Abstract
Introduction
To assess time in range (TIR) (70–180 mg/dL) with postprandial glucose (PPG)-focused titration of ultra rapid lispro (URLi; Lyumjev®) in combination with insulin degludec in people with type 1 diabetes (T1D).
Methods
This phase 2, single-group, open-label, exploratory study was conducted in 31 participants with T1D on multiple daily injection therapy. Participants were treated with insulin degludec and Lispro for an 11-day lead-in and then URLi for a 46-day treatment period consisting of 35-day titration and 11-day endpoint maintenance period. Glucose targets for the titration period were PPG < 140 mg/dL or < 20% increase from premeal, fasting glucose 80–110 mg/dL, and overnight excursion ± 30 mg/dL or less. Participants used the InPen™ bolus calculator and Dexcom G6 continuous glucose monitoring (CGM).
Results
Primary endpoint mean TIR (70–180 mg/dL) with URLi during the maintenance period was 70.2%. TIR (70–180 mg/dL) and times below/above range were not significantly different with URLi (maintenance) versus lispro (lead-in). HbA1c decreased from 7.1% at screening to 6.8% at endpoint (least squares mean [LSM] change from baseline, − 0.36%; P < 0.001). Fructosamine and 1,5-anhydroglucitol improved (P < 0.001). Mean hourly glucose using CGM was reduced from 8:00 am to 4:00 pm with URLi. Overall highest PPG excursion across meals was significantly reduced at URLi endpoint compared with lispro lead-in (mean 56.5 vs 72.4 mg/dL; P < 0.001). Insulin-to-carbohydrate ratio (U/X g) was reduced (more insulin given) at breakfast at URLi endpoint vs lead-in (LSM 9.0 vs 9.7 g; P = 0.002) and numerically decreased at other meals. Total daily insulin dose (TDD) was higher at URLi endpoint compared with lispro lead-in (mean 50.2 vs 47.0 U; P = 0.046) with similar prandial/TDD ratio (mean 52.1% vs 51.2%). There were no severe hypoglycemia events during the study.
Conclusions
URLi in a basal-bolus regimen focusing on PPG targets demonstrated improved overall glycemic control and reduced PPG excursions without increased hypoglycemia in participants with T1D.
Trial Registration
ClinicalTrial.gov, NCT04585776.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Commonly used titration schemes may not be optimized for the ultra-rapid time-action profile of URLi |
This phase 2 study assessed time in range using continuous glucose monitoring with postprandial-focused titration of URLi in combination with degludec in people with type 1 diabetes |
URLi demonstrated improved overall glycemic control without evidence of increased hypoglycemia/time below range, as well as reduced overall postprandial glucose excursions |
Treatment with URLi in a basal-bolus regimen using a dosing algorithm focused on postprandial glucose targets was efficacious and well tolerated in people with type 1 diabetes |
Introduction
Minimizing postprandial glucose (PPG) spikes is an important component of managing overall glycemic control in people with diabetes [1,2,3]. The development of rapid-acting prandial insulin analogues such as insulin lispro, aspart, and glulisine have improved control of PPG [4, 5]; however, the time-action of these may not be fast enough to match carbohydrate absorption [6, 7]. Ultra rapid lispro (URLi; Lyumjev®) is a formulation of insulin lispro with a faster onset and shorter duration of action compared to lispro (Humalog®) and was developed to more closely match physiological insulin secretion and improve PPG control [6]. URLi contains two active excipients, treprostinil and citrate, that act to accelerate insulin lispro absorption and insulin time-action [8, 9]. Compared with lispro, URLi has consistently shown faster onset of appearance, greater early insulin exposure, and reduced overall duration of action in healthy participants and people with type 1 diabetes (T1D) and type 2 diabetes (T2D) [10]. In phase 3 studies in people with T1D (PRONTO-T1D) and T2D (PRONTO-T2D) on multiple daily injection (MDI) therapy, URLi demonstrated non-inferiority to mealtime lispro in HbA1c change from baseline after 26 weeks while providing superior postprandial glucose (PPG) control when dosed at mealtime [11, 12]. The PRONTO T1D study featured a continuous glucose monitoring (CGM) substudy, which found that improvements in PPG control observed with mealtime URLi versus lispro were also associated with increased time in range (TIR) during the daytime period [13].
