomatic hypoglycemic events occurred.
The study consisted of seven pre-specified on-site visits at weeks − 1, 0, 4, 11, 12 and 24 and three telephone visits. Following randomization at week 0, phone visits were conducted at week 1, 2 and 8 to report on SMBG, insulin titration, and any hypoglycemia or adverse events. Patients were instructed on the self-titration algorithm at randomization, reinforced as necessary during the first 12 weeks of self-titration. Masked CGMs for determination of GV and TIR were collected for 7 consecutive days at baseline (week – 1), week 11 and week 24 using the Medtronic iPro2™ with Elite sensor (Medtronic, Northridge, CA, USA) with frequency of calibrations as recommended by the manufacturer. Raw CGM data were inspected and only patients with at least 70% valid sensor data were included in the analysis. Other secondary outcomes were measured at weeks 0, 12 and 24.
Rescue Medications
The doses and regimen of concomitant OGLDs remained stable throughout the study unless the patient fulfilled the rescue criteria. After week 12, rapid-acting acting prandial insulin (Actrapid or Humulin R) was added before the largest meal at the investigators’ discretion, if FPG could not be lowered below < 11 mmol/l or HbA1c < 8.5% (< 69.4 mmol/mol). Participants were continued on OGLDs throughout the study, with doses of sulfonylureas/glinides adjusted if ≥ 2 symptomatic or one severe hypoglycemic episode occurred, even after adjustment of insulin.
Outcome Measures
The primary outcome was described by the standard deviation (SD) of CGM glucose. The coefficient of variation of CGM glucose (CV%) was defined as SD/average glucose × 100%. Secondary CGM-based endpoints included average sensor glucose (SG), mean amplitude of glucose excursions (MAGE), glucose management index (GMI), mean percentage time in range (< 3.0 mmol/l, < 3.9 mmol/l 3.9–10.0 mmol/l, > 10.0 mmol/l, > 13.9 mmol/l) [7]. GMI, a correlate of HbA1c based on CGM mean glucose, was defined according to the formula GMI (%) = 3.31 + 0.02392 × [mean glucose in mg/l] [8]. Other glycemic endpoints included mean change in laboratory HbA1c and FPG at week 24, daily fasting SMBG and 7-point SMBG profiles at week 24, insulin dose (units per kilogram) and changes in body weight. The proportion of patients achieving target HbA1c < 7%, with and without biochemically confirmed hypoglycemia, respectively, was calculated.
Self-reported hypoglycemic events were collected throughout the on-treatment study period. The number of biochemically confirmed level 1 (< 3.9 mmol/l) and level 2 (< 3.0 mmol/l) hypoglycemic events was reported [9]. A severe hypoglycemic event was denoted by severe cognitive impairment requiring assistance for recovery. The incidence and number of hypoglycemic events per patient-year were calculated using the above thresholds; 00:00–05:59 h was categorized as nocturnal and between 0600 to 2359 h as diurnal. We also analyzed events during titration (weeks 0–12) and maintenance (weeks 13–24) periods.
Statistical Methods
Data were expressed as mean (SD) unless otherwise stated. All efficacy endpoints were assessed in the intention-to-treat (ITT) population (all randomized patients who received at least one dose of study insulin, analyzed according to the treatment group allocated by randomization). Change in GV and secondary endpoints (average SG, TIRs, HbA1c, FPG) during the 24-week period were analyzed by a mixed effect model with repeated measures (MMRM) using a missing at random framework, with treatment group, visit and treatment-by-visit interaction as fixed effects, participant as random effect, and baseline efficacy variable and baseline efficacy variable-by-visit interaction as fixed continuous covariates. From the model, relevant treatment differences were estimated as least squares (LS) means with standard errors (SEs) and LS mean difference with 95% confidence interval [CI]. Twenty-four-hour glucose profiles were generated by calculating the mean and 95% CI pooled across all patients within each treatment group. The proportion of patients with one or more self-reported hypoglycemic episodes was estimated. Annualized hypoglycemia event rates were calculated as events per patient-year, and the relative risk between treatment groups was estimated using the overinflated Poisson regression model.
