Abstract
Aims
The aim was to systematically review the efficacy and safety of sodium–glucose cotransporter inhibitor (SGLT2i) as an adjunct to insulin at different follow-up durations in randomized, double-blind clinical trials in patients with type 1 diabetes.
Methods
We conducted a search on Medline, Embase, and the Cochrane Library for relevant studies published before May 2020. According to the duration of follow-up, the subgroup analysis included four periods: 1–4, 12–18, 24–26, and 52 weeks. In the five trials included both 24–26 and 52 weeks of follow-up, we compared the efficacy by the placebo-subtracted difference and changes in SGLT2i groups.
Results
Fifteen trials including 7109 participants were analyzed. The combination of SGLT2i and insulin improved hemoglobin A1c (HbA1c), fasting plasma glucose (FPG), daily insulin dose, body weight, and blood pressure, which varied greatly by different follow-ups. Compared with %HbA1c at 24–26 weeks, placebo-subtracted differences and changes in the SGLT2i groups slightly increased. SGLT2i plus insulin treatment showed no difference in the occurrence of urinary tract infections (UTIs), hypoglycemia, or severe hypoglycemia but increased the risk of genital tract infections (GTIs) in a duration-dependent manner. SGLT2i treatment was associated with a significantly higher rate of ketone-related SAEs and diabetic ketoacidosis (DKA) at 52 weeks.
Conclusion
SGLT2i as an add-on therapy to insulin improved glycemic control and body weight and decreased the required dose of insulin without increasing the risk of hypoglycemia. However, after 6 months the benefits of SGLT2is on glycemic control may weaken and the risks of GTIs and DKA increased.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Diabetes mellitus (DM) is a systemic disease associated with an increased risk of adverse vascular events. DCCT-EDIC and UKPDS have shown that improved glucose control through an increase in insulin therapy is associated with reductions in the long-term risks of both microvascular and macrovascular events [1, 2]. However, the achievement and maintenance of glycemic targets have proven both difficult and hazardous, especially in type 1 diabetes (T1DM) [3, 4].
Over several decades, the T1DM prevalence has increased, seriously affecting the whole world [5, 6]. Although advances in medical and management technology for T1DM have been made, mortality remains almost unchanged among adults aged 20–44 years [7]. The mainstay of treatment for T1DM is still insulin therapy, with a varying degree of side effects including hypoglycemia and weight gain. Consequently, there is an unmet need for adjunctive treatment plus insulin in T1DM to meet the twin challenges of hyperglycemia and hypoglycemia [8, 9]. Many different oral antidiabetic drugs (OADs) combined with insulin have been approved for type 2 diabetes (T2DM), including metformin, incretin analogs, and sodium–glucose cotransporter (SGLT) 2 inhibitors (SGLT-2is) [10,11,12]. These drugs can improve insulin resistance and blood glucose levels, reduce the incidence of hypoglycemia, and manage body weight. Unfortunately, unlike for patients with T2DM, the options for those with T1DM are limited [8, 9, 13].
SGLT-2is are a novel class of antidiabetic agents, such as canagliflozin, dapagliflozin, empagliflozin, ipragliflozin, and tofogliflozin. SGLT-2is reduce glucose reabsorption at the proximal nephron, leading to increased glucose excretion through a mechanism that is independent of insulin [14,15,16]. Recently, SGLT-2is have become an attractive therapeutic proposition for diabetes patients due to their additional beneficial biological effects other than glycemic control, including decreased blood pressure, body weight loss, and reduced cardiovascular mortality in patients with T2DM [14, 15, 17, 18]. The recent publication of several large randomized controlled trials (RCTs) reported the benefits of SGLT2 inhibitors on the decrease in hemoglobin A1c (HbA1c), fasting plasma glucose (FPG) levels in T1DM with insulin therapy [19,20,21,22,23,24,25,26,27,28,29,30,31,32,33,34]. Nevertheless, questions remain regarding the long-term efficacy and safety of SGLT2 inhibitors as add-ons to insulin in the treatment of T1DM. There is no study to systematically review the efficacy and safety of the combination of SGLT2 inhibitors and insulin compared with insulin monotherapy in the different treatment periods. Therefore, the aim of the present study was to evaluate the relative effectiveness and safety of this important therapeutic course by a meta-analysis of randomized controlled trials in T1DM with insulin therapy.
Methods
Materials and methods
This study is a systematic review and meta-analysis assessing the duration of effects of SGLT2is for adjunctive treatment of T1DM. The extensive searches were carried out in PubMed, EMBASE, and the Cochrane Central Register of Controlled Trials (CENTRAL) through May 2020, using both Medical Subject Heading (MeSH) and free text terms. We also searched ClinicalTrials.gov to identify additional relevant trials. This meta-analysis was performed by following Cochrane Collaboration guidelines and is reported in accordance with the preferred reporting items for systematic reviews and meta-analyses (PRISMA) statement.
Primary and secondary endpoints
Studies included in the meta-analyses are randomized controlled trials (RCTs) that evaluated the efficacy, safety, and tolerability of SGLT2is as add-ons to insulin compared with placebo combination. Participants were T1DM patients having inadequate control of disease by insulin therapy (multiple daily insulin injections or insulin pump). Randomized trials that fulfilled the following criteria were eligible: (1) comparison of SGLT2-i therapy with placebo in adult patients (≥ 18 years old) with type 1 diabetes, and (2) reporting efficacy and safety outcomes of interest. Studies were excluded if other aspects of treatment were targeted, if the design was not double-blind (e.g., open-label or crossover). Studies of children and observational studies were also ineligible.