It has been recommended to titrate prandial insulin in patients with T1D on the basis of postprandial glucose values [14, 15], but frequent PPG testing was difficult for many patients to perform on a regular basis before CGM was available. The design of the PRONTO-T1D and -T2D studies included an 8-week basal insulin optimization lead-in period with glargine U-100 or degludec treatment in combination with prandial lispro before randomization [11, 12]. This might have favored aggressive titration of the basal insulin and limited titration of URLi and lispro during the subsequent treatment period, as the mean HbA1c decreased from 8.0% at screening to 7.3–7.4% at the end of the 8-week lead-in period in PRONTO-T1D [11]. It is possible that patient outcomes could be further optimized with alternate dosing regimens focused on prandial insulin optimization.
The aim of this study was to explore PPG-focused titration of URLi when administered as bolus insulin in combination with insulin degludec in participants with T1D on basal-bolus MDI therapy. The primary objective was to assess the percentage of TIR (70–180 mg/dL) using CGM after 35 days of URLi titration.
Methods
Study Design
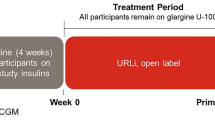
This phase 2 trial was a single-group, open-label, exploratory study conducted in participants with T1D currently treated with insulin degludec and a rapid-acting insulin analogue in a basal-bolus MDI regimen. This study consisted of a screening visit, 11-day lead-in period, 46-day treatment period consisting of a 35-day titration period and 11-day maintenance period, and a safety follow-up approximately 7 days after the last treatment visit (Fig. 1).
The study was conducted at three centers in the USA in accordance with the Declaration of Helsinki, the International Conference on Harmonization Guidelines for Good Clinical Practice, and applicable local laws and regulations. The study protocol and all procedures were reviewed and approved by an ethics review board for each study center (Healthpartners Institute Dba International Diabetes Center, Bloomington, MN; or Western Institutional Review Board – Connexus, Puyallup, WA). All participants provided written informed consent before participating in the study. All authors gave approval for the final version of the manuscript to be published. The study was registered at ClinicalTrials.gov (NCT04585776).
Participants
Adults aged 18–65 years with T1D, continuously treated with intensive insulin therapy for at least 1 year were eligible for inclusion if they met the following criteria: HbA1c 6.0–8.0%; BMI ≤ 35.0 kg/m2; treated with insulin degludec and rapid-acting insulin analogue in an MDI regimen for at least 30 days before screening; had been using unblinded CGM for at least two of the 6 months prior to screening; and were routinely using carbohydrate counting. Key exclusion criteria were more than one episode of severe hypoglycemia within the 90 days before screening; more than one emergency room visit due to hyperglycemia or diabetic ketoacidosis within 6 months before screening; or hypoglycemia unawareness as judged by the investigator.
Interventions and Treatment
Overall glycemic goals for participants during the study were a fasting glucose of 80–110 mg/dL, PPG < 140 mg/dL or < 20% increase from the premeal level, and overnight glucose excursion ± 30 mg/dL or less (Table S1 in the supplementary material). These targets were generally similar to those recommended by the American Association of Clinical Endocrinologists (AACE) [16]. Participants continued to receive insulin degludec U-100 as their basal insulin during lead-in at their pre-study dose unless adjustments were necessary for safety reasons. After lead-in, all participants were switched to morning dosing of degludec (if applicable). During the insulin titration period, the basal insulin dose was titrated on the basis of CGM readings approximately twice weekly to a fasting glucose target of 100 mg/dL (range 80–110 mg/dL) and an overnight excursion target of ± 30 mg/dL or less. During the maintenance period, the insulin degludec dose was kept unchanged unless adjustments were needed for safety reasons.
All participants were treated with prandial insulin lispro during the lead-in period. After lead-in, participants were transitioned to URLi, initiated unit-for-unit on the basis of the pre-study insulin-to-carbohydrate ratio (ICR) and insulin sensitivity factor (ISF), also referred to as the correction factor. URLi was administered immediately (0–2 min) before the start of each meal. Dosing was individualized by investigators and site staff on the basis of CGM data and the study glycemic targets. There were frequent interactions/visits with participants, and sites had access to the CGM data for remote review for telephone visits. During the titration period, ICR and ISF were adjusted by the investigator on the basis of CGM readings approximately twice per week as needed to achieve glycemic targets of PPG peak < 140 mg/dL or < 20% rise from premeal level. The postprandial glucose peak was evaluated by the investigators on the basis of CGM data. The duration of insulin action (DIA) could be adjusted per investigator discretion during the titration period. During the maintenance period, ICR, ISF, and DIA were unchanged unless for safety reasons.
