Abstract
Introduction
The aim of this study was to assess the efficacy and safety of sodium-glucose cotransporter 2 (SGLT2) inhibitors in East Asians with type 2 diabetes mellitus (T2DM).
Methods
A literature search that focused primarily on the PubMed, Embase, and Cochrane library databases was performed. All randomized controlled trials (RCTs) which satisfied the inclusion and exculsion criteria were eligible to be included in the meta-analysis. Risk ratios (RRs) and weighted mean differences (WMDs) were used as statistical indicators for the analysis of dichotomous data and continuous outcomes, respectively. Pooled estimates were obtained using random-effects models in RevMan version 5.3.5.
Results
Thirty-three RCTs (8496 randomized patients) fulfilled the eligibility criteria for inclusion in the meta-analysis. The meta-analysis showed that, compared with the control group, the use of SGLT2 inhibitors improved both glycated hemoglobin (HbA1c) in patients (WMD − 0.73%; 95% confidence interval [CI] − 0.84, − 0.61) and the percentage of patients with HbA1c < 7% (RR 2.33; 95% CI 1.74, 3.12); lowered both fasting plasma glucose (WMD − 28.47 mg/dl; 95% CI − 32.86, − 24.08) and postprandial glucose (WMD − 52.32 mg/dl; 95% CI − 67.67, − 39.96); reduced body weight (WMD − 1.73 kg; 95% CI − 2.28, − 1.17); and did not increase the risk of hypoglycemia (RR 1.27; 95% CI 0.89, 1.82) and urinary tract infections (RR 0.93; 95% CI 0.68, 1.27). However, SGLT2 inhibitors did increase the risk of genital tract infections (GTIs) (RR 1.73; 95% CI 1.02, 2.96). The stratified analysis showed that patients with higher HbA1c levels at baseline may achieve a greater improvement in HbA1c after taking SGLT2 inhibitors, while those with higher body weight or a longer history of diabetes may have an increased risk of developing GTIs.
Conclusion
Current research suggests that SGLT2 inhibitors have favorable efficacy and safety in East Asian patients with T2DM.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The burden of diabetes continues to increase globally, especially in developing countries [1]. Sodium-glucose cotransporter 2 (SGLT2) inhibitors are a new type of anti-hyperglycemic drug and have been recommended as first-line or second-line drugs for the treatment of diabetes by the European Diabetes Association and the American Diabetes Association. Recent studies have shown that SGLT2 inhibitors are beneficial for cardiovascular and renal outcomes in type 2 diabetes mellitus (T2DM) [2]. However, a systematic analysis of their efficacy in East Asian populations is lacking. Studies have shown that East Asian patients with T2DM have a lower body mass index (BMI), reduced insulin secretion, and higher insulin sensitivity than Caucasian patients with T2DM [3, 4]. It has also been reported that Chinese American and Japanese American premenopausal or early perimenopausal women without diabetes have a lower homeostatic model assessment (HOMA) of steady state beta cell function (%-β) than their non-Hispanic White counterparts [5]. These differences between East Asians and Western subjects in T2DM pathophysiology lead to discrepancies in therapeutic approaches. For example, studies suggest that dipeptidyl peptidase-4 (DPP-4) inhibitors and glucagon-like peptide-1 receptor agonists may be the more suitable therapeutic options for East Asian patients with T2DM [6]. Therefore, the aim of our study was to perform a systematic review and meta-analysis to evaluate the efficacy and safety profiles of SGLT2 inhibitors for the treatment East Asian patients with T2DM.
Methods
Search Strategy
This systematic review and meta-analysis were developed according to Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) Statement [7] (Electronic Supplemental Material [ESM] Table S1). We performed a systematic search of the PubMed, Embase, and the Cochrane Library databases to identify eligible studies published before October 2018 without the restrictions of country, language, or race. The following search terms were used: “East Asian” OR “East Asia” OR “China” OR “Japan” OR “Mongolia” OR “North Korea” OR “South Korea” AND “sodium glucose co-transporter 2 inhibitors” OR “dapagliflozin” OR “canagliflozin” OR “empagliflozin” OR “ipragliflozin” OR “tofogliflozin” OR “luseogliflozin” OR “sergliflozin” OR “remogliflozin.” Search conditions were adjusted to comply with the provisions of each database.
This article is based on previously conducted studies and does not contain any studies with human participants or animals performed by any of the authors.
Outcome Measures of Efficacy and Safety
The primary outcome of efficacy was the change in glycated hemoglobin (HbA1c) from baseline. The secondary results covered the proportion of patients with HbA1c < 7.0% (53 mmol/mol) and mean change in fasting plasma glucose (FPG), postprandial glucose (PPG) and body weight. The indicators of safety and tolerability events were hypoglycemia, urinary tract infections (UTIs), genital tract infections (GTIs), cardiovascular safety, acute renal failure, hypotension, and bone fractures.