Primary outcome measures of interest were the changes from baseline in percent HbA1c, FPG levels, and body weight. Secondary endpoints were the changes from baseline in systolic blood pressure (SBP), diastolic blood pressure (DBP), and daily insulin doses (basal, bolus, and total insulin doses). Safety endpoints were the incidence of hypoglycemia (including severe hypoglycemia), genital tract infections, urinary tract infections, and ketoacidosis. The definitions of severe hypoglycemia and diabetic ketoacidosis (DKA) were based on those of a previous meta-analysis [35].
Data extraction, synthesis, and statistical analysis
Data extraction was carried out by two reviewers (Lunwen Rao and Chenhong Ren) independently by adapting a standardized procedure. Data pertaining to the participants’ demographic and pathological characteristics, intervention design, trial eligibility criteria, outcome measures, and outcomes were extracted from the selected research articles. According to the duration of follow-up, the subgroup analysis from the consolidation of ranges included four periods: 1–4, 12–18, 24–26, and 52 weeks. Changes from baseline in the endpoints were either extracted directly from the respective research articles if provided or calculated from the baseline values and experimental values noted. Quality assessment of the RCTs included in this meta-analysis was carried out by using the Cochrane risk of bias tool [36]. For continuous outcomes, mean differences and 95% confidence intervals were calculated by an inverse variance random-effects model. For dichotomous outcomes, risk ratios and 95% confidence intervals were calculated by the random-effects Mantel–Haenszel approach [37]. Data and analysis module of RevMan (version 5.2; Cochrane Collaboration) was used for the meta-analyses. Between-studies (heterogeneity) was tested by I2 statistics, and a p value of less than 0.05 was considered statistically significant.
Results
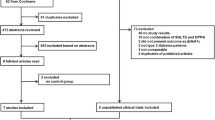
A total of 324 articles were retrieved initially utilizing the search strategy (Fig. 1). After the removal of 77 duplicate articles, 247 articles remained for title and abstract screening. A total of 222 articles were ruled out on the basis of titles and abstracts. Nine articles were included for full-text screening. There were two studies in which the risk of bias could not be judged due to inadequate information [38, 39]. Fifteen randomized placebo-controlled trials (n = 7,109 patients) satisfied the inclusion criteria. We included five trials of sotagliflozin, four trials of empagliflozin, three trials of dapagliflozin, two trials of ipragliflozin, and one trial of canagliflozin. The baseline characteristics and results were obtained in trials in Tables S1 and S2 of Supplementary Materials. The mean hemoglobin A1C (HbA1c), total insulin dose, and body mass index (BMI) were 8.0–8.5%, 0.6–0.7 units/kg/day, and 23–29 kg/m2, respectively. These factors were balanced between groups.
The duration of follow-up varied widely, including four trials at 1–4 weeks, two trials at 12–16 weeks, eight trials at 24–26 weeks, and five trials at 52 weeks. And the five trials included both 24–26 and 52 weeks of follow-up [23, 25, 29, 31, 32, 34]. Definitions of hypoglycemia were similar in all trials and followed the American Diabetes Association criteria. Not all studies reported all the outcomes. In general, the majority of the domains for the seventeen studies were considered to have a high quality and a low risk of bias. Risks of bias assessments are included in Figures S1 and S2.
Efficacy of SGLT-2i intervention as add-ons
Pooled analysis
Not all studies reported all the outcomes (Table 1). There was no significant heterogeneity between studies in terms of HbA1c, fasting plasma glucose (FPG), daily insulin dose (total, basal, and bolus), and seated blood pressure (systolic and diastolic) (P > 0.05, respectively). Large heterogeneity was noted between studies only in terms of body weight (P < 0.001, I2 = 82%). However, the combination of SGLT2is and insulin treatment markedly reduced HbA1c, fasting plasma glucose (FPG), body weight, daily insulin dose (total, basal, and bolus), and seated blood pressure (systolic and diastolic) (P < 0.001).
Subgroup analysis by SGLT2 inhibitors
We also observed the efficacy of different SGLT2is, including canagliflozin, dapagliflozin, empagliflozin, ipragliflozin, and sotagliflozin (Table 1). Insulin treatment plus dapagliflozin, empagliflozin, or sotagliflozin markedly decreased %HbA1c, FPG, body weight, daily insulin dose (total, basal, and bolus), and seated systolic blood pressure. Canagliflozin which is a drug significantly lowered %HbA1c, body weight, and daily basal insulin dose, but demonstrated no significant effect on body weight or daily insulin dose (total, bolus). Only two RCTs on ipragliflozin were conducted in Japan, with a small sample size, and ipragliflozin was shown to abate %HbA1c and daily insulin dose (total, basal, and bolus). Interestingly, only sotagliflozin significantly decreased SBP (by − 1.52 mmHg [ − 2.24, − 0.80], P < 0.001) and empagliflozin slightly changed SBP (by − 1.14 [ − 2.36, 0.07], P = 0.06).
Subgroup analysis by the duration of follow-up
According to the duration of follow-up, the subgroup analysis from the consolidation of ranges included four periods: 1–4, 12–18, 24–26, and 52 weeks (Table 2). Two studies had follow-ups of only 12–18 weeks. We found that different follow-up periods in different outcome measures had a great impact on the results. The combination of SGLT2 inhibitors and insulin treatment reduced %HbA1c and body weight at all follow-up durations (P < 0.05). An SGLT2 inhibitor plus insulin treatment reduced FPG and total, basal, and bolus insulin doses at 1–4, 24–26, and 52 weeks (P < 0.05) not at 12–16 weeks. Both SBP and DBP markedly decreased at 24–26 weeks (P < 0.001). At 52 weeks, the combination treatment significantly decreased seated systolic blood pressure ( − 3.29[ − 4.37, − 2.21], P < 0.001) but only slightly decreased diastolic blood pressure ( − 1.73[ − 2.14, − 1.32], P = 0.06).
