Abstract
Introduction
We aimed to investigate whether treatment with exenatide could increase time in range (TIR) and decrease glycemic variability, and to evaluate the association between TIR and endothelial injury in patients with type 2 diabetes mellitus (T2DM).
Methods
Two-hundred patients with T2DM treated with exenatide for 16 weeks were included in this study. Seven-point fingerstick blood glucose was used to evaluate derived TIR and glycemic variability. The serum levels of soluble endothelial cell protein C receptor (sEPCR) and von Willebrand factor (vWF) were measured. Ninety-three patients having the data of endothelial injury markers were categorized as derived TIR > 70% or ≤ 70% after the treatment and the association between TIR and endothelial injury were evaluated.
Results
Treatment with exenatide for 16 weeks resulted in a significant reduction in fasting blood glucose, postprandial 2 h blood glucose, and glycated hemoglobin A1c (HbA1c) levels in patients with T2DM. Compared with baseline, derived TIR value was significantly increased [85.7 (57.1, 100.0) % vs. 42.9 (14.9, 71.4) %, P < 0.001], and the parameters of glycemic variability were remarkably decreased after the treatment. After the treatment, serum sEPCR level was significantly decreased from baseline in patients with TIR > 70% [74.5 (32.8, 122.5) ng/mL vs. 96.9 (48.5, 150.9) ng/mL, P = 0.006] but not in those with TIR ≤ 70%; serum vWF level was remarkably decreased in patients with TIR > 70% [from 1166.2 (848.1, 1335.5) mIU/mL to 907.4 (674.3, 1335.1) mIU/mL, P = 0.001] while this effect was modest in those with TIR ≤ 70%.
Conclusions
Treatment with exenatide increases TIR and decreases glycemic variability in patients with T2DM. Moreover, the amelioration of endothelial injury is more pronounced in patients with TIR > 70% after the treatment.
Trial Registration
ChiCTR-IPR-15006558 (registered, 27 May 2015).
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Why carry out this study? |
Intensive glycemic control can decrease the incidence of diabetic vascular complications and reduce all-cause mortality. |
Whether time in range (TIR) is associated with vascular endothelial dysfunction in patients with type 2 diabetes treated with exenatide remains to be clarified. |
This study aimed to investigate the association between TIR and endothelial injury markers in patients with type 2 diabetes treated with exenatide. |
What was learned from the study? |
Treatment with exenatide increases TIR and decreases glycemic variability in patients with T2DM. |
The amelioration of endothelial injury is more pronounced in patients with TIR > 70% after the treatment with exenatide. |
Introduction
The increasing prevalence and incidence of diabetes and its complications represent a global public health burden. According to data released by the International Diabetes Federation, there are 537 million diabetic adults aged 20–79 years old in 2021, and this number is expected to reach 783 million in 2045 [1]. Intensive glycemic control can decrease the incidence of diabetic vascular complications and reduce all-cause mortality [2]. Although glycated hemoglobin A1c (HbA1c) is the gold standard for monitoring glycemic control at 2–3 months prior to the measurement, it provides no information of glycemic variability, which can exert deleterious effects on the endothelium and lead to diabetic vascular complications in type 2 diabetes mellitus (T2DM) [3, 4]. Time in range (TIR) refers to the percentage of time that glucose is within the target range (usually 3.9–10.0 mmol/L in patients with type 1 diabetes mellitus and T2DM) per day, and is recommended as a parameter of glycemic control that provides valuable information beyond HbA1c alone [5]. The increased TIR is associated with lower incidence of diabetic macrovascular and microvascular complications, making TIR to become a key metric of glycemic control in clinical practice [6, 7].