Participants used the InPen™ (Medtronic, Minneapolis, MN, USA) smart insulin pen for prandial insulin administration during the lead-in and treatment periods. Participants used the bolus calculator function on the InPen mobile app to determine all meal/snack and correction doses. Prandial insulin doses were calculated on the basis of the estimated carbohydrate content of the meal and the investigator prescribed ICR and ISF. The investigator also determined the DIA with the InPen.
Continuous Glucose Monitoring
All participants used study provided unblinded CGM (Dexcom G6) for glucose data collection during the study. CGM data collected during the lead-period were used as baseline CGM data. Participants were required to have at least 5 days with a minimum of 70% CGM measures per day during the lead-in period in order to proceed to the treatment period. All CGM outcome variables were derived for baseline and day 46 on the basis of CGM data collected from valid CGM days defined as a day with at least 70% of the total measures that are supposed to be obtained. For calculation of CGM outcome variables, daytime was defined as 6:00 am to 11:59 pm and nighttime as 12:00 am to 5:59 am.
Assessments
The primary endpoint was the percentage of time with glucose levels within the target range (70–180 mg/dL) during the maintenance period. Secondary endpoints were ICR, ICR × total daily insulin dose (TDD), and prandial to TDD ratio for the maintenance period. Prespecified exploratory endpoints included adverse events (AEs); time below range (< 54 mg/dL) and time above range (> 250 mg/dL); ISF; within-day glucose variability; highest PPG and time to highest PPG within 4 h after meals; and HbA1c, fructosamine, and 1,5-anhydroglucitol (1,5-AG) levels at the end of the maintenance period compared to baseline.
Statistical Methods
It was estimated that approximately 34 participants assigned to study treatment would result in 30 evaluable participants completing the maintenance period, assuming a 10% dropout rate. The study was not strictly powered to demonstrate a statistically significant change from baseline in the primary endpoint because of the exploratory nature of the study. Using a standard deviation of 12%, the sample would provide approximately 80% probability that the half-width of the 95% confidence interval of the change from baseline in the primary endpoint falls within 4.93%.
Unless otherwise specified, all efficacy and safety analyses were conducted on the treated population of participants that received at least one dose of study drug after the lead-in period. Statistical tests were conducted at a two-sided alpha level of 0.05, and confidence intervals calculated at 95%, two-sided. Comparison between baseline and endpoints were performed at the full significance level of 0.05. No multiplicity adjustment was made. Baseline was defined as the last non-missing measurement at or before the treatment assignment. Significance tests were based on least squares mean (LSM) and type III tests and conducted using SAS PROC MIXED (SAS Institute, Cary, NC, USA).
An analysis of covariance (ANCOVA) was used to analyze continuous variables that were collected only at baseline and endpoint and included baseline as a covariate. CGM outcome variables were derived for baseline (lispro lead-in) and treatment endpoint (URLi maintenance period) based upon the CGM data collected from valid CGM days. A restricted-maximum-likelihood-based mixed model repeated measures (MMRM) analysis was used to analyze continuous longitudinal variables collected at baseline and more than one post-baseline visit. The model for the analysis included the fixed class effect of visit, and the random effect of patient.
Safety measures included AEs, vital signs, and treatment exposure. Analyses of AEs were descriptive and included all data collected during the treatment period. Serious AEs (SAEs), AEs reported as the reason for discontinuation from the treatment or study, and treatment-emergent AEs (TEAEs) were summarized by preferred term using the Medical Dictionary for Regulatory Activities (MedDRA) version 24.0. Severe hypoglycemia, defined as requiring the assistance of others because of cognitive impairment, was reported as an SAE.
Results
Participants
A total of 31 participants were enrolled in the study and all 31 competed treatment and safety follow-up. Demographics and baseline characteristics are provided in Table 1. The mean age was 42 years, BMI, 26.7 kg/m2, duration of diabetes, 20.3 years, and screening HbA1c, 7.13%. Participants entered the study using insulin lispro (64.5%) or aspart (35.5%) as their bolus insulin.