Subgroup analysis of placebo-subtracted differences between 24–26 and 52 weeks
To further explore the long-term effects of SGLT2is on T1DM, we compared the placebo-subtracted difference between 24–26 and 52 weeks among the five trials [23, 25, 29, 31, 32, 34]. In the five trials, two trials were on sotagliflozin, two trials on dapagliflozin, and one trial on empagliflozin. The placebo-subtracted difference in body weight at 52 weeks was further reduced by 0.60 kg ([0.37, 0.82]; P < 0.001) and daily basal insulin dose (0.72 [0.27, 1.17]; P = 0.002), while the placebo-subtracted difference in %HbA1c increased by ( − 0.11 [ − 0.14, − 0.07]; P < 0.001) (Fig. 2).
Subgroup analysis of changes in SGLT2i or placebo groups between 24–26 and 52 weeks
Similarly, in the SGLT2i groups and relative to the baseline level, changes in %HbA1c at 24–26 weeks were larger than those at 52 weeks ( − 0.18 [ − 0.22, − 0.13]; P < 0.001) (Fig. S3). Compared with 24–26 weeks, body weight at 52 weeks was also slightly reduced by 0.26 kg ([0.01, 0.50]; P = 0.04)). In addition, we also compared the changes in insulin monotherapy groups between 24–26 and 52 weeks (Fig. S4). Surprisingly, we found that body weight and seated systemic blood pressure at 52 weeks increased 0.35 kg ([ − 0.69, − 0.01]; P = 0.04) and − 1.83 mmHg [ − 3.14, − 0.51]; P = 0.007), respectively, and %HbA1c also slightly increased − 0.07 ([ − 0.14, − 0.00]; P = 0.05). These results demonstrated that there was weight gain, increased blood pressure, and poor glycemic control in studies of long-term insulin monotherapy in T1DM.
Safety of SGLT-2 inhibitor intervention
A summary of the overall safety and selected AEs is shown in Table 3. Regarding the comparisons of the plus treatment between the SGLT2i groups and placebo groups, the differences in the incidence of AEs were significant, with an RR of 1.20 ([1.05, 1.38], P = 0.008) at 24–26 weeks and 1.43 ([1.21, 1.69], P < 0.001) at 52 weeks relative to the placebo group. The rates of serious AEs were also higher for the combination treatment group, with an RR of 6.37 ([1.24, 32.62], P = 0.03) at 12–18 weeks, 1.54 ([1.14, 2.08], P = 0.005) at 24–26 weeks, and 1.40 ([1.12, 1.77], P = 0.004) at 52 weeks relative to the placebo.
Several specific AEs occurred more frequently than others, such as ketone-related AEs, DKA, hypoglycemia, urinary tract infections, and genital mycotic infection. We found no significant difference between the combination group and the monotherapy group for hypoglycemia, severe hypoglycemia, or UTIs (P > 0.05). However, compared with placebo, SGLT2i treatment was associated with a significantly higher rate of GTIs at 24–26 weeks (4.14[2.72, 6.29], P < 0.001) and 52 weeks (4.37 [3.15, 6.06], P < 0.001)] in patients with T1DM receiving insulin therapy (Table 3). Interestingly, at 52 weeks, we noted significantly increased risks of ketone-related SAEs and DKA (0.47[0.62, 1.21], 3.94[1.81, 8.58], respectively) in the SGLT2i plus insulin groups compared with those in the insulin monotherapy group, but we noted no effects at other follow-up time points.
Discussion
Main findings
In our study, initial combination therapy with a SGLT2i and insulin was more efficacious in terms of glycemic control, body weight, and seated blood pressure control than treatment with insulin alone in T1DM. However, the subgroup analysis by the length of follow-up also showed that SGLT2is as add-on therapy to insulin did provide insulin-independent glucose lowering after 6 months, but the effects might weaken. With the extension of the follow-up, especially at 52 weeks, the frequency of genital infections and DKA significantly increased in T1DM patients treated with SGLT2is as an adjunct to insulin. The above findings warrant careful consideration of long-term benefits and potential undesirable effects of these SGLT2is as add-on treatment to insulin.
In our study, subgroup analysis showed that short-term (1–4 weeks) to long-term (52 weeks) SGLT2is plus insulin resulted in a larger reduction in HbA1c and FPG levels compared with placebo in T1DM patients. In a meta-analysis, SGLT2is ameliorated glycemic efficacy outcomes accompanied by a lower insulin dose requirement without increasing the risk of hypoglycemia [40]. Importantly, SGLT2is improving glycemic control by increasing time in range on average while reducing glycemic variability [41, 42]. SGLT2is are particularly attractive for add-on therapy to insulin in T1DM because they are oral agents that decrease the reabsorption of glucose in the kidney and increase its excretion via the urine, a mechanism that is not dependent of islet cell functionality [14,15,16, 43, 44]. In addition, sotagliflozin, a dual SGLT1 and SGLT2 inhibitor, can delay and blunt intestinal glucose absorption after meals, resulting in lower PPG and insulin levels [45].
Consistent with previous studies, our study confirmed that weight gain may be the side effect of long-term insulin monotherapy in T1DM, which is the main reason that intensive insulin therapy fails to improve the microvascular and macrovascular complications of diabetes [1, 2, 46,47,48]. So noninsulin pharmacological therapies as an add-on treatment to insulin have received a surge of interest in T1DM patients. We found that SGLT2is not only offset the weight gain induced by insulin treatment but also reduced the blood pressure. Osmotic diuresis and natriuresis are the reasons for weight loss, which is also the reason for the improvement in blood pressure [17, 49]. The use of a SGLT2i as an add-on therapy to insulin may be a preferred option for patients with T1DM.