Based on estimated mean scores for anytime glucose SD and a standard deviation of 0.5 mmol/l, a total sample size of 44 (22 in each arm) would have a power of ~ 80% at alpha 0.05 to detect a 0.5 mmol/l difference in the primary outcome at week 24 using sample size estimation for a multilevel design with repeated measures [10]. Fifty patients were enrolled allowing for a 12% drop-out rate. Data were analyzed with R version 3.3.2 (R Foundation for Statistical Computing) and SPSS software package version 25.0 (IBM Corp., Armonk, NY, USA). A p value < 0.05 was considered statistically significant.
Results
Study Population
A total of 50 insulin-naïve patients with T2D were enrolled, 1 withdrew consent with 25 randomized to the NPH group and 24 to Gla-300. Twenty-three patients in the Gla-300 group and 23 patients in the NPH group received at least one dose of study medication and were included in the modified ITT analysis. Eighty-seven percent of patients in the Gla-300 group and 78% of patients in the NPH group completed the study. A summary of patient flow is shown in Fig S1. The baseline characteristics of randomized patients are shown in Table 1. Age, mean duration of diabetes, baseline HbA1c and FPG were similar between groups. Over half of patients were treated with three non-insulin OGLDs in both arms.
HbA1c, FPG and 7-point SMBG
There were no overall differences in HbA1c at week 12 and week 24 between the two intervention groups. At week 24, HbA1c was 7.44 (0.54)% versus 7.43 (0.79)% in the Gla-300 and NPH groups, respectively (LS mean difference − 0.16%, 95% CI [− 0.57–0.25], p = 0.43). FPG was 6.92 (1.96) mmol/l versus 7.36 (2.08) mmol/l in the Gla-300 and NPH groups at week 24, respectively (LS mean difference − 0.30 mmol/l, 95% CI [− 1.89–1.30], p = 0.70) (Fig S2). At week 11, the 7-point SMBG tended to be lower in the Gla-300 compared with the NPH group but these differences did not persist at week 24. Twenty-one percent of patients in the Gla-300 group versus 4% in the NPH group achieved HbA1c < 7% without confirmed hypoglycemia (Fig S3).
Glycemic Variability
GV increased in both groups compared with baseline following initiation of insulin (Table 2). Mean change ± SE in CGM glucose SD was 0.15 ± 0.11 and 0.23 ± 0.12 mmol/l in the Gla-300 and NPH groups at week 24 (LS mean difference − 0.08 mmol/l, 95% CI [− 0.42–0.26], p = 0.63). There were no significant differences in change in %CV between groups over the study period (LS mean difference − 1.4, 95% CI [− 4.8–2.0] %, p = 0.40). MAGE was also similar between the two groups during the study.
We further analyzed GV indices by time of day. The diurnal %CV was similar between the two arms (LS mean difference − 2.8%, 95% CI [− 3.9–3.4], p = 0.88). However, change in nocturnal CV was significantly lower in the Gla-300 group compared with the NPH group at week 24 (3.9 ± 1.2 versus 8.3 ± 1.3%, LS mean difference − 4.5%, 95% CI [− 8.1 to − 0.8], p = 0.018) (Table 2).
The average sensor glucose was mean (SD) 9.23 (1.40) and 8.81 (1.21) mmol/l in the Gla-300 and NPH groups, respectively, at week 24 (LS mean difference − 0.081 mmol/l, 95% CI [− 0.61–0.77], p = 0.83) There were no significant differences in the GMI between groups at week 24 (Table 2).
Time in range
Overall, the mean percentage TIR 3.9–10.0 mmol/l at week 24 was similar between the Gla-300 and NPH groups (LS mean difference 0.47%, 95% CI [− 7.39–8.33], p = 0.91). There were no significant differences in time above range (TAR) (> 10.0 mmol/l) (Table 3).
We further analyzed TIR by time of day. TIR, TAR and time below range (TBR) during the diurnal hours were similar between treatment groups (Fig. 1). Diurnal TIR (3.9–10.0 mmol/l) was higher in the Gla-300 versus NPH group (65.6 versus 56.5%) at week 11 but these differences did not persist at week 24 (Fig. 1). Nocturnal TBR (≤ 3.9 mmol/l) was significantly lower in Gla-300- versus NPH-treated patients at week 24 (LS mean difference − 5.03% [− 9.92 to − 0.14], p = 0.04, Fig. 1). The mean percentage time in level 2 nocturnal hypoglycemia (< 3.0 mmol/l) was also numerically lower in the Gla-300 versus NPH group (Fig. 1). The percentage nocturnal TIR was higher in the Gla-300 versus NPH arms (80.2% versus 76.5%) at week 24.