Inclusion Criteria and Exclusion Criteria
Inclusion criteria were: (1) randomized clinical trials (RCTs) that compared SGLT2 inhibitors as either monotherapy or add-on therapy with other hyperglycemic drugs or placebo in T2DM subjects; (2) patients aged ≥ 18 years who were diagnosed with T2DM mellitus according to the 1999 World Health Organization diagnostic criteria or the 1997 American Diabetes Association [8, 9]; (3) treatment duration of at least 12 weeks; and (4) a percentage of East Asian patients in the study of > 50%.
The exclusion criteria were: (1) subgroup analysis, retrospective study, meta-analysis, uncontrolled trials, reviews, research in which HbA1c information cannot be extracted, duplicate publications, and case reports; and (2) patients with severe cardiac, hepatic, and renal insufficiency, as well as pregnant or lactating women.
Data Extraction
Two researchers independently screened and extracted the data according to the eligibility and exclusion criteria; any disagreements were discussed and a consensus reached. Following screening of the literature, two researchers assessed whether the trials would eventually be incorporated into the meta-analysis. Data on baseline demographics, interventions, efficacy, and safety outcomes were extracted.
Risk of Bias Assessment
The methodological quality of the included RCTs was assessed using the Cochrane Collaboration risk of bias tool (RevMan software version 5.3.5; Cochrane, London, UK). This tool assesses selection bias (random sequence generation; allocation concealment); performance bias (blinding of participants and personnel); detection bias (blinding of outcome assessors); attrition bias (incomplete outcome data); reporting bias (selective outcome reporting); and other bias parameters. Three levels were used to judge the risk bias of each study: “high risk,” “low risk,” and “unclear risk.” Two of the researchers conducted the quality assessment and consulted with a third reviewer if disagreements arose. Publication bias was assessed using funnel plots.
Statistical Analysis
RevMan (version 5.3.5; Cochrane Collaboration) was used for the analysis. For dichotomous data and continuous outcomes, risk ratios (RRs) and weighted mean differences (WMDs) were used. The Chi-square test (χ2) and I2 statistics were used to assess heterogeneity, and the random effect model was adopted regardless of I2. If the primary outcome data (such as the standard deviation and variance measures) were missing or incomplete, then we sent emails to the corresponding authors or sponsors of the study. If necessary, the standard deviation was calculated according to the confidence interval (CI) or standard error as described in the Cochrane Handbook. In addition, we conducted stratified analyses of the characteristic profiles based on HbA1c improvement and the risk of GTIs, respectively. The unpaired t test was selected in the GraphPad Prism 7.00 (Graphpad Software Inc., San Diego, CA, USA) to complete the analysis.
Results
Literature Search and Characteristics of the Included Studies
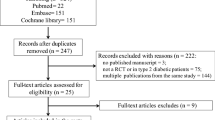
The selection steps and results are outlined in Fig. 1. Using the described search strategies, we screened 1450 items. After reviewing the articles according to titles, abstracts, and full texts in detail, we selected 33 trials for inclusion in the meta-analysis. Compared to placebo or other active antidiabetic agents (metformin, teneligliptin, sitagliptin, etc.), SGLT2 inhibitors were studied as monotherapy in 13 RCTs [10–22] and as add-on therapy in 20 RCTs [23–42]. Specifically, in these trials a total of 8496 patients were randomized, of which 6711 were assigned to an SGLT2 inhibitor: dapagliflozin (10 RCTs), canagliflozin (5 RCTs), empagliflozin (3 RCTs), ipragliflozin (9 RCTs), tofogliflozin (2 RCTs), and luseogliflozin (4 RCTs). The characteristics of the included studies are shown in ESM Table S2. Participants were aged > 18 years with HbA1c between 7% (53 mmol/mol) and 12% (107 mmol/mol). The BMI of most patients was > 24 kg/m2.
Risk of Bias Assessment
The risk of bias was estimated according to the Cochrane Collaboration risk of bias tool; the data are shown in ESM Fig. S1. Random sequence generation was evident in all but two of the RCTs [39, 42]; similarly, allocation concealment was obtained in 31 RCTs but was not explicitly described in these same two RCTs [39, 42]. In addition, four studies [39–42] were open label trials, and the blind law principle was not strictly enforced. The overall risk of bias in the included studies was low. Funnel charts showing publication bias are shown in ESM Fig. S2.
Efficacy of SGLT2 inhibitors
All 33 RCTs, involving 8469 patients, reported the change in HbA1c from baseline. The heterogeneity, as assessed by I2, was 92% (P < 0.00001). The meta-analysis (Fig. 2) using the random-effects model showed that the SGLT2 inhibitors improved HbA1c compared with the control group (WMD − 0.73%; 95% CI − 0.84, − 0.61; P < 0.00001). A subgroup analysis was also conducted to estimate the impact of different therapeutic regimens compared with placebo or active control. The results indicated that SGLT2 inhibitors as both a monotherapy and add-on therapy could significantly reduce HbA1c (monotherapy vs. control: WMD − 0.78%; 95% CI − 0.90, − 0.65; add-on therapy vs. control: WMD − 0.68%; 95% CI − 0.87, − 0.50) (Fig. 2).