As the most important complications of diabetes, vascular complications are the major cause of death and disability in patients with diabetes, and vascular endothelial injury is their primary pathogenesis [8, 9]. Therefore, in the treatment of diabetes, glucose-lowering agents are expected to prevent and/or treat diabetic vascular complications beyond their glucose-lowering effect. Glucagon-like peptide-1 receptor agonists (GLP-1RAs), a class of glucose-lowering agents, which can enhance glucose-dependent insulin secretion, inhibit glucagon secretion, suppress appetite, and delay gastric emptying, are widely used in the treatment of patients with T2DM [10]. Data from cardiovascular outcome trials show that GLP-1RAs have cardiovascular protective effects. Moreover, meta-analysis indicates that GLP-1RAs also possess potential effects against diabetic microvascular complications [11, 12]. However, whether increment of TIR is associated with improvement of vascular endothelial dysfunction in patients with type 2 diabetes treated with GLP-1RAs remains to be clarified.
In the present study, we aimed to investigate whether treatment with exenatide, the first GLP-1RA approved for the treatment of T2DM in China in 2009, could increase TIR and decrease glycemic variability, and to identify the association between TIR and endothelial injury markers in patients with T2DM. Our study supplies evidence for the diabetes treatment and helps precise diabetes management.
Methods
Study Design and Participants
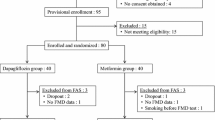
The participants in this study were from a randomized, multicenter, non-inferiority clinical trial. We enrolled patients with T2DM aged 20–70 years, with the HbA1c levels ranging from 7.0% to 10.0%. All patients included in the trial had received monotherapy or combination treatment with metformin and insulin secretagogues. Patients were randomized to receive branded exenatide (Byetta®, Lilly Pharma Fertigung und Distribution GmbH & Co. KG, Giessen, Germany) or generic exenatide (Qinghai Chenfei Pharmaceutical Co. Ltd, Qinghai, China). In both groups, exenatide was administered subcutaneously at an initial dose of 5 μg twice daily for 4 weeks, followed by 10 μg twice daily for an additional 12 weeks (keeping the primary dosage if patients were unable to tolerate the increase). Full details of study design and protocol have been published elsewhere [13]. The trial was registered at www.chictr.org.cn (ChiCTR-IPR-15006558). A total of 240 patients were treated with either of the exenatides in this study. Forty patients were excluded because of protocol violation, loss to follow-up, withdrawal due to adverse events, refusal to continue participation, or missing baseline data for derived TIR calculation. Therefore, 200 patients were included in the final analyses (Supplementary Fig. 1).
This study was approved by the Ethics Committee of Peking University Third Hospital and conducted in accordance with the ethical principles of the Declaration of Helsinki of 1964 and its later amendments. All patients signed written informed consent before enrollment.
Blood Collection and Measurement
Blood samples were collected from each patient at baseline and after 16 weeks of treatment with exenatide, and centrifuged at 4000 rpm for 10 min for serum collection. The laboratory tests performed for serum chemistry analysis included HbA1c, lipid profiles, hepatic parameters, and renal function. Biochemical analysis of endothelial injury marker levels in serum was performed by using enzyme-linked immunosorbent assay (ELISA) for soluble endothelial cell protein C receptor (sEPCR; Dogesce Biotechnology, Beijing, China) and von Willebrand factor (vWF; Abcam, Cambridge, MA, USA). To avoid the possible bias induced by different exenatide origins on the levels of serum endothelial injury markers, only 93 patients who were treated with the branded exenatide injection were selected to evaluate the effect of exenatide on endothelial injury markers. The clinical characteristics of patients with and without the evaluation of endothelial injury markers were almost identical (Supplementary Table 1).
Glycemic Metrics
Fingerstick blood glucose levels that were monitored seven times a day (before three meals, 2 h after three meals, and at bedtime) were obtained from all patients at baseline and at the interval of every 2 weeks after treatment with exenatide. The values of derived TIR, time above range (TAR), and time below range (TBR) were calculated as the percentage of blood glucose values within the target range of 3.9–10.0 mmol/L, above 10 mmol/L and below 3.9 mmol/L, respectively. Moreover, the parameters of glycemic variability, including standard deviation of blood glucose (SDBG), largest amplitude of glycemic excursions (LAGE), and postprandial glucose excursions (PPGE), were calculated as reported previously [14,15,16].