Twenty-four-hour Glucose Profile
Averaged 24-h CGM profiles at baseline, weeks 11 and 24 are shown in Fig. 2. The lowest mean nocturnal sensor glucose occurred between 04:00 and 05:00 h in the NPH group. In the Gla-300 group, this occurred between 06:00 and 07:00 h. We examined differences in mean SG in the last 4 h of the injection interval (19:00–23:00 h). Mean SG was lower in Gla-300- compared with NPH-treated patients at week 11 (9.38 mmol/l versus 10.60 mmol/l). However, at week 24, the mean SG was similar in both groups between 19:00 and 23:00 h (Fig. 2).
Self-reported Hypoglycemia by SMBG
The incidence and rates of hypoglycemia are shown in Table 4. The incidence of biochemically confirmed hypoglycemia ≤ 3.9 mmol/l by SMBG was significantly lower in the Gla-300 group compared with NPH group throughout the 24-week period (30.4 versus 73.9%, RR 0.41, 95% CI [0.21–0.8], p = 0.003). The incidence of hypoglycemia < 3.0 mmol/l was also lower in the Gla-300 group but this did not reach statistical significance (Table 3). Most of the hypoglycemic events occurred during weeks 0–12. Incidence of nocturnal SMBG confirmed hypoglycemia ≤ 3.9 mmol/l was significantly lower in the Gla-300 group (4.3 vs. 39.1%, RR 0.11, 95% CI [0.02–0.81], p = 0.004) (Table 4). There were no severe hypoglycemic episodes in either arm.
Insulin Dose and Body Weight
The starting dose in both groups was similar: 0.20 (0.01) U/kg in the Gla-300 and 0.19 (0.01) U/kg in the NPH groups. In the Gla-300 group, the insulin dose increased to 0.23 (0.05) U/kg at week 12 and 0.25 (0.07) U/kg at 24 weeks. In the NPH group, the insulin dose was static at 0.21 (0.06) U/kg and 0.21 (0.05) U/kg at 12 and 24 weeks, respectively. Body weight increased by mean (SD) Δ1.5 (2.7) kg in the Gla-300 group versus Δ1.2 (3.1) kg in the NPH group at the end of the study.
Seven patients in the Gla-300 group and six patients in the NPH group had sulfonylureas reduced after week 12 because of recurrent hypoglycemia. No patient in the Gla-300 group received rescue therapy while two patients in the NPH group received pre-prandial Actrapid insulin with the largest meal at week 12.
Discussion
In this study, we have compared CGM profiles in Chinese insulin-naïve type 2 diabetes patients initiated on a second-generation basal analogue Gla-300 compared with NPH insulin following a self-titrated regimen. Similar HbA1c levels and average glucose were achieved at week 24 but Gla-300 was associated with fewer episodes of self-reported biochemically confirmed hypoglycemia and CGM-detected hypoglycemia.. Gla-300 was associated with lower nocturnal GV and CGM detected hypoglycemia. However, there were no differences in anytime or diurnal GV and TIR between the Gla-300 and NPH groups.
Our data are unique in studying CGM profiles during insulin initiation with self-titration in Asian T2D patients, where few data exist for both NPH and second-generation insulin analogues. As expected, Gla-300 was associated with lower rates of hypoglycemia compared with NPH insulin. Our findings are consistent with previous results in the EDITION trials in T2D patients where Gla-300 consistently reduced nocturnal hypoglycemia compared with Gla-100 with no differences in HbA1c [2, 3]. With comparable HbA1c and FPG achieved between treatment groups, there were notable differences in GV and TIR between the Gla-300 and NPH groups that varied by time of day. Percentage TBR was lower in the Gla-300 group compared with the NPH group during nocturnal hours. Nocturnal %CV was also significantly lower in the Gla-300 group. This is probably not due to the reduction in sulfonylurea dose after week 12 as the number of affected patients was similar in each arm. Indeed, our CGM data support the observations that a more consistent PK profile of Gla-300 could lead to lower nocturnal variability and risk of hypoglycemia compared with NPH administered at bedtime.