Twelve trials (1275 patients) reported the percentage of patients with HbA1c < 7% at the end of the study The heterogeneity, as assessed by I2, was 80% (P < 0.00001). The meta-analysis showed that the percentage of patients with HbA1c < 7% was 2.33-fold higher in the patients receiving SGLT2 inhibitors than in the control group [RR 2.33; 95% CI 1.74, 3.12). Compared with the control group, SGLT2 inhibitors significantly lowered the percentage of patients with HbA1c < 7% , whether as monotherapy (RR 4.53; 95% CI 2.18, 9.40) or as add-on therapy (RR 1.81; 1.40, 2.33) (Fig. 3). The change in FPG and PPG was reported in 29 trials (7764 patients) and ten trials (2500 patients), respectively; the respective heterogeneities, as assessed by I2, were 90% (P < 0.00001) and 95% (P < 0.00001). Our meta-analysis (ESM Figs. S3, S4) showed that both FPG (WMD − 28.47 mg/dl; 95% CI − 32.86, − 24.08)] and PPG (WMD − 52.32 mg/dl; 95% CI − 67.67, − 39.96) were improved in patients treated with SGLT2 inhibitors compared with the control group. Subgroup analysis revealed that SGLT2 inhibitors significantly lowered FPG compared to the control as both monotherapy (WMD − 29.99 mg/dl; 95% CI − 34.17, − 25.81) and as add-on therapy (WMD − 27.00 mg/dl; 95% CI − 34.71, − 19.30) (ESM Fig. S3). Similar results were found for PPG, with SGLT2 inhibitors clearly decreasing PPG as monotherapy (WMD − 58.14 mg/dl; 95% CI − 67.56, − 48.73) and as add-on therapy (WMD − 43.08 mg/dl; 95% CI − 68.82, − 17.35) (ESM Fig. S4). In the subgroup analysis, both monotherapy and add-on therapy distinctly improved FPG (monotherapy vs. control: WMD − 29.99 mg/dl, 95% CI − 34.17, − 25.81; add-on therapy vs. control: WMD − 27.00 mg/dl, 95% − 34.71, − 19.30) and PPG (monotherapy vs. control: WMD − 58.14 mg/dl, 95% CI − 67.56, − 48.73; add-on therapy vs. control: WMD − 43.08 mg/dl, 95% CI − 68.82, − 17.35).
The change in body weight was evaluated in 29 trials (7818 patients). The heterogeneity, as assessed by I2, was 98% (P < 0.00001). The meta-analysis showed (ESM Fig. S5) a significant reduction in body weight of − 1.73 kg (95% CI − 2.28, − 1.17) in patients receiving SGLT2 inhibitors as compared with those in the control group. In addition, compared with the control group, SGLT2 inhibitors significantly reduced body weight, whether as monotherapy or as add-on therapy (monotherapy vs. control: WMD − 1.73 kg, 95% CI − 1.56, − 1.90; add-on therapy vs. control: WMD − 1.70 kg, 95% CI − 2.53, − 0.87).
Safety of SGLT2 Inhibitors
The risk of all adverse events was reported in 30 trials (8163 patients). The heterogeneity, as assessed by I2, was 49% (P = 0.001). The meta-analysis (Fig. 4) using the random effects model showed that patients who received a therapeutic intervention with a SGLT2 inhibitor had a higher risk of adverse events than did the control group (RR 1.09; 95% CI 1.02, 1.12). Hypoglycemia was defined as a plasma glucose level of ≤ 3.9 mmol/L (70 mg/dl) with or without symptoms [43]. Thirty-one trials (8405 patients) reported hypoglycemia. The heterogeneity, as assessed by I2, was 50% (P = 0.001). The meta-analysis (ESM Fig. S6) showed that SGLT2 inhibitors did not increase the risk of hypoglycemia compared with the control group [RR 1.27; 95% CI 0.89, 1.82).
UTI events were reported in 23 trials (7284 patients). The heterogeneity, as assessed by I2, was 0% (P = 0.97). The meta-analysis showed no higher risk of UTIs in patients taking SGLT2 inhibitors than in patients taking placebo or active controls (RR 0.93; 95% CI 0.68, 1.27) (ESM Fig. S7). Twenty-six trials (7687 patients) reported the GTI events. The heterogeneity as assessed by I2 was 0% (P = 0.89). The meta-analysis using the random-effects model showed that patients taking SGLT2 inhibitors were 1.73-fold more likely to develop GTIs than patients taking placebo or active controls (RR 1.73; 95% CI 1.02, 2.96) (Fig. 5).