In addition to insulin side effects, another disadvantage of insulin replacement treatment for T1DM patients is the lack of a longer-term glycemic benefit [50]. To explore the longer-term glycemic effects, we first compared them with four trials to simultaneously investigate the efficacy at 24–26 and 52 weeks in the same population [23, 25, 29, 31, 32, 34]. Notably, the placebo-subtracted difference and changes in %HbA1c in the SGLT2i group at 24–26 weeks were larger than those at 52 weeks (Fig. 2). Furthermore, we also found that decreases from 24–26 to 52 weeks were dose-independent for the different SGLT2is (including dapagliflozin, sotagliflozin, and empagliflozin) [23, 25, 29, 31, 32, 34]. This phenomenon shows that the addition of an SGLT2i to insulin did provide insulin-independent glucose lowering after 6 months, but the effects weaken, which seems to be a contradiction because an SGLT2i as an add-on to metformin treatment gradually reduced %HbA1c from 24 to 104 weeks in T2DM patients [51, 52]. In this regard, the 52-week study period may have been too brief to show longer beneficial effects of SGLT2is on glycemic control. A possible mechanism for the contradiction is lower renal threshold for glucose reabsorption in T1DM patients [53, 54]. The renal threshold for glucose reabsorption in T1DM patients with T1DM was near the normal range and significantly lower than that in T2DM patients [53].
In our meta-analysis, genital tract infections (GTIs) occurred more often in SGLT2i plus insulin therapy than in insulin monotherapy, and there was no difference in the occurrence of urinary tract infections (UTIs). Subgroup analysis on treatment duration showed that the effects of SGLT inhibition plus insulin on safety outcomes were duration-dependent, although there were slight effects at 1–4 and 12–18 weeks. This is consistent with the previous literature that SGLT2is increase the risk of GTIs [55, 56], but we first reported that a longer duration might confer a higher risk of GTI events in T1DM.
Recently, the FDA warned that SGLT2is can produce too many ketoacids in some diabetes patients [57]. Our meta-analysis also demonstrated that the use of SGLT2i as an add-on therapy was associated with long-term risks in the incidence of ketone-related SAEs and DKA in T1DM patients receiving insulin therapy.
Therefore, to sufficiently comprehend the treatment benefits and risks of SGLT2is over a long period of follow-up, future RCTs should be more effective.
Comparison with other studies
Five previous meta-analyses reported that an SGLT2i as an add-on therapy to insulin is effective in improving glycemic and blood pressure control and decreasing body weight and total daily insulin dose in patients with T1DM [35, 40, 58,59,60]. Four of these meta-analyses researched studies evaluating the use of SGLT2is in patients with T1DM before 2018 [40, 58,59,60]. In addition, two meta-analyses confirmed that dual SGLT 1/2 inhibitor sotagliflozin adjuvant therapy improves glycemic and nonglycemic outcomes and reduces the rate of hypoglycemia and severe hypoglycemia [37, 61].
In contrast, two early meta-analyses reported that, compared with a control treatment, SGLT2 inhibitors did not increase the risk of adverse events [58, 59]. A third study reported that only the risk of DKA should be carefully monitored in SGLT2 inhibitors as adjunctive therapy [50, 60]. The latest four studies confirmed that add-on SGLT-2i therapy increased diabetic ketoacidosis and genital tract infections [35, 37, 40, 61].
In our study, we identified eligible RCTs from inception through May 2020, including 15 trials of 7,109 patients. Our results regarding the efficacy and safety of SGLT2i as an add-on therapy were generally consistent with previous findings. However, we assessed the safety and efficacy of SGLT2is (including canagliflozin, dapagliflozin, empagliflozin, sotagliflozin, and ipragliflozin) at different follow-up periods through subgroup analysis. In addition to the data on efficacy in long-term treatment, we also analyzed events suggestive of effective outcomes in the same four RCTs regardless of biases.
Limitations
Our study also has some limitations. Although the majority of trials used the same classification system for efficacy and safety, some other trials may have overreported adverse events using symptoms alone. Second, a wide variation in the duration of follow-up of the included studies was noted, from 1 to 52 weeks. Third, for the outcomes of efficacy and safety, trials of dapagliflozin, canagliflozin, empagliflozin, sotagliflozin, and ipragliflozin accounted for the majority of the evidence. Long-term treatment merely focused on empagliflozin, sotagliflozin, and dapagliflozin, and the follow-up of only four studies reached 52 weeks. RCTs on canagliflozin or ipragliflozin were few and had a small sample sizes [50].
Conclusion
In summary, SGLT2is as adjunctive therapy improved glycemic control and body weight and decreased the required dose of insulin without increasing the risk of hypoglycemia. However, the subgroup analysis by the length of follow-up also showed that SGLT2is as add-on therapy to insulin did provide insulin-independent glucose lowering after 6 months, but the effects might weaken.