Time in Range (70–180 mg/dL)
The primary endpoint of mean TIR (70–180 mg/dL) during the URLi maintenance period over 24 h was 70.2% (1010.7 min [16 h 50.7 min]) (Fig. 2a), which was a numerical increase compared with lispro lead-in (67.9%; 977.8 min [16 h 17.8 min]). Similarly, a numerical increase in daytime TIR (70–180 mg/dL) was observed during the URLi maintenance period (71.6%; 773.0 min [12 h 53 min]) compared to lispro lead-in (68.3%; 738.0 min [12 h 18 min) (Fig. 2b).
Time Below Range
During the URLi maintenance period, the percentage of time below range (TBR) (< 70 mg/dL or < 54 mg/dL) during the 24-h period was 2.4% and 0.3% respectively (Fig. 2a). There were no significant differences between the URLi maintenance period and lispro lead-in. This trend was also observed for the daytime period with similar TBR (< 70 mg/dL or < 54 mg/dL) during the URLi maintenance period and lispro lead-in (Fig. 2b).
Time Above Range
During the URLi maintenance period, the percentage of time above range (TAR) (> 180 mg/dL or > 250 mg/dL) during the 24-h period were 27.5% and 5.5% respectively (Fig. 2a). There were no significant differences between the URLi maintenance period and lispro lead-in. This trend was also observed for the daytime period with similar TAR (> 180 mg/dL or > 250 mg/dL) during the URLi maintenance period and lispro lead-in (Fig. 2b).
Continuous Glucose Monitoring Consensus Targets
The proportion of participants achieving key CGM-based glycemic targets as recommended by the International Consensus on Time in Range [17, 18] are shown in Table S2. The proportions of patients that achieved the recommended CGM target of > 70% TIR (70–180 mg/dL) and < 5% TAR (> 250 mg/dL) were numerically increased during the URLi maintenance period compared with lispro lead-in. The proportion of patients that achieved the recommended CGM target of < 1% TBR (< 54 mg/dL) decreased slightly during the URLi maintenance period (93.5%) compared with lispro lead-in (96.8%).
Insulin Dose
The mean TDD was statistically significantly higher at the end of the URLi maintenance period (50.22 U/day) compared to lispro lead-in (47.03 U/day; LSM change from baseline, 3.28 U/day [P = 0.046]). At the end of the URLi maintenance period compared to lispro lead-in, there were numerical increases in the prandial (LSM change from baseline, 2.46 U/day) and basal insulin dose (LSM change from baseline, 1.70 U/day). The prandial-to-TDD ratio at the end of the URLi maintenance period (52.1%) was similar to that at the lispro lead-in (51.2%) (Fig. 3).
Insulin-to-Carbohydrate Ratio and Insulin-to-Carbohydrate Ratio × Total Daily Insulin Dose
There was statistically significant reduction in ICR (more insulin administered) at breakfast at the end of the URLi maintenance period compared with lispro lead-in (LSM change from baseline = − 0.67 g carbohydrate/U; P = 0.002) and numerical reductions at the end of the URLi maintenance period compared with lispro lead-in for lunch and dinner and across all meals (Table S3).
To evaluate the relationship between ICR and TDD, the product of ICR (g/U) and TDD (U/day) was assessed. The product of ICR and TDD was numerically higher at the end of the URLi maintenance period compared to lispro lead-in for breakfast, lunch, dinner, and the average of the three meals (Table S4). Pairwise comparisons of ICR × TDD between meals were similar at both the end of the URLi maintenance period and lispro lead-in.
Laboratory Parameters
At the end of the URLi maintenance period, the mean HbA1c was 6.76%, which was a significant improvement compared to screening (7.11%; LSM change from screening, − 0.36% [P < 0.001]) (Table 2). At the end of the URLi maintenance period, there were also significant improvements in fructosamine levels (LSM change from screening, − 25.0 μmol [P < 0.001]) and 1,5-AG concentration (LSM change from screening, 1.88 mg/L [P < 0.001]) (Table 2).
Hourly Glucose Profiles, Mean Sensor Glucose, and Glucose Variability
Figure 4a displays mean hourly glucose profiles over 24 h. Mean glucose was lower during the URLi maintenance period compared with the lispro lead-in from around 8:00 am to 4:00 pm and similar at other timepoints. The median and percentile hourly glucose profiles over 24 h are provided in Supplementary Fig. S1. There were no significant differences between the URLi maintenance period and lispro lead-in in mean sensor glucose during the 24-h or daytime periods. Within-day glucose variability, as measured by %CV of sensor glucose, was statistically significantly lower during the URLi maintenance period compared to lispro lead-in for the 24-h (LSM change from baseline = − 1.55%; p = 0.010) and daytime (LSM change from baseline = − 1.50%; p = 0.026) periods (Table S5).