Surprisingly, no differences were observed with the anytime and diurnal GV and TIR range between study arms at week 24. The mean SG tended to be lower in the last 4 h of the insulin injection interval (19:00–23:00) at %TAR at week 11, but not at week 24. A similar trend was observed with 7-point SMBG profiles. Gla-300 has an extended terminal half-life with coverage up to 36 h compared with NPH insulin. In a previous study involving people with type 1 diabetes, lower SGs were observed in the last 4 h of Gla-300 administration compared with Gla-100 [5]. Overall, percentage time in hyperglycemia remained high in both treatment arms during the day at the end of the study. These differences could be ethnic-specific reflecting a greater contribution of daytime postprandial glucose compared with fasting glucose toward overall glycemic control in Asian patients. In a previous analysis of Gla-100, Asian patients with T2D had higher HbA1c despite optimization of FPG compared with non-Asian patients [11].
Our study is also unique in comparing CGM profiles during insulin titration and maintenance periods. We note a larger number of self-reported hypoglycemic events occurred during the initial 12-week titration period, which was significantly higher in the NPH group. A lower risk of hypoglycemia in the initial titration period with Gla-300 has also been observed in head-to-head trials with insulin degludec [4]. CGM has the advantage of detecting asymptomatic hypoglycemia particularly during the nocturnal hours. Here we note that the time in nocturnal hypoglycemia decreased from week 11 and week 24 for the Gla-300 group and remained lower overall compared with NPH-treated patients.
The final insulin dose was 0.25 U/kg in Gla-300 versus 0.21 U/kg in the NPH group with the same starting dose of 0.2 U/kg. Dose requirements of Gla-300 are generally 10–15% higher than those of Gla-100 or NPH. In the NPH group, up-titration of insulin was frequently limited by hypoglycemia. The overall basal insulin requirements were lower in this trial compared with Caucasians and could be linked to ethnic differences, as has been observed in lower dose requirements in phase 3 trials of Gla-300 in Asians in a physician-titrated setting [3]. Due to ethnic differences in basal insulin requirements, our self-titration regime was designed to be less aggressive, titrating by 1 IU weekly compared with other studies where insulin was titrated every few days [6]. We cannot exclude the possibility that the adequacy of self-titration could have influenced our results. In our study, generally both arms adhered to the self-titration protocol to the same extent. The two patients lost to follow-up in the NPH group also defaulted other usual care appointments; therefore, this was unlikely related to the allocated intervention per se. We cannot exclude the possibility the results may be different under a physician-titrated setting, although other studies have shown better or similar glycemic control with self-titration versus titration by care team [6]. Another reason for differences in insulin requirement may be the higher number of background GLDs compared with previous studies. Over half of the patients in this trial were treated with three background OGLDs.
Increased GV has been shown to be associated with increased oxidative stress [12]. In patients with T2D and acute coronary syndrome, GV was found to be predictive of midterm cardiovascular (CV) events, independent of mean HbA1c levels [13]. Nocturnal hypoglycemia has been associated with cardiac arrhythmias in patients with T2D in studies using ambulatory Holter and CGM monitoring [14]. Hypoglycemia may elicit sustained pro-inflammatory and prothrombotic effects that predispose to adverse CV events downstream of the episode [15]. This underscores the importance of reducing GV and minimizing hypoglycemia, while optimizing glucose control, especially in patients with T2D with advanced disease and high CV risk [16].
The strength of this study relates to the detailed 24-h CGM profiles throughout the insulin initiation, titration and maintenance phases. There are limitations to this study including the small sample size and open-label design. Only 7 days of blinded CGM data were collected. We acknowledge that 14 days of CGM may provide more representative data but the study was designed and received ethical approval prior to the publication of the 2017 international consensus on CGM reporting [17]. We compared initiation of Gla-300 versus NPH insulin because of their relevance to the local setting where the majority of T2D patients would be initiated on NPH insulin as first line in the absence of risk factors for hypoglycemia. However, future head-to-head trials should compare CGM profiles of Gla-300 versus other basal insulin analogues. There are subtle differences in PK between second-generation basal insulin analogues, and it would be of interest to explore whether these translate into clinically relevant differences in 24-h glucose profiles and GV [18]. Studies comparing CGM profiles with evening and morning administration of Gla-300 are also needed.