References
Van Dieren S, Peelen LM, Nothlings U, van der Schouw YT, Rutten GE, Spijkerman AM, Van der AD, Sluik D, Boeing H, Moons KG, Beulens JW (2011) External validation of the UK Prospective Diabetes Study (UKPDS) risk engine in patients with type 2 diabetes. Diabetologia 54(2):264–270. https://doi.org/10.1007/s00125-010-1960-0
Subramanian S, Hirsch IB (2018) Intensive Diabetes Treatment and Cardiovascular Outcomes in Type 1 Diabetes Mellitus: Implications of the Diabetes Control and Complications Trial/Epidemiology of Diabetes Interventions and Complications Study 30-Year Follow-up. Endocrinol Metab Clin North Am 47(1):65–79. https://doi.org/10.1016/j.ecl.2017.10.012
Miller KM, Foster NC, Beck RW, Bergenstal RM, DuBose SN, DiMeglio LA, Maahs DM, Tamborlane WV, Network TDEC (2015) Current state of type 1 diabetes treatment in the U.S.: updated data from the T1D Exchange clinic registry. Diabet Care 38(6):971–978. https://doi.org/10.2337/dc15-0078
McKnight JA, Wild SH, Lamb MJ, Cooper MN, Jones TW, Davis EA, Hofer S, Fritsch M, Schober E, Svensson J, Almdal T, Young R, Warner JT, Delemer B, Souchon PF, Holl RW, Karges W, Kieninger DM, Tigas S, Bargiota A, Sampanis C, Cherubini V, Gesuita R, Strele I, Pildava S, Coppell KJ, Magee G, Cooper JG, Dinneen SF, Eeg-Olofsson K, Svensson AM, Gudbjornsdottir S, Veeze H, Aanstoot HJ, Khalangot M, Tamborlane WV, Miller KM (2015) Glycaemic control of Type 1 diabetes in clinical practice early in the 21st century: an international comparison. Diabet Med 32(8):1036–1050. https://doi.org/10.1111/dme.12676
Mayer-Davis EJ, Lawrence JM, Dabelea D, Divers J, Isom S, Dolan L, Imperatore G, Linder B, Marcovina S, Pettitt DJ, Pihoker C, Saydah S, Wagenknecht L (2017) Incidence Trends of Type 1 and Type 2 Diabetes among Youths, 2002–2012. N Engl J Med 376(15):1419–1429. https://doi.org/10.1056/NEJMoa1610187
Patterson CC, Harjutsalo V, Rosenbauer J, Neu A, Cinek O, Skrivarhaug T, Rami-Merhar B, Soltesz G, Svensson J, Parslow RC, Castell C, Schoenle EJ, Bingley PJ, Dahlquist G, Jarosz-Chobot PK, Marciulionyte D, Roche EF, Rothe U, Bratina N, Ionescu-Tirgoviste C, Weets I, Kocova M, Cherubini V, Rojnic Putarek N, deBeaufort CE, Samardzic M, Green A (2019) Trends and cyclical variation in the incidence of childhood type 1 diabetes in 26 European centres in the 25 year period 1989–2013: a multicentre prospective registration study. Diabetologia 62(3):408–417. https://doi.org/10.1007/s00125-018-4763-3
Gregg EW, Cheng YJ, Srinivasan M, Lin J, Geiss LS, Albright AL, Imperatore G (2018) Trends in cause-specific mortality among adults with and without diagnosed diabetes in the USA: an epidemiological analysis of linked national survey and vital statistics data. Lancet 391(10138):2430–2440. https://doi.org/10.1016/S0140-6736(18)30314-3
Pettus JH, Kushner JA, Valentine V, Wood R, Pang C, Paranjape S, Berria R, Deluzio A, Edelman SE (2019) Adjunct Therapy in Type 1 Diabetes: A Survey to Uncover Unmet Needs and Patient Preferences Beyond HbA1c Measures. Diabet technol ther 21(6):336–343. https://doi.org/10.1089/dia.2019.0027
Cefalu WT, Tamborlane WV, Skyler JS (2015) Type 1 diabetes at a crossroads! Diabetes Care 38(6):968–970. https://doi.org/10.2337/dc15-0615
Aschner P (2019) Insulin Therapy in Type 2 Diabetes. Am J Ther. https://doi.org/10.1097/MJT.0000000000001088
Patel JJ, Mundi MS (2019) Metformin for Type 2 Diabetes. JAMA 322(13):1312–1313. https://doi.org/10.1001/jama.2019.11497
Yoon JH, Min SH, Ahn CH, Cho YM, Hahn S (2018) Comparison of non-insulin antidiabetic agents as an add-on drug to insulin therapy in type 2 diabetes: a network meta-analysis. Sci rep 8(1):4095. https://doi.org/10.1038/s41598-018-22443-1
Wright LA, Hirsch IB (2019) Non-insulin treatments for Type 1 diabetes: critical appraisal of the available evidence and insight into future directions. Diabet Med 36(6):665–678. https://doi.org/10.1111/dme.13941
Mahaffey KW, Neal B, Perkovic V, de Zeeuw D, Fulcher G, Erondu N, Shaw W, Fabbrini E, Sun T, Li Q, Desai M, Matthews DR (2018) Canagliflozin for Primary and Secondary Prevention of Cardiovascular Events: Results From the CANVAS Program (Canagliflozin Cardiovascular Assessment Study). Circulation 137(4):323–334. https://doi.org/10.1161/CIRCULATIONAHA.117.032038
Zinman B, Wanner C, Lachin JM, Fitchett D, Bluhmki E, Hantel S, Mattheus M, Devins T, Johansen OE, Woerle HJ, Broedl UC, Inzucchi SE, Investigators E-RO (2015) Empagliflozin, Cardiovascular Outcomes, and Mortality in Type 2 Diabetes. N Engl J Med 373(22):2117–2128. https://doi.org/10.1056/NEJMoa1504720
Wright EM, Loo DD, Hirayama BA (2011) Biology of human sodium glucose transporters. Physiol Rev 91(2):733–794. https://doi.org/10.1152/physrev.00055.2009
Lee PC, Ganguly S, Goh SY (2018) Weight loss associated with sodium-glucose cotransporter-2 inhibition: a review of evidence and underlying mechanisms. Obesity reviews : an official journal of the International Association for the Study of Obesity 19(12):1630–1641. https://doi.org/10.1111/obr.12755