Statistical Analyses
The Shapiro–Wilk test was applied to test for the normality of continuous variables. Data are presented as mean ± standard deviation (SD) or median (interquartile range), as appropriate. For comparison of two groups, Student’s t test and Wilcoxon signed-rank test were used for the normally distributed variables and non-normally distributed variables, respectively. Categorical variables were reported as number (%) and Pearson’s chi-squared test was used to compare differences between groups. Univariate and multivariate logistic regression analyses were performed to evaluate the baseline parameters predicting derived TIR value after the exenatide treatment. P < 0.05 was considered as statistical significance.
Results
Clinical Characteristics of Participants at Baseline and After Treatment with Exenatide for 16 Weeks
A total of 200 patients with T2DM receiving the exenatide treatment were included in this study. Compared with baseline, a significant reduction in body weight (77.7 ± 14.8 kg vs. 79.7 ± 15.0 kg, P < 0.001), body mass index (27.7 ± 4.04 kg/m2 vs. 28.4 ± 4.14 kg/m2, P < 0.001), diastolic blood pressure (76.9 ± 7.38 mmHg vs. 78.9 ± 8.27 mmHg, P = 0.001), fasting blood glucose (8.09 ± 2.20 mmol/L vs. 9.39 ± 1.79 mmol/L, P < 0.001), postprandial 2 h blood glucose (13.3 ± 4.05 mmol/L vs. 16.2 ± 3.48 mmol/L, P < 0.001), and HbA1c (7.07 ± 1.16 % vs. 8.19 ± 0.90 %, P < 0.001) level was observed after treatment with exenatide for 16 weeks in patients with T2DM whose blood glucose levels were inadequately controlled with monotherapy or combination therapy of metformin and insulin secretagogues (Table 1). There were no significant differences between baseline and post-treatment in terms of lipid profiles, hepatic parameters, and renal function (all P > 0.05).
Effects of Exenatide Treatment on Derived TIR and Glycemic Variability
After treatment with exenatide for 16 weeks, derived TIR value increased from baseline in a time-dependent manner, with a significant difference starting from the second week of treatment (Fig. 1). At the end of the 16-week treatment, the derived TIR value significantly increased in patients with T2DM when compared with baseline [85.7 (57.1, 100.0) % vs. 42.9 (14.9, 71.4) %, P < 0.001] (Table 1 and Fig. 1). Compared with baseline, the parameters of glycemic variability, such as SDBG [1.64 (1.11, 2.39) mmol/L vs. 2.36 (1.74, 2.98) mmol/L, P < 0.001], LAGE [4.50 (3.00, 6.75) mmol/L vs. 6.40 (4.80, 8.00) mmol/L, P < 0.001], and PPGE [2.20 (1.38, 3.22) mmol/L vs. 3.27 (2.01, 4.43) mmol/L, P < 0.001] significantly decreased after the treatment (Table 1 and Fig. 2). In addition, the similar effects of exenatide on the derived TIR and the parameters of glycemic variability were observed in subgroups of patients with and without the evaluation of endothelial injury markers (Supplementary Figs. 2 and 3).
Baseline Variables Predicting Derived TIR Value Induced by Exenatide Treatment
Results of univariate logistic regression analysis of baseline variables in relation to derived TIR value are listed in Table 2. The results showed that younger age, shorter duration of diabetes, lower blood glucose concentration at postprandial 2 h or fasting state, lower HbA1c level, smaller alkaline phosphatase value, and higher high-density lipoprotein cholesterol level at baseline were associated with higher derived TIR value after treatment with exenatide. To investigate the independent effects, the factors with significant differences in univariate logistic analysis were further analyzed by using stepwise multivariate logistic regression. As shown in Fig. 3, HbA1c level (OR 0.534; 95% CI 0.359, 0.793) and duration of diabetes (OR 0.885; 95% CI 0.807, 0.971) at baseline were associated with higher derived TIR value after the treatment.