In conclusion, our data suggest that Gla-300 once daily at bedtime was associated with similar HbA1c but lower nocturnal %CV, TBR and self-reported hypoglycemia in insulin-naïve T2D patients over a 24-week study period compared with NPH insulin. No differences were observed in diurnal GV or TIR at 24 weeks between groups. Use of second-generation analogues may promote more effective self-titration for optimizing fasting blood glucose without fear of hypoglycemia, particularly nocturnal hypoglycemia; however, these findings need to be confirmed in larger studies.
References
Porcellati F, Lucidi P, Candeloro P, et al. Pharmacokinetics, pharmacodynamics, and modulation of hepatic glucose production with insulin glargine U300 and glargine U100 at steady state with individualized clinical doses in type 1 diabetes. Diabetes Care. 2019;42(1):85–92. https://doi.org/10.2337/dc18-0706.
Bolli GB, Riddle MC, Bergenstal RM, et al. New insulin glargine 300 U/ml compared with glargine 100 U/ml in insulin-naïve people with type 2 diabetes on oral glucose-lowering drugs: a randomized controlled trial (EDITION 3). Diabetes Obes Metab. 2015;17(4):386–94. https://doi.org/10.1111/dom.12438.
Ji L, Kang ES, Dong XL, et al. Efficacy and safety of insulin glargine 300 U/mL versus insulin glargine 100 U/mL in Asia Pacific insulin-naïve people with type 2 diabetes: the EDITION AP randomized controlled trial. Diabetes Obes Metab. 2019. https://doi.org/10.1111/dom.13936.
Rosenstock J, Cheng A, Ritzel R, et al. More similarities than differences testing insulin glargine 300 units/mL versus insulin degludec 100 units/mL in insulin-naive type 2 diabetes: the randomized head-to-head bright trial. Diabetes Care. 2018;41:2147–54. https://doi.org/10.2337/dc18-0559
Bergenstal RM, Bailey TS, Rodbard D, et al. Comparison of insulin glargine 300 units/mL and 100 units/mL in adults with type 1 diabetes: continuous glucose monitoring profiles and variability using morning or evening injections. Diabetes Care. 2017;40(4):554–60. https://doi.org/10.2337/dc16-0684.
Russell-Jones D, Dauchy A, Delgado E, et al. Take Control: A randomized trial evaluating the efficacy and safety of self- versus physician-managed titration of insulin glargine 300 U/mL in patients with uncontrolled type 2 diabetes. Diabetes, Obes Metab. 2019;21(7):1615–24. https://doi.org/10.1111/dom.13697.
Battelino T, Danne T, Bergenstal RM, et al. Clinical targets for continuous glucose monitoring data interpretation: recommendations from the international consensus on time in range. Diabetes Care. 2019;42(8):1593–603. https://doi.org/10.2337/dci19-0028.
Bergenstal RM, Beck RW, Close KL, et al. Glucose management indicator (GMI): a new term for estimating A1C from continuous glucose monitoring. Diabetes Care. 2018;41(11):2275–80. https://doi.org/10.2337/dc18-1581.
Heller SR. Glucose concentrations of less than 3.0 mmol/L (54 mg/dL) should be reported in clinical trials: a joint position statement of the American diabetes association and the European association for the study of diabetes. Diabetes Care. 2017;40(1):155–7. https://doi.org/10.2337/dc16-2215.
Kreidler SM, Muller KE, Grunwald GK, et al. GLIMMPSE: online power computation for linear models with and without a baseline covariate. J Stat Softw. 2013;54(10):1–26. https://doi.org/10.18637/jss.v054.i10.
Chan JCN, Bunnag P, Chan SP, et al. Glycaemic responses in Asian and non-Asian people with type 2 diabetes initiating insulin glargine 100 units/mL: a patient-level pooled analysis of 16 randomised controlled trials. Diabetes Res Clin Pract. 2018;135:199–205. https://doi.org/10.1016/j.diabres.2017.11.025.
Monnier L, Colette C. Glycemic variability: should we and can we prevent it? Diabetes Care. 2008;31(Suppl):2. https://doi.org/10.2337/dc08-s241.
Gerbaud E, Darier R, Montaudon M, et al. Glycemic variability is a powerful independent predictive factor of midterm major adverse cardiac events in patients with diabetes with acute coronary syndrome. Diabetes Care. 2019;42(4):674–81. https://doi.org/10.2337/dc18-2047.