Monami M, Dicembrini I, Mannucci E (2017) Effects of SGLT-2 inhibitors on mortality and cardiovascular events: a comprehensive meta-analysis of randomized controlled trials. Acta Diabetol 54(1):19–36. https://doi.org/10.1007/s00592-016-0892-7
Kaku K, Isaka H, Toyoshima J, Sakatani T (2019) Clinical pharmacology study of ipragliflozin in Japanese patients with type 1 diabetes mellitus: A phase 2, randomized, placebo-controlled trial. Diabet Obes Metab. https://doi.org/10.1111/dom.13679
Baker C, Wason S, Banks P, Sawhney S, Chang A, Danne T, Gesty-Palmer D, Kushner JA, McGuire DK, Mikell F, O’Neill M, Peters AL, Strumph P (2019) Dose-dependent glycometabolic effects of sotagliflozin on type 1 diabetes over 12 weeks: The inTandem4 trial. Diabet Obes Metab. https://doi.org/10.1111/dom.13825
Garg SK, Henry RR, Banks P, Buse JB, Davies MJ, Fulcher GR, Pozzilli P, Gesty-Palmer D, Lapuerta P, Simó R et al (2017) Effects of Sotagliflozin Added to Insulin in Patients with Type 1 Diabetes. New Engl J Med 377(24):2337–2348. https://doi.org/10.1056/NEJMoa1708337
Henry RR, Thakkar P, Tong C, Polidori D, Alba M (2015) Efficacy and Safety of Canagliflozin, a Sodium-Glucose Cotransporter 2 Inhibitor, as Add-on to Insulin in Patients With Type 1 Diabetes. Diabetes Care 38(12):2258–2265. https://doi.org/10.2337/dc15-1730
Dandona P, Mathieu C, Phillip M, Hansen L, Griffen SC, Tschöpe D, Thorén F, Xu J, Langkilde AM (2017) Efficacy and safety of dapagliflozin in patients with inadequately controlled type 1 diabetes (DEPICT-1): 24 week results from a multicentre, double-blind, phase 3, randomised controlled trial. lancet Diabetes endocrinol 5(11):864–876. https://doi.org/10.1016/S2213-8587(17)30308-X
Mathieu C, Dandona P, Gillard P, Senior P, Hasslacher C, Araki E, Lind M, Bain SC, Jabbour S, Arya N, Hansen L, Thoren F, Langkilde AM (2018) Efficacy and Safety of Dapagliflozin in Patients With Inadequately Controlled Type 1 Diabetes (the DEPICT-2 Study): 24-Week Results From a Randomized Controlled Trial. Diabetes Care 41(9):1938–1946. https://doi.org/10.2337/dc18-0623
Dandona P, Mathieu C, Phillip M, Hansen L, Tschöpe D, Thorén F, Xu J, Langkilde AM (2018) Efficacy and Safety of Dapagliflozin in Patients With Inadequately Controlled Type 1 Diabetes: the DEPICT-1 52-Week Study. Diabetes Care 41(12):2552–2559. https://doi.org/10.2337/dc18-1087
Kaku K, Isaka H, Sakatani T, Toyoshima J (2019) Efficacy and safety of ipragliflozin add-on therapy to insulin in Japanese patients with type 1 diabetes mellitus: A randomized, double-blind, phase 3 trial. Diabetes Obes Metab. https://doi.org/10.1111/dom.13807
Shimada A, Hanafusa T, Yasui A, Lee G, Taneda Y, Sarashina A, Shiki K, George J, Soleymanlou N, Marquard J (2018) Empagliflozin as adjunct to insulin in Japanese participants with type 1 diabetes: results of a 4-week, double-blind, randomized, placebo-controlled phase 2 trial. Diabet Obes Metab 20(9):2190–2199. https://doi.org/10.1111/dom.13351
Pieber TR, Famulla S, Eilbracht J, Cescutti J, Soleymanlou N, Johansen OE, Woerle HJ, Broedl UC, Kaspers S (2015) Empagliflozin as adjunct to insulin in patients with type 1 diabetes: a 4-week, randomized, placebo-controlled trial (EASE-1). Diabet Obes Metab 17(10):928–935. https://doi.org/10.1111/dom.12494
Rosenstock J, Marquard J, Laffel LM, Neubacher D, Kaspers S, Cherney DZ, Zinman B, Skyler JS, George J, Soleymanlou N, Perkins BA (2018) Empagliflozin as adjunctive to insulin therapyin type 1 diabetes: The EASE trials. Diabetes Care 41(12):2560–2569. https://doi.org/10.2337/dc18-1749
Henry RR, Rosenstock J, Edelman S, Mudaliar S, Chalamandaris AG, Kasichayanula S, Bogle A, Iqbal N, List J, Griffen SC (2015) Exploring the potential of the SGLT2 inhibitor dapagliflozin in type 1 diabetes: a randomized, double-blind, placebo-controlled pilot study. Diabetes Care 38(3):412–419. https://doi.org/10.2337/dc13-2955
Danne T, Cariou B, Banks P, Brandle M, Brath H, Franek E, Kushner JA, Lapuerta P, McGuire DK, Peters AL, Sawhney S, Strumph P (2018) HbA1c and Hypoglycemia Reductions at 24 and 52 Weeks With Sotagliflozin in Combination With Insulin in Adults With Type 1 Diabetes: The European inTandem2 Study. Diabetes Care 41(9):1981–1990. https://doi.org/10.2337/dc18-0342
Buse JB, Garg SK, Rosenstock J, Bailey TS, Banks P, Bode BW, Danne T, Kushner JA, Lane WS, Lapuerta P, McGuire DK, Peters AL, Reed J, Sawhney S, Strumph P (2018) Sotagliflozin in Combination With Optimized Insulin Therapy in Adults With Type 1 Diabetes: The North American inTandem1 Study. Diabetes Care 41(9):1970–1980. https://doi.org/10.2337/dc18-0343
Sands AT, Zambrowicz BP, Rosenstock J, Lapuerta P, Bode BW, Garg SK, Buse JB, Banks P, Heptulla R, Rendell M et al (2015) Sotagliflozin, a Dual SGLT1 and SGLT2 Inhibitor, as Adjunct Therapy to Insulin in Type 1 Diabetes. Diabetes Care 38(7):1181–1188. https://doi.org/10.2337/dc14-2806