Five trials (1115 patients) using SGLT2 inhibitors for add-on therapy reported fractures. The heterogeneity, as assessed by I2, was 0% (P = 0.83). The meta-analysis showed that the risk of fracture in the experimental group did not increase compared with that of the control group (RR 1.60; 95% CI 0.48, 5.29) (ESM Fig. S8). Three trials [10, 24, 27] (1502 patients) focused on the indicator of hypotension (ESM Fig. S9), of which only one trial [24] reported a case of hypotension in the SGLT2 inhibitor (canagliflozin 100 mg) group. Ten trials (3333 patients) focused on the indicator of mortality (ESM Fig. S10); of these only one trial [16] reported a case of mortality in the placebo group.
The study duration of the RCTs included in this meta-analysis was mostly ≤ 24 weeks and, consequently, any long-term safety evaluation, such as cardiovascular mortality or other outcomes of special interest, could not be performed. Cardiovascular-related adverse events were reported in one article [25], with one event in the experimental group (teneligliptin + canagliflozin, n = 70) and two events in the control group (teneligliptin + placebo, n = 68), but no deaths were reported.
In addition, the total change in HbA1c was − 0.73%. Using this cutoff value, we performed a stratified analysis and compared the characteristics profile (age, duration of T2DM, HbA1c, FPG, body weight) of patients with a change in HbA1c levels of < 0.73% (Group 1) and those of patients with a change in HbA1c levels of > 0.73% (Group 2). The results showed that baseline HbA1c levels were higher in Group 2 patients (8.16% vs. 7.93%; P < 0.05), while the differences in other indicators were not statistically significant (ESM Table S3). At the same time, we used the stratified analysis to compare the characteristics of baseline data between people with no increased risk of GTIs (Group 3) and those with increased risk of GTIs (Group 4). The results of this analysis showed that the population in Group 4 had a higher mean body weight (69.95 vs. 67.69 kg; P < 0.05) and a longer mean duration of T2DM (8.18 vs. 6.48 years; P < 0.05) at the start of the respective study (ESM Table S4).
Discussion
In our systematic review, we evaluated the safety and efficacy of SGLT2 inhibitors (as monotherapy and add-on therapy) in East Asians with diabetes mellitus.
SGLT2 inhibitors are relatively new antihyperglycemic drugs and are not cheap. However, for patients who need to add other oral antihyperglycemic agents to metformin, SGLT2 inhibitors appear to be superior to other second-line drugs (such as DPP-4 inhibitors, sulfonylureas, etc.) [44]. Studies have shown that SGLT2 inhibitors reduce plasma glucose levels and body weight by increasing urine glucose excretion and thereby caloric loss, which is a different mode of action from several other hypoglycemic agents [45, 46]. This mechanism also plays a role in East Asian patients with T2DM. Our meta-analysis shows that compared with the control group, SGLT2 inhibitors improved both glycated hemoglobin (HbA1c) in patients (WMD − 0.73%; 95% CI − 0.84, − 0.61) and the percentage of patients with HbA1c < 7% (RR 2.33; 95% CI 1.74, 3.12); lowered both FPG (WMD − 28.47 mg/dl; 95% CI − 32.86, − 24.08) and PPG (WMD − 52.32 mg/dl; 95% CI − 67.67, − 39.96); reduced body weight (WMD − 1.73 kg; 95% CI − 2.28, − 1.17); and did not increase the risk of hypoglycemia, UTIs, hypotension, fractures, and mortality. However, SGLT2 inhibitors did increased the risk of GTIs (RR 1.73; 95% CI 1.02, 2.96). The meta-analysis based on the overall population showed that SGLT2 inhibitors significantly improved HbA1c levels (WMD − 0.66%; 95% CI − 0.73, − 0.58) compared with placebo [47, 48]. A meta-analysis aimed at evaluating the effects of SGLT2 inhibitors on UTIs and GTIs in subjects with T2DM reported that SGLT2 inhibitors did not increase the risk of UTIs, but did increase risk of GTIs [49]. These results were similar to those of our study.
The antihyperglycemic mechanism of SGLT2 inhibitors underlies the increased risk of polyuria, dehydration, and genitourinary infections due to increased levels of sugar in the urine [50]. Western population-based studies have shown that the risk of hypoglycemia with SGLT2 inhibitors is similar to that with other drugs, but that the risk of genitourinary infections increases [47, 51, 52]. Only the increased risk of urinary and genital infections has been consistently reported in clinical trials and observational studies [53]. Our meta-analysis indicates a slight increase in the risk of adverse reactions to SGLT2 inhibitors in East Asian T2DM patients compared to controls. In particular, the risk of developing GTIs was 1.73-fold higher in patients receiving SGLT2 inhibitors than in the control group. This result is consistent with the conclusion drawn by the authors of the Asian population-based study [54]. A meta-analysis involving a large population (n = 50,880) and no ethnic restrictions [55] found that SGLT-2 inhibitors did not appear to increase the risk of UTIs in patients with T2DM—with the exception of high-dose dapagliflozin (10 mg daily). In addition to race, sample size, drug type, and dose, study duration may also be a factor influencing the results of any analysis. To summarize, our results lead us to suggest that the treating physician should focus on GTIs in East Asian patients who use SGLT2 inhibitors and be alert to UTIs. The cardiovascular and renal safety of SGLT2 inhibitors in East Asian patients with T2DM could not be adequately assessed in our meta-analysis due to the limitation in study duration of the trials included in the meta-analysis. Therefore, further research is needed.