Attainment of TIR Target is Associated with Improvement of Endothelial Injury in Patients Treated with Exenatide
According to the values of derived TIR at 16 weeks, the patients were divided into two groups (TIR > 70% and TIR ≤ 70%). Compared with baseline, a significant reduction in body weight (78.1 ± 14.3 kg vs. 80.6 ± 14.8 kg, P < 0.001), fasting blood glucose (7.30 ± 1.52 mmol/L vs. 9.12 ± 1.73 mmol/L, P < 0.001), alkaline phosphatase [65.0 (56.0, 79.0) U/L vs. 67.0 (57.0, 80.6) U/L, P = 0.005], SDBG [1.30 (0.98, 1.87) mmol/L vs. 2.21 (1.65, 2.87) mmol/L, P < 0.001], LAGE [3.80 (2.50, 5.20) mmol/L vs. 6.00 (4.30, 7.90) mmol/L, P < 0.001], and PPGE [1.87 (1.20, 2.60) mmol/L vs. 3.07 (1.73, 4.10) mmol/L, P < 0.001] was observed in patients with TIR > 70% but not in those with TIR ≤ 70% after treatment with exenatide (Table 3). Importantly, serum sEPCR level was significantly decreased from baseline in patients with TIR > 70% [74.5 (32.8, 122.5) ng/mL vs. 96.9 (48.5, 150.9) ng/mL, P = 0.006] but not in those with TIR ≤ 70% after the treatment. Serum vWF level was remarkably decreased from baseline in patients with TIR > 70% [907.4 (674.3, 1335.1) mIU/mL vs. 1166.2 (848.1, 1335.5) mIU/mL, P = 0.001], while this effect was modest in those with TIR ≤ 70% [1168.2 (802.2, 1455.3) mIU/mL vs. 1245.8 (1022.0, 1812.6) mIU/mL, P = 0.009] after the exenatide treatment (Fig. 4 and Supplementary Table 2).
Changes of endothelial injury markers in patients with derived TIR > 70% and ≤ 70% after the exenatide treatment. Data are presented as median (interquartile range) and each dot represents an individual value. *P < 0.05 vs. baseline. sEPCR soluble endothelial cell protein C receptor, vWF von Willebrand factor
Discussion
In the present study, we demonstrated that treatment with exenatide for 16 weeks improved glycemic control and lowered body weight and diastolic blood pressure in patients with T2DM whose HbA1c levels were inadequately controlled by either monotherapy or combined therapy with metformin and insulin secretagogues. Moreover, the exenatide treatment resulted in an increased derived TIR value and a decreased glycemic variability as indicated by SDBG, LAGE, and PPGE. In addition, lower baseline HbA1c level and shorter duration of diabetes were identified as independent predictors for higher derived TIR value after treatment with exenatide. Notably, the amelioration of endothelial injury, characterized by the decreased levels of sEPCR and vWF, was more pronounced in patients with TIR > 70% than in those with TIR ≤ 70% after the exenatide treatment.
Several clinical studies have shown that treatment with exenatide improves glycemic control and reduces body weight in patients with T2DM [17, 18]. Similarly, this study indicated that treatment with exenatide for 16 weeks in patients with T2DM not only significantly lowered fasting blood glucose, postprandial 2 h blood glucose, and HbA1c levels but also remarkably reduced body weight and body mass index.
With growing evidence of correlation between TIR and diabetic vascular complications, TIR has been recommended as an important parameter to evaluate the favorable glycemic control by recent international guidelines and consensus [5, 19]. Glucose-lowering agents, such as teneligliptin (a dipeptidyl peptidase 4 inhibitor), dapagliflozin (a sodium-glucose cotransporter 2 inhibitor), and acarbose (an α-glucosidase inhibitor), have been demonstrated to increase TIR value in patients with T2DM [20,21,22]. However, whether exenatide, the first approved GLP-1RA, can increase TIR value is still unknown. In the present study, we found that treatment with exenatide for 16 weeks in patients with T2DM significantly increased derived TIR value. To the best of our knowledge, this is the first report to evaluate the effect of exenatide on TIR value in patients with diabetes.
Our study also showed that the parameters of glycemic variability, including SDBG, LAGE, and PPGE, were significantly decreased after treatment with exenatide, indicating that exenatide was potent in reducing blood glucose fluctuations in patients with T2DM. These results are in accordance with findings from a previous study, in which treatment with exenatide for 16 weeks markedly decreased SDBG, LAGE, and mean amplitude of glycemic excursions. However, a small sample size of participants (only 19 patients with T2DM) was included in that study [23]. This study included 200 patients with T2DM and made the observations that exenatide decreased glycemic variability more concrete.