CGM mean glucose profiles during the URLi maintenance period and lispro lead-in a over 24-h (n = 31) and b 0–4 h postmeal (URLi n = 30; lispro n = 29). CGM, continuous glucose monitoring; n = number of participants number of participants who had valid CGM data per prespecified criteria; URLi, ultra rapid lispro
Postprandial Glucose Levels and Excursions
Figure 4b displays mean glucose profiles for the period 0–4 h after breakfast, lunch, and dinner. The median and percentile glucose profiles for the 0–4 h postmeal period are provided in Supplementary Fig. S2. Postprandial incremental area under curve (iAUC) within 2 and 4 h after the start of a meal were calculated (Fig. 5). During the URLi maintenance period compared to lispro lead-in, there was a statistically significant reduction in mean iAUC0–2h and iAUC0–4h for breakfast, lunch, and across all meals.
To further assess PPG, the highest PPG excursion from 0 to 4 h after the start of a meal, the highest PPG level within 4 h after the start of a meal and the time to highest PPG level were evaluated. During the URLi maintenance period compared to lispro lead-in, there was a statistically significant reduction in highest PPG excursion from 0 to 4 h after the start of a meal at breakfast and lunch and across all meals (Fig. 6). There was also a statistically significant reduction in highest PPG level within 4 h after the start of a meal at breakfast and lunch during the URLi maintenance period compared to lispro lead-in. There were no statistically significant changes in the time from the start of meal to highest PPG level within 4 h after the start of a meal.
Insulin Sensitivity Factor
At the end of the URLi maintenance period, ISF was numerically lower compared to lispro lead-in at breakfast, lunch, dinner, and across all meals (Table S6).
Duration of Insulin Action
The duration of insulin action was statistically significantly lower at the end of the URLi maintenance period compared to lispro lead-in (mean 3.34 h vs 3.52 h; LSM change from baseline, − 0.19 h [p = 0.007]).
Safety
There were no SAEs, deaths, severe hypoglycemia events, or discontinuations during the study. Four participants (12.9%) reported at least one TEAE from the first dose of investigational product through safety follow-up, including one participant (3.2%) who reported an injection site reaction of mild injection site erythema.
There were no statistically significant changes in vital signs, body weight, or BMI at endpoint compared with baseline.
Discussion
This study examined titration of URLi based on peak PPG values or the change from premeal levels using CGM in people with T1D. This approach may be more suited to titrating ultra-rapid-acting prandial insulins with a faster on–faster off profile like URLi and could allow for more aggressive prandial dosing to further improve PPG without increasing the risk of late postmeal hypoglycemia. We found that, in participants with T1D on MDI therapy, 46 days of treatment with URLi resulted in improved overall glycemic control without increased time below range/hypoglycemia. There was a numerical increase in TIR (70–180 mg/dL), as well as a statistically significant improvement in HbA1c, fructosamine, and 1,5-AG. In addition, URLi treatment demonstrated improved PPG control characterized by reduced highest PPG within 4 h after the start of a meal, glucose iAUC0–2h and iAUC0–4h, and highest PPG excursions 0 to 4 h after the start of a meal. This study builds on the findings of the phase 3 PRONTO diabetes studies including PRONTO-T1D, and its CGM substudy, which found that mealtime URLi was non-inferior to mealtime lispro for HbA1c change from baseline and superior for controlling 1- and 2-h PPG excursions as well as improving daytime TIR (70–180 mg/dL) [11, 13].
The primary endpoint of TIR (70–180 mg/dL) over the 24-h period was 70.2% during the URLi maintenance period, which was above the International Consensus Guidelines on CGM recommendations that people with diabetes should aim for > 70% TIR (70–180 mg/dL) [17]. There was a numerical increase in 24-h and daytime TIR (70–180 mg/dL) during the URLi maintenance period compared to lispro lead-in. There were no significant differences in TBR (< 70 mg/dL or < 54 mg/dL) or TAR (> 180 mg/dL or > 250 mg/dL) between the maintenance period and lead-in during either the daytime or 24-h periods. It is noteworthy in the context of these results that participants already had good glycemic control prior to URLi treatment with a screening HbA1c of 7.13% and TIR (70–180 mg/dL) of 67.9% at the end of the lispro lead-in. Therefore, it may be expected that improvement in glycemic control over the 46-day treatment period may be modest.