Mathieu C, Rudofsky G, Phillip M, Araki E, Lind M, Arya N, Thorén F, Scheerer MF, Iqbal N, Dandona P (2020) Long-term efficacy and safety of dapagliflozin in patients with inadequately controlled type 1 diabetes (the DEPICT-2 study): 52-week results from a randomized controlled trial. Diabet Obes Metab. https://doi.org/10.1111/dom.14060
Lu J, Tang L, Meng H, Zhao J, Liang Y (2019) Effects of sodium-glucose cotransporter (SGLT) inhibitors in addition to insulin therapy on glucose control and safety outcomes in adults with type 1 diabetes: A meta-analysis of randomized controlled trials. Diabet Metab Res Rev 35(7):e3169. https://doi.org/10.1002/dmrr.3169
Cumpston M, Li T, Page MJ, Chandler J, Welch VA, Higgins JP, Thomas J (2019) Updated guidance for trusted systematic reviews: a new edition of the Cochrane for Interventions. Cochrane Database Syst Rev. https://doi.org/10.1002/14651858.ED000142
Musso G, Gambino R, Cassader M, Paschetta E (2019) Efficacy and safety of dual SGLT 1/2 inhibitor sotagliflozin in type 1 diabetes: meta-analysis of randomised controlled trials. BMJ 365:l1328. https://doi.org/10.1136/bmj.l1328
Lunder M, Janić M, Japelj M, Juretič A, Janež A, Šabovič M (2018) Empagliflozin on top of metformin treatment improves arterial function in patients with type 1 diabetes mellitus. Cardiovasc Diabetol 17(1):153. https://doi.org/10.1186/s12933-018-0797-6
Watada H, Shiramoto M, Ueda S, Tang W, Asano M, Thoren F, Kim H, Yajima T, Boulton DW, Araki E (2018) Pharmacokinetics and pharmacodynamics of dapagliflozin in combination with insulin in Japanese patients with type 1 diabetes. Diabet Obes Metab. https://doi.org/10.1111/dom.13593
Yamada T, Shojima N, Noma H, Yamauchi T, Kadowaki T (2018) Sodium-glucose co-transporter-2 inhibitors as add-on therapy to insulin for type 1 diabetes mellitus: Systematic review and meta-analysis of randomized controlled trials. Diabet Obes Metab 20(7):1755–1761. https://doi.org/10.1111/dom.13260
Biester T, Muller I, von dem Berge T, Atlas E, Nimri R, Phillip M, Battelino T, Bratina N, Dovc K, Scheerer MF, Kordonouri O, Danne T (2021) Add-on therapy with dapagliflozin under full closed loop control improves time in range in adolescents and young adults with type 1 diabetes: The DAPADream study. Diabetes Obes Metab 23(2):599–608. https://doi.org/10.1111/dom.14258
Danne T, Cariou B, Buse JB, Garg SK, Rosenstock J, Banks P, Kushner JA, McGuire DK, Peters AL, Sawhney S et al (2019) Improved time in range and glycemic variability with sotagliflozin in combination with insulin in adults with type 1 diabetes: a pooled analysis of 24-week continuous glucose monitoring data from the IntanDEM program. Diabetes Care 42(5):919–930. https://doi.org/10.2337/dc18-2149
Evans M, Hicks D, Patel D, Patel V, McEwan P, Dashora U (2020) Optimising the Benefits of SGLT2 Inhibitors for Type 1 Diabetes. Diabetes ther: res treat educ diabetes relat disord 11(1):37–52. https://doi.org/10.1007/s13300-019-00728-6
Sokolov V, Yakovleva T, Ueda S, Parkinson J, Boulton DW, Penland RC, Tang W (2019) Urinary glucose excretion after dapagliflozin treatment: an exposure-response modelling comparison between Japanese and non-Japanese patients diagnosed with type 1 diabetes mellitus. Diabetes Obes Metab 21(4):829–836. https://doi.org/10.1111/dom.13586
Powell DR, Zambrowicz B, Morrow L, Beysen C, Hompesch M, Turner S, Hellerstein M, Banks P, Strumph P, Lapuerta P (2020) Sotagliflozin Decreases Postprandial Glucose and Insulin Concentrations by Delaying Intestinal Glucose Absorption. J Clin Endocrinol Metab 105(4):e1235–e1249. https://doi.org/10.1210/clinem/dgz258
Fahrmann ER, Adkins L, Loader CJ, Han H, Rice KM, Denvir J, Driscoll HK (2015) Severe hypoglycemia and coronary artery calcification during the diabetes control and complications trial/epidemiology of diabetes interventions and complications (DCCT/EDIC) study. Diabetes Res Clin Pract 107(2):280–289. https://doi.org/10.1016/j.diabres.2014.10.007
Beall C, Ashford ML, McCrimmon RJ (2012) The physiology and pathophysiology of the neural control of the counterregulatory response. Am J Physiol Regul Integr Comp Physiol 302(2):R215-223. https://doi.org/10.1152/ajpregu.00531.2011
The DCCT Research Group (1988) Weight gain associated with intensive therapy in the diabetes control and complications trial. Diabetes Care 11(7):567–573. https://doi.org/10.2337/diacare.11.7.567
Heerspink HJL, Kosiborod M, Inzucchi SE, Cherney DZI (2018) Renoprotective effects of sodium-glucose cotransporter-2 inhibitors. Kidney Int 94(1):26–39. https://doi.org/10.1016/j.kint.2017.12.027
Warnes H, Helliwell R, Pearson SM, Ajjan RA (2018) Metabolic Control in Type 1 Diabetes: Is Adjunctive Therapy the Way Forward? Diabetes ther: res treat educ diabetes relat disord 9(5):1831–1851. https://doi.org/10.1007/s13300-018-0496-z