The results of the stratified analysis suggest that for East Asian patients with T2DM who use SGLT2, the level of HbA1c was higher at baseline and the improvement might be more pronounced at the end of the trial, compared with the control group. Our analysis also suggests that patients with higher body weight or a longer history of T2DM have an increased risk of developing GTIs after treatment with SGLT2 inhibitors. The specific mechanisms still need to be researched.
The efficacy data had a heterogeneity of > 90%, whereas the heterogeneity of the safety data was close to 0%. An important for this difference may be that the indicators involved in the effectiveness analysis (such as HbA1c) were reported in all 33 RCTs included in our meta-analysis, but the indicators involved in the safety analysis (such as UTIs) were only reported in 26 studies. The second explanation may be the data types. HbA1c is the continuous variable and GTIs are the dichotomous outcomes. Taking into account the obvious heterogeneity associated with HbA1c, body weight, FPG, PPG, and other indicators, we grouped the studies according to study protocol and performed a subgroup analysis based on drug type to explore the sources of heterogeneity. We divided the included studies into four groups (monotherapy vs. placebo; add-on therapy vs. placebo; monotherapy vs. active control; add-on therapy vs. active control) and established six subgroups (dapagliflozin, canagliflozin, empagliflozin, ipragliflozin, tofogliflozin, and luseogliflozin). The results are shown in ESM Figs. S12–20). Our final conclusion is that the heterogeneity may be due to the type of drug; however, we could not rule out the effect of drug dose. In general, this did not affect our assessment of the overall efficacy of SGLT2 inhibitors.
Our research had several potential limitations. First, considering the clinical heterogeneity of different randomized clinical trials, such as study design methods, drug doses, and medication regimens, we used a random effects model for all of the meta-analyses. Moreover, we excluded meeting abstracts, posters, and articles in which we did not have access to raw data.
Conclusion
This meta-analysis evaluated the efficacy and safety of SGLT2 inhibitors in the treatment of T2DM in East Asian patients. First, SGLT2 inhibitors exhibited beneficial effects in terms of the control of HbA1c, either as monotherapy or as an add-on to oral antihyperglycemic agents, compared to the control group. At the same time, SGLT2 inhibitor therapies achieved both significant reductions in FPG and PPG and a decrease in body weight. Our safety analysis showed a statistically significant increase in the risk of GTIs, while the risk of UTIs, hypoglycemia, hypotension, fractures, and mortality did not appear to increase. The stratified analysis showed that patients with higher HbA1c levels at baseline might achieve a greater improvement in HbA1c after taking SGLT2 inhibitors; those with higher body weight or a longer history of diabetes might have an increased risk of developing GTIs. In conclusion, further long-term studies are needed to fully assess the benefit/risk profiles of SGLT2 inhibitors, which in turn will help to determine the strengths and weaknesses of the use of SGLT2 inhibitors to treat East Asian patients with T2DM.
References
Chan JCN, Bunnag P, Chan SP, et al. Glycaemic responses in Asian and non-Asian people with type 2 diabetes initiating insulin glargine 100 units/mL: a patient-level pooled analysis of 16 randomised controlled trials. Diabetes Res Clin Pract. 2018;135:199–205.
Zelniker TA, Wiviott SD, Raz I, et al. SGLT2 inhibitors for primary and secondary prevention of cardiovascular and renal outcomes in type 2 diabetes: a systematic review and meta-analysis of cardiovascular outcome trials. Lancet. 2019;393(10166):31–9.
Ma RC, Chan JC. Type 2 diabetes in East Asians: similarities and differences with populations in Europe and the United States. Ann NY Acad Sci. 2013;1281:64–91.
Yabe D, Seino Y, Fukushima M, Seino S. Beta cell dysfunction versus insulin resistance in the pathogenesis of type 2 diabetes in East Asians. Curr Diabetes Rep. 2015;15(6):602.
Torrens JI, Skurnick J, Davidow AL, et al. Ethnic differences in insulin sensitivity and beta-cell function in premenopausal or early perimenopausal women without diabetes: the Study of Women’s Health Across the Nation (SWAN). Diabetes Care. 2004;27(2):354–61.
Seino Y, Kuwata H, Yabe D. Incretin-based drugs for type 2 diabetes: focus on East Asian perspectives. J Diabetes Investig. 2016;7[Suppl 1]:102–9.
Moher D, Liberati A, Tetzlaff J, Altman DG. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med. 2009;6(7):e1000097.
American Diabetes Association. Report of the Expert Committee on the Diagnosis and Classification of Diabetes Mellitus. Diabetes Care. 1997;20(7):1183–97.
World Health Organization. Definition, Diagnosis and Classification of Diabetes Mellitus and its Complications: Report of a WHO consultation. Part 1: Diagnosis and classification of diabetes mellitus. Geneva, WHO, 1999.