Identification of the baseline parameters to predict which patients derive the most benefit from treatment with exenatide will help precision diabetes management [24]. We demonstrated that shorter duration of diabetes and lower HbA1c level at baseline were independently associated with higher derived TIR value, which indicated that patients with T2DM whose duration of diabetes was shorter and baseline HbA1c level was lower would benefit more from the exenatide treatment in terms of TIR control. This finding might be partly explained by the fact that shorter duration of diabetes and lower HbA1c level reflected better pancreatic β cell function, and exenatide was more prone to increase TIR in this subgroup of patients with T2DM [25, 26].
The results of cardiovascular outcome trials and meta-analysis show that GLP-1RAs can not only reduce the risk of major adverse cardiovascular events but also prevent the development of diabetic microvascular complications [11, 12], indicating that GLP-1RAs have preventive and therapeutic effects on diabetic vascular complications. It has been demonstrated that endothelial dysfunction is a critical mechanism of diabetic vascular complications, and amelioration of endothelial injury can help to prevent the progression of these complications [27]. In our previous studies, treatment with exenatide in patients with T2DM significantly decreased the levels of endothelial injury markers like soluble vascular cell adhesion molecule 1, intercellular cell adhesion molecule 1, soluble thrombomodulin, sEPCR, and vWF [28, 29]. However, which subgroup of people with T2DM will benefit more from the amelioration of endothelial injury remains unclear. In the present study, we found that the levels of the endothelial injury markers sEPCR and vWF were more significantly decreased in patients who achieved the goal of TIR > 70% than in those with TIR ≤ 70% after the exenatide treatment. These results indicated that elevation of TIR value had an important role in the improvement of endothelial dysfunction in patients with T2DM treated with exenatide.
There are some limitations in our study. First, seven-point fingerstick blood glucose monitoring is somewhat limited in assessing TIR and glycemic variability for individuals with diabetes. However, TIR derived from the seven-point blood glucose monitoring has been reported to have a strong association with the risk of the development and/or progression of diabetic retinopathy and diabetic kidney disease in several other studies [6, 30]. Second, even though 200 patients with T2DM were included in the present study, larger number of patients with T2DM would be required to confirm our findings. Third, this is a post hoc analysis with only single-arm intervention. Randomized clinical trials having a control group without treatment with exenatide can provide stronger evidence.
Conclusions
Our study demonstrates that treatment with exenatide for 16 weeks improves glycemic control and lowers body weight in patients with T2DM. Moreover, treatment with exenatide results in improved TIR control and decreased glycemic variability. In addition, lower baseline HbA1c level and shorter duration of diabetes are identified as independent predictors for better TIR control in the patients treated with exenatide. Importantly, the amelioration of endothelial injury is more pronounced in patients with TIR > 70% than in those with TIR ≤ 70% after treatment with exenatide.
References
Ogurtsova K, Guariguata L, Barengo NC, et al. IDF diabetes Atlas: Global estimates of undiagnosed diabetes in adults for 2021. Diabetes Res Clin Pract. 2022;183: 109118.
Holman RR, Paul SK, Bethel MA, et al. 10-year follow-up of intensive glucose control in type 2 diabetes. N Engl J Med. 2008;359(15):1577–89.
Vigersky RA. Going beyond HbA1c to understand the benefits of advanced diabetes therapies. J Diabetes. 2019;11(1):23–31.
Ceriello A, Monnier L, Owens D. Glycaemic variability in diabetes: clinical and therapeutic implications. Lancet Diabetes Endocrinol. 2019;7(3):221–30.
Battelino T, Danne T, Bergenstal RM, et al. Clinical targets for continuous glucose monitoring data interpretation: recommendations from the international consensus on time in range. Diabetes Care. 2019;42(8):1593–603.
Beck RW, Bergenstal RM, Riddlesworth TD, et al. Validation of time in range as an outcome measure for diabetes clinical trials. Diabetes Care. 2019;42(3):400–5.