The proportion of participants meeting the key consensus guideline recommended CGM target [17] of > 70% TIR (70–180 mg/dL) numerically increased during the URLi maintenance period compared to lead-in as did the proportion with < 5% TAR (> 250 mg/dL). Over 90% of participants had < 1% TBR (< 54 mg/dL) although the proportion was slightly lower during the maintenance period (93.5%) compared to lead-in (96.8%).
Mean CGM hourly glucose profiles showed that glucose was lower during the URLi maintenance period compared to lispro lead-in from around 8:00 am to 4:00 pm. At the end of the maintenance period, within-day sensor variability (%CV) was statistically significantly lower compared to lead-in for the daytime and 24-h periods. During both the daytime and 24-h periods, within-day glucose variability was lower than the consensus guidelines target %CV of ≤ 36%, considered as the cutoff to distinguish between low and high variability [18, 19].
Postprandial glucose control improved with significantly lower iAUC0–2h and iAUC0-4 and highest PPG excursion within 4 h after the start of a meal at breakfast, lunch, and across all meals during the URLi maintenance period. The PPG excursion at dinner was not significantly different and could reflect greater variability in glucose levels later on in the day in comparison to in the morning period which may be more standardized. The highest PPG levels were also significantly lower during the URLi maintenance period at breakfast and lunch. This improved PPG control with URLi reflects the findings of previous studies. In PRONTO-T1D, URLi, administered at mealtime, demonstrated superiority to mealtime lispro in reducing 1-h and 2-h PPG excursions during a 4-h standardized mixed meal tolerance test (approx. 100 g carbohydrate) [11]. These results were supported by the PRONTO-T1D CGM substudy, which found that mealtime URLi was superior in reducing iAUC0–2h at breakfast (primary endpoint) and for all meals combined [13], and mealtime URLi statistically significantly reduced PPG excursions up to 3 h compared to mealtime lispro for all meals combined.
There was a significant reduction in HbA1c at the end of the URLi maintenance period compared with screening, demonstrating an improvement in overall glycemic control following treatment with URLi. Other laboratory parameters, fructosamine and 1,5-AG, which provide measures of medium-term glycemic control and hyperglycemia exposure respectively, were also significantly improved at the end of the maintenance period compared to screening. During the study, insulin dosing was intensified with increased total daily insulin dose and reduced ICR, indicating that more prandial insulin was being administered. However, the prandial-to-TDD ratio was unchanged and was similar to that reported in the PRONTO-T1D study after 26 weeks of URLi treatment (approx. 52%) [11]. There was also a statistically significant reduction in investigator-prescribed duration of insulin action at the end of the URLi maintenance period compared with lispro lead-in.
Treatment with URLi was well tolerated. There were no SAEs, severe hypoglycemia events, or study discontinuations.
Strengths of the study were that unblinded CGM data was collected throughout the whole treatment period and used to inform insulin dosing decisions. The study was conducted at experienced study sites in the USA with close patient involvement and follow-up. This enabled participants and investigators to thoroughly evaluate postmeal glucose peaks to focus on reaching postprandial glycemic targets; however, as a result, study outcomes may not be widely generalizable. Limitations included that no comparator was included in this study and so comparison to other therapies was not possible. However, the efficacy and safety of URLi compared with lispro in people with T1D on MDI therapy have been evaluated in the 52-week phase 3 PRONTO T1D study and its CGM substudy [11, 13]. It is also likely that the frequent monitoring that occurred as part of the clinical study played a role in the improvement in glycemic control. Furthermore, this study had a short duration with a 46-day treatment period and the study had a small population with people from ethnic minority groups underrepresented in the study. Because of the exploratory nature of the study, it was not strictly powered to demonstrate a statistically significant change from baseline in the primary endpoint. Conducting a study with more participants and/or a longer study duration may allow for further evaluation of prandial insulin optimization and improvements to glycemic control and CGM parameters.
Conclusions
In this exploratory study in participants with T1D with quite good baseline glycemic control, URLi in a basal-bolus MDI regimen with a dosing algorithm focused on PPG targets demonstrated improved overall glycemic control and reduced overall PPG excursions without evidence of increased hypoglycemia/TBR. These results indicate the need for further study of the ability to intensify prandial insulin therapy on the basis of postprandial CGM glucose in the treatment of people with T1D.