Chow E, Bernjak A, Williams S, et al. Risk of cardiac arrhythmias during hypoglycemia in patients with type 2 diabetes and cardiovascular risk. Diabetes. 2014;63(5):1738–1747. https://doi.org/10.2337/db13-0468.
Chow E, Iqbal A, Walkinshaw E, et al. Prolonged prothrombotic effects of antecedent hypoglycemia in individuals with type 2 diabetes. Diabetes Care. 2018;41(12):2625–2633. https://doi.org/10.2337/dc18-0050.
Lau E, Salem A, Chan JCN, et al. Insulin glargine compared to neutral protamine Hagedorn (NPH) insulin in patients with type-2 diabetes uncontrolled with oral anti-diabetic agents alone in Hong Kong: a cost-effectiveness analysis. Cost Eff Resour Alloc. 2019;17(1):13. https://doi.org/10.1186/s12962-019-0180-9.
Danne T, Nimri R, Battelino T, et al. International consensus on use of continuous glucose monitoring. Diabetes Care. 2017;40(12):1631–40. https://doi.org/10.2337/dc17-1600.
Bailey TS, Pettus J, Roussel R, et al. Morning administration of 0.4 U/kg/day insulin glargine 300 U/mL provides less fluctuating 24-hour pharmacodynamics and more even pharmacokinetic profiles compared with insulin degludec 100 U/mL in type 1 diabetes. Diabetes Metab. 2018;44(1):15–21. https://doi.org/10.1016/j.diabet.2017.10.001.
Acknowledgements
We thank Cherry Chiu, Candice Lau, Lei Ka Ying and all staff at the Diabetes Mellitus and Endocrine Centre at Prince of Wales Hospital for their support in conduct of this study. We thank all participants for their valuable time and contribution toward the study.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
This study was supported by a Sanofi investigator-initiated study grant. Sanofi reviewed the final manuscript but had no influence in the design, conduct of the trial or final analyses. The journal’s Rapid Service Fee was supported by Sanofi.
Authorship
All named authors meet the International Committee of Medical Journal Editors (ICMJE) criteria for authorship for this article, take responsibility for the integrity of the work as a whole, and have given their approval for this version to be published.
Disclosures
Elaine Chow has received travel and conference, honoraria for giving lectures and research support from Sanofi, Medtronic and Novartis. Juliana C.N. Chang has received research grants and/or honoraria for consultancy or giving lectures from Astra Zeneca, Bayer, Bristol-Myers Squibb, Boehringer Ingelheim, Daiichi-Sanyko, Eli-Lilly, GlaxoSmithKline, Merck Serono, Merck Sharp & Dohme, Novo Nordisk, Pfizer and Sanofi. Alice P.S. Kong has received research grants and/or speaker honoraria from Abbott, Astra Zeneca, Eli-Lilly, Merck Serono, Nestle and Novo Nordisk. The proceeds have been donated to the Chinese University of Hong Kong and other charity organizations to support diabetes research and education. James Ling, Emily W. M. Poon, Aimin Yang, Theresa Yeung, Kitman Loo, Risa Ozaki, Ronals CW and Andrea OY Luk have nothing to disclose.
Compliance with Ethics Guidelines
The study was approved by the Joint Chinese University of Hong Kong-New Territories East Cluster Clinical Research Ethics Committee. The study was performed in accordance with Good Clinical Practice and the Declaration of Helsinki 1964 and its later amendments. All patients provided voluntary written informed consent prior to trial participation.
Data Availability
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
Supplementary Information
Below is the link to the electronic supplementary material.
13300_2021_1046_MOESM1_ESM.docx
Supplementary file1 Fig. S1 Participant flow. Fig. S2 Fasting plasma glucose, glycated hemoglobin A1c (HbA1c) and 7-point self-monitored blood glucose during the 24-week study period. Fig. S3 Percentage of patients achieving HbA1c target without confirmed hypoglycemia at week 24 (DOCX 340 KB)
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License, which permits any non-commercial use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc/4.0/.
About this article
Cite this article
Ling, J., Poon, E.W.M., Yang, A. et al. Glycemic Variability and Time in Range During Self-titration of Once Daily Insulin Glargine 300 U/ml Versus Neutral Protamine Hagedorn Insulin in Insulin-naïve Chinese Type 2 Diabetes Patients. Diabetes Ther 12, 1399–1413 (2021). https://doi.org/10.1007/s13300-021-01046-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13300-021-01046-6