Inagaki N, Kondo K, Yoshinari T, Maruyama N, Susuta Y, Kuki H. Efficacy and safety of canagliflozin in Japanese patients with type 2 diabetes: a randomized, double-blind, placebo-controlled, 12-week study. Diabetes Obes Metab. 2013;15(12):1136–45.
Inagaki N, Kondo K, Yoshinari T, Takahashi N, Susuta Y, Kuki H. Efficacy and safety of canagliflozin monotherapy in Japanese patients with type 2 diabetes inadequately controlled with diet and exercise: a 24-week, randomized, double-blind, placebo-controlled, Phase III study. Expert Opin Pharmacother. 2014;15(11):1501–15.
Ji L, Ma J, Li H, et al. Dapagliflozin as monotherapy in drug-naive Asian patients with type 2 diabetes mellitus: a randomized, blinded, prospective phase III study. Clin Ther. 2014;36(1):84.
Kaku K, Kiyosue A, Inoue S, et al. Efficacy and safety of dapagliflozin monotherapy in Japanese patients with type 2 diabetes inadequately controlled by diet and exercise. Diabetes Obes Metab. 2014;16(11):1102–10.
Kaku K, Inoue S, Matsuoka O, et al. Efficacy and safety of dapagliflozin as a monotherapy for type 2 diabetes mellitus in Japanese patients with inadequate glycaemic control: a phase II multicentre, randomized, double-blind, placebo-controlled trial. Diabetes Obes Metab. 2013;15(5):432–40.
Kadowaki T, Haneda M, Inagaki N, et al. Empagliflozin monotherapy in Japanese patients with type 2 diabetes mellitus: a randomized, 12-week, double-blind, placebo-controlled, phase II trial. Adv Ther. 2014;31(6):621–38.
Roden M, Weng J, Eilbracht J, et al. Empagliflozin monotherapy with sitagliptin as an active comparator in patients with type 2 diabetes: a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet Diabetes Endocrinol. 2013;1(3):208–19.
Kashiwagi A, Kazuta K, Takinami Y, Yoshida S, Utsuno A, Nagase I. Ipragliflozin improves glycemic control in Japanese patients with type 2 diabetes mellitus: the BRIGHTEN study. BRIGHTEN: double-blind randomized study of ipragliflozin to show its efficacy as monotherapy in T2DM patients. Diabetol Int. 2015;6(1):8–18.
Kashiwagi A, Kazuta K, Yoshida S, Nagase I. Randomized, placebo-controlled, double-blind glycemic control trial of novel sodium-dependent glucose cotransporter 2 inhibitor ipragliflozin in Japanese patients with type 2 diabetes mellitus. J Diabetes Investig. 2014;5(4):382–91.
Seino Y, Sasaki T, Fukatsu A, Sakai S, Samukawa Y. Efficacy and safety of luseogliflozin monotherapy in Japanese patients with type 2 diabetes mellitus: a 12-week, randomized, placebo-controlled, phase II study. Curr Med Res Opin. 2014;30(7):1219–30.
Seino Y, Sasaki T, Fukatsu A, Ubukata M, Sakai S, Samukawa Y. Efficacy and safety of luseogliflozin as monotherapy in Japanese patients with type 2 diabetes mellitus: a randomized, double-blind, placebo-controlled, phase 3 study. Curr Med Res Opin. 2014;30(7):1245–55.
Seino Y, Sasaki T, Fukatsu A, Ubukata M, Sakai S, Samukawa Y. Dose-finding study of luseogliflozin in Japanese patients with type 2 diabetes mellitus: a 12-week, randomized, double-blind, placebo-controlled, phase II study. Curr Med Res Opin. 2014;30(7):1231–44.
Kaku K, Watada H, Iwamoto Y, et al. Efficacy and safety of monotherapy with the novel sodium/glucose cotransporter-2 inhibitor tofogliflozin in Japanese patients with type 2 diabetes mellitus: a combined Phase 2 and 3 randomized, placebo-controlled, double-blind, parallel-group comparative study. Cardiovasc Diabetol. 2014;13:65.
Inagaki N, Harashima S, Maruyama N, Kawaguchi Y, Goda M, Iijima H. Efficacy and safety of canagliflozin in combination with insulin: a double-blind, randomized, placebo-controlled study in Japanese patients with type 2 diabetes mellitus. Cardiovasc Diabetol. 2016;15:89.
Ji L, Han P, Liu Y, et al. Canagliflozin in Asian patients with type 2 diabetes on metformin alone or metformin in combination with sulphonylurea. Diabetes Obes Metab. 2015;17(1):23–31.
Kadowaki T, Inagaki N, Kondo K, et al. Efficacy and safety of canagliflozin as add-on therapy to teneligliptin in Japanese patients with type 2 diabetes mellitus: results of a 24-week, randomized, double-blind, placebo-controlled trial. Diabetes Obes Metab. 2017;19(6):874–82.