Data Availability
Eli Lilly and Company provides access to all individual participant data collected during the trial, after anonymization, with the exception of pharmacokinetic or genetic data. Data are available to request 6 months after the indication studied has been approved in the United States and European Union and after primary publication acceptance, whichever is later. No expiration date of data requests is currently set once data are made available. Access is provided after a proposal has been approved by an independent review committee identified for this purpose and after receipt of a signed data sharing agreement. Data and documents, including the study protocol, statistical analysis plan, clinical study report, blank or annotated case report forms, will be provided in a secure data sharing environment. For details on submitting a request, see the instructions provided at www.vivli.org.
References
Monnier L, Colette C, Boniface H. Contribution of postprandial glucose to chronic hyperglycaemia: from the “glucose triad” to the trilogy of “sevens”. Diabetes Metab. 2006;32:2S6–2S11.
Ceriello A. The glucose triad and its role in comprehensive glycaemic control: current status, future management. Int J Clin Pract. 2010;64(12):1705–11.
Beisswenger P, Heine RJ, Leiter LA, Moses A, Tuomilehto J. Prandial glucose regulation in the glucose triad. Endocrine. 2004;25(3):195–202.
Hirsch IB. Insulin analogues. N Engl J Med. 2005;352(2):174–83.
Hirsch IB, Juneja R, Beals JM, Antalis CJ, Wright EE Jr. The evolution of insulin and how it informs therapy and treatment choices. Endocr Rev. 2020;41(5):733–55.
Heise T, Piras de Oliveira C, Juneja R, Ribeiro A, Chigutsa F, Blevins T. What is the value of faster acting prandial insulin? Focus on ultra rapid lispro. Diabetes Obes Metab. 2022;24(9):1689–701.
Evans M, Wilkinson M, Giannpolou A. Fast-acting insulin aspart: the rationale for a new mealtime insulin. Diabetes Ther. 2019;10(5):1793–800.
Pratt E, Leohr J, Heilmann C, Johnson J, Landschulz W. Treprostinil causes local vasodilation, is well tolerated, and results in faster absorption of insulin lispro. Diabetes. 2017;66(Suppl. 1):A253.
Michael MD, Zhang C, Siesky AM, et al. Exploration of the mechanism of accelerated absorption for a novel insulin lispro formulation. Diabetes. 2017;66(Suppl. 1):A250.
Leohr J, Dellva MA, Carter K, LaBell E, Linnebjerg H. Ultra Rapid Lispro (URLi) Accelerates insulin lispro absorption and insulin action vs Humalog® consistently across study populations: a pooled analysis of pharmacokinetic and glucodynamic data. Clin Pharmacokinet. 2021;60(11):1423–34.
Klaff L, Cao D, Dellva MA, et al. Ultra rapid lispro improves postprandial glucose control compared with lispro in patients with type 1 diabetes: results from the 26-week PRONTO-T1D study. Diabetes Obes Metab. 2020;22(10):1799–807.
Blevins T, Zhang Q, Frias JP, Jinnouchi H, Chang AM. Randomized double-blind clinical trial comparing ultra rapid lispro with lispro in a basal-bolus regimen in patients with type 2 diabetes: PRONTO-T2D. Diabetes Care. 2020;43(12):2991–8.
Malecki MT, Cao D, Liu R, et al. Ultra-rapid lispro improves postprandial glucose control and time in range in type 1 diabetes compared to lispro: PRONTO-T1D Continuous Glucose Monitoring Substudy. Diabetes Technol Ther. 2020;22(11):853–60.
Walsh J. Pumping insulin: everything for success on an insulin pump and CGM. San Diego: Torrey Pines; 2016.
Scheiner G. Think like a pancreas: a practical guide to managing diabetes with insulin. New York: Hachette Go; 2020.
Bailey TS, Grunberger G, Bode BW, et al. American Association of Clinical Endocrinologists and American College of Endocrinology 2016 outpatient glucose monitoring consensus statement. Endocr Pract. 2016;22(2):231–61.
Battelino T, Danne T, Bergenstal RM, et al. Clinical targets for continuous glucose monitoring data interpretation: recommendations from the International Consensus on Time in Range. Diabetes Care. 2019;42(8):1593–603.