Lu J, Ma X, Shen Y, et al. Time in range is associated with carotid intima-media thickness in type 2 diabetes. Diabetes Technol Ther. 2020;22(2):72–8.
Cho NH, Shaw JE, Karuranga S, et al. IDF Diabetes Atlas: global estimates of diabetes prevalence for 2017 and projections for 2045. Diabetes Res Clin Pract. 2018;138:271–81.
Santilli F, D’Ardes D, Davì G. Oxidative stress in chronic vascular disease: from prediction to prevention. Vascul Pharmacol. 2015;74:23–37.
Campbell JE, Drucker DJ. Pharmacology, physiology, and mechanisms of incretin hormone action. Cell Metab. 2013;17(6):819–37.
Lim S, Oh TJ, Dawson J, et al. Diabetes drugs and stroke risk: intensive versus conventional glucose-lowering strategies, and implications of recent cardiovascular outcome trials. Diabetes Obes Metab. 2020;22(1):6–15.
Avgerinos I, Karagiannis T, Malandris K, et al. Glucagon-like peptide-1 receptor agonists and microvascular outcomes in type 2 diabetes: a systematic review and meta-analysis. Diabetes Obes Metab. 2019;21(1):188–93.
Yang J, Xiao W, Guo L, et al. Efficacy and safety of generic exenatide injection in Chinese patients with type 2 diabetes: a multicenter, randomized, controlled, non-inferiority trial. Acta Diabetol. 2020;57(8):991–1000.
Tang X, Li S, Wang Y, et al. Glycemic variability evaluated by continuous glucose monitoring system is associated with the 10-y cardiovascular risk of diabetic patients with well-controlled HbA1c. Clin Chim Acta. 2016;461:146–50.
Wang C, Lv L, Yang Y, et al. Glucose fluctuations in subjects with normal glucose tolerance, impaired glucose regulation and newly diagnosed type 2 diabetes mellitus. Clin Endocrinol (Oxf). 2012;76(6):810–5.
Bonora E, Calcaterra F, Lombardi S, et al. Plasma glucose levels throughout the day and HbA(1c) interrelationships in type 2 diabetes: implications for treatment and monitoring of metabolic control. Diabetes Care. 2001;24(12):2023–9.
DeFronzo RA, Ratner RE, Han J, et al. Effects of exenatide (exendin-4) on glycemic control and weight over 30 weeks in metformin-treated patients with type 2 diabetes. Diabetes Care. 2005;28(5):1092–100.
Fehse F, Trautmann M, Holst JJ, et al. Exenatide augments first- and second-phase insulin secretion in response to intravenous glucose in subjects with type 2 diabetes. J Clin Endocrinol Metab. 2005;90(11):5991–7.
American Diabetes Association. 6. Glycemic targets: standards of medical care in diabetes-2021. Diabetes Care. 2021;44(Suppl 1):S73–S84.
Saboo B, Erande S, Unnikrishnan AG. A prospective multicentre open label study to assess effect of teneligliptin on glycemic control through parameters of time in range (TIR) metric using continuous glucose monitoring (TOP-TIR study). Diabetes Metab Syndr. 2022;16(2): 102394.
Henry RR, Strange P, Zhou R, et al. Effects of dapagliflozin on 24-hour glycemic control in patients with type 2 diabetes: a randomized controlled trial. Diabetes Technol Ther. 2018;20(11):715–24.
Gao F, Ma X, Peng J, et al. The effect of acarbose on glycemic variability in patients with type 2 diabetes mellitus using premixed insulin compared to metformin (AIM): an open-label randomized trial. Diabetes Technol Ther. 2020;22(4):256–64.
Yin T-T, Bi Y, Li P, et al. Comparison of glycemic variability in chinese T2DM patients treated with exenatide or insulin glargine: a randomized controlled trial. Diabetes Ther. 2018;9(3):1253–67.
Heo CU, Choi C-I. Current progress in pharmacogenetics of second-line antidiabetic medications: towards precision medicine for type 2 diabetes. J Clin Med. 2019;8(3):393.