Li J, Gong Y, Li C, Lu Y, Liu Y, Shao Y (2017) Long-term efficacy and safety of sodium-glucose cotransporter-2 inhibitors as add-on to metformin treatment in the management of type 2 diabetes mellitus: A meta-analysis. Medicine 96(27):e7201. https://doi.org/10.1097/MD.0000000000007201
Ridderstrale M, Andersen KR, Zeller C, Kim G, Woerle HJ, Broedl UC (2014) Comparison of empagliflozin and glimepiride as add-on to metformin in patients with type 2 diabetes: a 104-week randomised, active-controlled, double-blind, phase 3 trial. lancet Diabetes endocrinol 2(9):691–700. https://doi.org/10.1016/S2213-8587(14)70120-2
Osaki A, Shimoda Y, Okada J, Yamada E, Saito T, Nakajima Y, Ozawa A, Niijima Y, Okada S, Yamada M (2019) Lower Renal Threshold for Glucose Reabsorption in Type 1 Diabetes Mellitus (T1DM) May Explain the Smaller Contribution of SGLT2 Inhibitors to the Improvement of Plasma Glucose Control Compared with T2DM. Diabetes ther : res treat educ diabetes relat disord 10(4):1531–1534. https://doi.org/10.1007/s13300-019-0649-8
Mondick J, Riggs M, Kaspers S, Soleymanlou N, Marquard J, Nock V (2018) Population Pharmacokinetic- Pharmacodynamic Analysis to Characterize the Effect of Empagliflozin on Renal Glucose Threshold in Patients With Type 1 Diabetes Mellitus. J Clin Pharmacol 58(5):640–649. https://doi.org/10.1002/jcph.1051
Liu J, Li L, Li S, Jia P, Deng K, Chen W, Sun X (2017) Effects of SGLT2 inhibitors on UTIs and genital infections in type 2 diabetes mellitus: a systematic review and meta-analysis. Sci rep 7(1):2824. https://doi.org/10.1038/s41598-017-02733-w
Puckrin R, Saltiel MP, Reynier P, Azoulay L, Yu OHY, Filion KB (2018) SGLT-2 inhibitors and the risk of infections: a systematic review and meta-analysis of randomized controlled trials. Acta Diabetol 55(5):503–514. https://doi.org/10.1007/s00592-018-1116-0
Perkins BA, Cherney DZ, Partridge H, Soleymanlou N, Tschirhart H, Zinman B, Fagan NM, Kaspers S, Woerle HJ, Broedl UC, Johansen OE (2014) Sodium-glucose cotransporter 2 inhibition and glycemic control in type 1 diabetes: results of an 8-week open-label proof-of-concept trial. Diabetes Care 37(5):1480–1483. https://doi.org/10.2337/dc13-2338
El Masri D, Ghosh S, Jaber LA (2018) Safety and efficacy of sodium-glucose cotransporter 2 (SGLT2) inhibitors in type 1 diabetes: A systematic review and meta-analysis. Diabetes Res Clin Pract 137:83–92. https://doi.org/10.1016/j.diabres.2018.01.004
Yang Y, Pan H, Wang B, Chen S, Zhu H (2017) Efficacy and Safety of SGLT2 Inhibitors in Patients with Type 1 Diabetes: A Meta-analysis of Randomized Controlled Trials. Chinese med sci j 32(1):22–27
Chen J, Fan F, Wang JY, Long Y, Gao CL, Stanton RC, Xu Y (2017) The efficacy and safety of SGLT2 inhibitors for adjunctive treatment of type 1 diabetes: a systematic review and meta-analysis. Sci rep 7:44128. https://doi.org/10.1038/srep44128
Chen MB, Xu RJ, Zheng QH, Zheng XW, Wang H, Ding YL, Yue MX (2019) Effectiveness and safety of sotagliflozin adjuvant therapy for type 1 diabetes mellitus: A protocol for Systematic review and Meta-analysis. Medicine (Baltimore) 98(33):e16850. https://doi.org/10.1097/md.0000000000016850
Acknowledgments
The present study was supported by grants provided by the natural science foundation of Hubei provincial department of education (D20182104), the scientific and technological project of Shiyan City of Hubei Province (16K67), and the initial project for post-graduates of Hubei University of Medicine (2016QDJZR08).
Author information
Authors and Affiliations
Contributions
Chenghu Huang and Xuefeng Li conceived and designed the review and contributed to data interpretation. Lunwen Rao and Chenhong Ren identified reports, extracted the data, and input and interpreted the data. Shan Luo provided statistical advice. Chenghu Huang drafted the manuscript, and all other authors (Lunwen Rao, Chenhong Ren, Shan Luo, and Xuefeng Li) critically reviewed it.
Corresponding authors
Ethics declarations
Conflicts of interest
The authors declare that there are no conflicts of interest that could be perceived as prejudicing the impartiality of the research reported.
Ethics statement
All collected data were extracted from published studies, and there is no ethical issue.
Additional information
Managed by Massimo Porta.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Below is the link to the electronic supplementary material.
Below is the link to the electronic supplementary material.
Below is the link to the electronic supplementary material.
Below is the link to the electronic supplementary material.
Below is the link to the electronic supplementary material.
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Rao, L., Ren, C., Luo, S. et al. Sodium–glucose cotransporter 2 inhibitors as an add-on therapy to insulin for type 1 diabetes mellitus: Meta-analysis of randomized controlled trials. Acta Diabetol 58, 869–880 (2021). https://doi.org/10.1007/s00592-021-01686-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00592-021-01686-x