Araki E, Onishi Y, Asano M, et al. Efficacy and safety of dapagliflozin in addition to insulin therapy in Japanese patients with type 2 diabetes: results of the interim analysis of 16-week double-blind treatment period. J Diabetes Investig. 2016;7(4):555–64.
Yang W, Han P, Min KW, et al. Efficacy and safety of dapagliflozin in Asian patients with type 2 diabetes after metformin failure: a randomized controlled trial. J Diabetes. 2016;8(6):796–808.
Yang W, Ma J, Li Y, et al. Dapagliflozin as add-on therapy in Asian patients with type 2 diabetes inadequately controlled on insulin with or without oral antihyperglycemic drugs: a randomized controlled trial. J Diabetes. 2018;10(7):589–99.
Kawamori R, Haneda M, Suzaki K, et al. Empagliflozin as add-on to linagliptin in a fixed-dose combination in Japanese patients with type 2 diabetes: glycaemic efficacy and safety profile in a 52-week, randomized, placebo-controlled trial. Diabetes Obes Metab. 2018;20(9):2200–9.
Han KA, Chon S, Chung CH, et al. Efficacy and safety of ipragliflozin as an add-on therapy to sitagliptin and metformin in Korean patients with inadequately controlled type 2 diabetes mellitus: a randomized controlled trial. Diabetes Obes Metab. 2018;20(10):2408–15.
Ishihara H, Yamaguchi S, Nakao I, Okitsu A, Asahina S. Efficacy and safety of ipragliflozin as add-on therapy to insulin in Japanese patients with type 2 diabetes mellitus (IOLITE): a multi-centre, randomized, placebo-controlled, double-blind study. Diabetes Obes Metab. 2016;18(12):1207–16.
Kashiwagi A, Shiga T, Yoshida A, et al. Efficacy and safety of ipragliflozin as an add-on to a sulfonylurea in Japanese patients with inadequately controlled type 2 diabetes: results of the randomized, placebo-controlled, double-blind, phase III EMIT study. Diabetol Int. 2015;6(2):125–38.
Kashiwagi A, Shiga T, Akiyama N, et al. Efficacy and safety of ipragliflozin as an add-on to pioglitazone in Japanese patients with inadequately controlled type 2 diabetes: a randomized, double-blind, placebo-controlled study (the SPOTLIGHT study). Diabetol Int. 2015;6(2):104–16.
Kashiwagi A, Kazuta K, Goto K, Yoshida S, Ueyama E, Utsuno A. Ipragliflozin in combination with metformin for the treatment of Japanese patients with type 2 diabetes: iLLUMINATE, a randomized, double-blind, placebo-controlled study. Diabetes Obes Metab. 2015;17(3):304–8.
Kashiwagi A, Takahashi H, Ishikawa H, et al. A randomized, double-blind, placebo-controlled study on long-term efficacy and safety of ipragliflozin treatment in patients with type 2 diabetes mellitus and renal impairment: results of the long-term ASP1941 safety evaluation in patients with type 2 diabetes with renal impairment (LANTERN) study. Diabetes Obes Metab. 2015;17(2):152–60.
Chuang LM, Min KW, Lu CH, Kokubo S, Yoshida S, Cha BS. Efficacy, safety, and tolerability of ipragliflozin in Asian type 2 diabetic patients with inadequate glycemic control with metformin Results of a phase 3 randomized, placebo-controlled, double-blind, multicenter trial. Diabetes Res Clin Pract. 2014;106:S4.
Seino Y, Inagaki N, Haneda M, et al. Efficacy and safety of luseogliflozin added to various oral antidiabetic drugs in Japanese patients with type 2 diabetes mellitus. J Diabetes Investig. 2015;6(4):443–53.
Terauchi Y, Tamura M, Senda M, Gunji R, Kaku K. Efficacy and safety of tofogliflozin in Japanese patients with type 2 diabetes mellitus with inadequate glycaemic control on insulin therapy (J-STEP/INS): results of a 16-week randomized, double-blind, placebo-controlled multicentre trial. Diabetes Obes Metab. 2017;19(10):1397–407.
Hayashi T, Fukui T, Nakanishi N, et al. Dapagliflozin decreases small dense low-density lipoprotein-cholesterol and increases high-density lipoprotein 2-cholesterol in patients with type 2 diabetes: comparison with sitagliptin. Cardiovasc Diabetol. 2017;16(1):8.
Shigiyama F, Kumashiro N, Miyagi M, et al. Effectiveness of dapagliflozin on vascular endothelial function and glycemic control in patients with early-stage type 2 diabetes mellitus: DEFENCE study. Cardiovasc Diabetol. 2017;16(1):84.
Jeon HJ, Ku EJ, Oh TK. Dapagliflozin improves blood glucose in diabetes on triple oral hypoglycemic agents having inadequate glucose control. Diabetes Res Clin Pract. 2018;142:188–94.