Rasouli N, Younes N, Utzschneider KM, et al. Association of baseline characteristics with insulin sensitivity and β-cell function in the glycemia reduction approaches in diabetes: a comparative effectiveness (GRADE) study cohort. Diabetes Care. 2021;44(2):340–9.
Funakoshi S, Fujimoto S, Hamasaki A, et al. Analysis of factors influencing pancreatic beta-cell function in Japanese patients with type 2 diabetes: association with body mass index and duration of diabetic exposure. Diabetes Res Clin Pract. 2008;82(3):353–8.
Teodoro JS, Nunes S, Rolo AP, et al. Therapeutic options targeting oxidative sttress, mitochondrial dysfunction and inflammation to hinder the progression of vascular complications of diabetes. Front Physiol. 2018;9:1857.
Wei R, Ma S, Wang C, et al. Exenatide exerts direct protective effects on endothelial cells through the AMPK/Akt/eNOS pathway in a GLP-1 receptor-dependent manner. Am J Physiol Endocrinol Metab. 2016;310(11):E947–57.
Yang J, Le Y, Wei T, et al. Non-targeted metabolomic analysis predicts the therapeutic effects of exenatide on endothelial injury in patients with type 2 diabetes. J Diabetes Complications. 2021;35(2): 107797.
Avari P, Uduku C, George D, et al. Differences for percentage times in glycemic range between continuous glucose monitoring and capillary blood glucose monitoring in adults with type 1 diabetes: analysis of the REPLACE-BG dataset. Diabetes Technol Ther. 2020;22(3):222–7.
Acknowledgements
The authors appreciate for Lixin Guo from Department of Endocrinology, Beijing Hospital; Quanmin Li from Department of Endocrinology, PLA Rocket Force Characteristic Medical Center; Liyong Zhong from Department of Endocrinology, Beijing Tiantan Hospital, Capital Medical University; Jinkui Yang from Department of Endocrinology, Beijing Tongren Hospital, Capital Medical University; Jing Yang from Department of Endocrinology, The First Hospital of Shanxi Medical University; and Yongyi Gao from Department of Endocrinology, People’s Hospital of Hainan Province, for their help with the enrollment of patients and data collection.
Funding
This study was partially supported by research grants from National Key Research and Development Program of China (2018YFC1313900), the National Natural Science Foundation of China (81830022, 82170875, and 82070319), the Natural Science Foundation of Beijing (7212127), and the Capital’s Funds for Health Improvement and Research (2020–3-40914). The journal’s Rapid Service Fee was funded by the authors.
Authorship
All named authors meet the International Committee of Medical Journal Editors (ICMJE) criteria for authorship for this article, take responsibility for the integrity of the work as a whole, and have given their approval for this version to be published.
Author Contributions
Tianpei Hong and Rui Wei made substantial contributions to conception, study design, and reviewing of the manuscript. Yunyi Le and Kun Yang performed the research experiments and statistical analyses and prepared the manuscript. Jin Yang, Wei Fu and Wenhua Xiao helped perform the research experiments and analyze data.
Disclosures
Yunyi Le, Kun Yang, Jin Yang, Wei Fu, Wenhua Xiao, Rui Wei and Tianpei Hong confirm that they have no competing interests to declare.
Compliance with Ethics Guidelines
This study was fully compliant with ethical guidelines and was approved by the Ethinic Committee of Peking University Third Hospital. This study was registered on the Chinese Clinical Trial Registry Database (ChiCTR-IPR-15006558) and was conducted in accordance with the ethical principles of the Declaration of Helsinki of 1964 and its later amendments. All participants provided written informed consent before enrollment.
Data Availability
The datasets generated and/or analyzed during the current study are available from the corresponding author on reasonable request.
Author information
Authors and Affiliations
Corresponding authors
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License, which permits any non-commercial use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc/4.0/.
About this article
Cite this article
Le, Y., Yang, K., Yang, J. et al. Association of Time in Range with Endothelial Injury in Patients with Type 2 Diabetes Treated with Exenatide. Diabetes Ther 13, 1755–1767 (2022). https://doi.org/10.1007/s13300-022-01310-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13300-022-01310-3