Danne T, Nimri R, Battelino T, et al. International consensus on use of continuous glucose monitoring. Diabetes Care. 2017;40(12):1631–40.
Monnier L, Colette C, Wojtusciszyn A, et al. Toward defining the threshold between low and high glucose variability in diabetes. Diabetes Care. 2016;40(7):832–8.
Acknowledgements
The authors thank the study participants, and the investigators and study coordinators who provided care for them.
Medical Writing or Editorial Assistance
The authors did not use any medical writing or editorial assistance for this article.
Prior Presentation
A portion of the data from this study was presented at the Advanced Technologies & Treatments for Diabetes (ATTD) 15th International Conference, held April 27–30, 2022, virtually and in Barcelona, Spain.
Funding
This study and the journal Rapid Service fee were funded by Eli Lilly and Company.
Author information
Authors and Affiliations
Contributions
Annette M. Chang was responsible for medical oversight during the trial and contributed to the study conception and design. Richard M. Bergenstal, Bruce W. Bode, and Anuj Bhargava were involved in the acquisition of data for the study. Qianqian Wang contributed to the statistical analyses. All author contributed to interpretation of results. Alastair W. Knights contributed to the drafting of the manuscript and all authors were involved in critical review and revision of the manuscript. All authors gave approval for the final version of the manuscript to be published.
Corresponding author
Ethics declarations
Conflict of interest
Richard M. Bergenstal has received research support, consulted, or has been on a scientific advisory board for Abbott Diabetes Care, Ascensia, CeQur, Dexcom, Eli Lilly and Company, Glooko, Hygieia, Johnson & Johnson, Medtronic, Merck, Novo Nordisk, Onduo, Roche, Sanofi, United Healthcare and Zealand. Richard M. Bergenstal’s employer, nonprofit HealthPartners Institute, contracts for his services, and no personal income goes to Richard M. Bergenstal. Bruce W. Bode is a shareholder of Aseko, Inc. and has received consultant fees from Eli Lilly and Company, Lexicon, Medtronic, Novo Nordisk, and Pfizer; and speaker honoraria from AstraZeneca, Boehringer Ingelheim, Eli Lilly and Company, Janssen, Mannkind, Medtronic, Novo Nordisk, Sanofi, Senseonics, and Xeris. Bruce W. Bode is an employee of Atlanta Diabetes Associates. Atlanta Diabetes Associates has received research grants and support from Boehringer Ingelheim, Dexcom, Diasome, Eli Lilly and Company, Insulet, Janssen, Lexicon, Mannkind, Medtronic, National Institutes of Health (NIH), Nova Biomedical, Novo Nordisk, Provention Bio, Sanofi, Senseonics, REMD Biotherapeutics, VtV Therapeutics LLC, and Xeris. Anuj Bhargava has received research grants from Abbott Diabetes Care, AbbVie, Boehringer Ingelheim, Boston Therapeutics, Covance, Dexcom, Eli Lilly and Company, Gan & Lee Pharmaceuticals, Insulet Corporation, Kowa Pharmaceuticals America, Lexicom, Madrigal Pharmaceuticals, Medtronic, Novo Nordisk, Poxel, Quintiles, Sanofi, Senseonics, Tolerion, Viking Therapeutics, and VtV Therapeutics. Qianqian Wang, Alastair W. Knights, and Annette M. Chang are employees and shareholders of Eli Lilly and Company.
Ethical Approval
The study was conducted at three centers in the United States in accordance with the Declaration of Helsinki, the International Conference on Harmonization Guidelines for Good Clinical Practice, and applicable local laws and regulations. The study protocol and all procedures were reviewed and approved by an ethics review board for each study center (Healthpartners Institute Dba International Diabetes Center, Bloomington, MN; or Western Institutional Review Board – Connexus, Puyallup, WA). All participants provided written informed consent before participating in the study. All authors gave approval for the final version of the manuscript to be published.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License, which permits any non-commercial use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc/4.0/.
About this article
Cite this article
Bergenstal, R.M., Bode, B.W., Bhargava, A. et al. Assessing Time in Range with Postprandial Glucose-Focused Titration of Ultra Rapid Lispro (URLi) in People with Type 1 Diabetes. Diabetes Ther 14, 1933–1945 (2023). https://doi.org/10.1007/s13300-023-01476-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13300-023-01476-4