Nomoto H, Miyoshi H, Sugawara H, et al. A randomized controlled trial comparing the effects of dapagliflozin and DPP-4 inhibitors on glucose variability and metabolic parameters in patients with type 2 diabetes mellitus on insulin. Diabetol Metab Syndr. 2017;9:54.
Seaquist ER, Anderson J, Childs B, et al. Hypoglycemia and diabetes: a report of a workgroup of the American Diabetes Association and the Endocrine Society. J Clin Endocrinol Metab. 2013;98(5):1845–59.
Reusch JE, Manson JE. Management of type 2 diabetes in 2017: getting to goal. JAMA. 2017;317(10):1015–6.
Vallon V. The mechanisms and therapeutic potential of SGLT2 inhibitors in diabetes mellitus. Annu Rev Med. 2015;66:255–70.
Morgan CL, Jenkins-Jones S, Evans M, Barnett AH, Poole CD, Currie CJ. Weight change in people with type 2 diabetes: secular trends and the impact of alternative antihyperglycaemic drugs. Diabetes Obes Metab. 2012;14(5):424–32.
Vasilakou D, Karagiannis T, Athanasiadou E, et al. Sodium-glucose cotransporter 2 inhibitors for type 2 diabetes: a systematic review and meta-analysis. Ann Intern Med. 2013;159(4):262–74.
Shyangdan DS, Uthman OA, Waugh N. SGLT-2 receptor inhibitors for treating patients with type 2 diabetes mellitus: a systematic review and network meta-analysis. BMJ Open. 2016;6(2):e009417.
Liu J, Li L, Li S, et al. Effects of SGLT2 inhibitors on UTIs and genital infections in type 2 diabetes mellitus: a systematic review and meta-analysis. Sci Rep. 2017;7(1):2824. https://doi.org/10.1038/s41598-017-02733-w.
Hattersley AT, Thorens B. Type 2 diabetes, SGLT2 inhibitors, and glucose secretion. New Engl J Med. 2015;373(10):974–6.
Monami M, Nardini C, Mannucci E. Efficacy and safety of sodium glucose co-transport-2 inhibitors in type 2 diabetes: a meta-analysis of randomized clinical trials. Diabetes Obes Metab. 2014;16(5):457–66.
Liu XY, Zhang N, Chen R, Zhao JG, Yu P. Efficacy and safety of sodium-glucose cotransporter 2 inhibitors in type 2 diabetes: a meta-analysis of randomized controlled trials for 1 to 2years. J Diabetes Complicat. 2015;29(8):1295–303.
Scheen AJ. An update on the safety of SGLT2 inhibitors. Expert Opin Drug Saf. 2019;18(4):295–311.
Cai X, Gao X, Yang W, et al. No disparity of the efficacy and all-cause mortality between Asian and non-Asian type 2 diabetes patients with sodium-glucose cotransporter 2 inhibitors treatment: a meta-analysis. J Diabetes Investig. 2018;9(4):850–61.
Puckrin R, Saltiel MP, Reynier P, Azoulay L, Yu OHY, Filion KB. SGLT-2 inhibitors and the risk of infections: a systematic review and meta-analysis of randomized controlled trials. Acta Diabetol. 2018;55(5):503–14.
Acknowledgements
Funding
This study was supported by the National Natural Science Foundation of China (Grant No. 81673803) and Chengdu University of Traditional Chinese Medicine Xinglin scholars program (No. QNXZ2018033). The journal’s Rapid Service Fee was funded by the authors.
Authorship
All named authors meet the International Committee of Medical Journal Editors (ICMJE) criteria for authorship for this article, take responsibility for the integrity of the work as a whole, and have given their approval for this version to be published.
Disclosures
Lidan Yang, Lin Zhang, He He, Zhenmei An and Mei Zhang have nothing to disclose.
Compliance with Ethics Guidelines
This article is based on previously conducted studies and does not contain any studies with human participants or animals performed by any of the authors.
Data Availability
The datasets during and/or analyzed during the current study are available from the corresponding author on reasonable request.
Open Access
This article is distributed under the terms of the Creative Commons Attribution-NonCommercial 4.0 International License (http://creativecommons.org/licenses/by-nc/4.0/), which permits any noncommercial use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
Author information
Authors and Affiliations
Corresponding author
Additional information
Enhanced Digital Features
To view enhanced digital features for this article go to https://doi.org/10.6084/m9.figshare.8792030.
Electronic Supplementary Material
Below is the link to the electronic supplementary material.
Rights and permissions
This article is published under an open access license. Please check the 'Copyright Information' section either on this page or in the PDF for details of this license and what re-use is permitted. If your intended use exceeds what is permitted by the license or if you are unable to locate the licence and re-use information, please contact the Rights and Permissions team.
About this article
Cite this article
Yang, L., Zhang, L., He, H. et al. Efficacy and Safety of Sodium-Glucose Cotransporter 2 Inhibitors in East Asians with Type 2 Diabetes: A Systematic Review and Meta-Analysis. Diabetes Ther 10, 1921–1934 (2019). https://doi.org/10.1007/s13300-019-0674-7
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13300-019-0674-7