Abstract
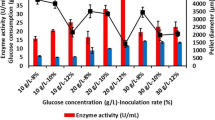
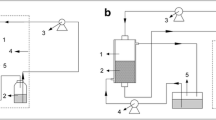
Mannanases, one of the important enzyme group for industry, are produced by numerous filamentous fungi, especially Aspergillus species with different fermentation methods. The aim of this study was to show the best fermentation method of β-mannanase production for fungal growth in fermenter. Therefore, different fermentation strategies in fed-batch fermentation (suspended, immobilized cell, biofilm and microparticle-enhanced bioreactor) were applied for β-mannanase production from glucose medium (GM) and carob extract medium (CEM) by using recombinant Aspergillus sojae. The highest β-mannanase activities were obtained from microparticle-enhanced bioreactor strategy. It was found to be 347.47 U/mL by adding 10 g/L of Al2O3 to GM and 439.13 U/mL by adding 1 g/L of talcum into CEM. The maximum β-mannanase activities for suspended, immobilization, and biofilm reactor remained at 72.55 U/mL in GM, 148.81 U/mL in CEM, and 194.09 U/mL in GM, respectively. The reason for that is the excessive, and irregular shaped growth and bulk formation, inadequate oxygen transfer or substrate diffusion in bioreactor. Consequently, the enzyme activity was significantly enhanced by addition of microparticles compared to other fed-batch fermentation strategies. Also, repeatable β-mannanase activities were obtained by controlling of the cell morphology by adding microparticle inside the fermenter.




Similar content being viewed by others
References
Bai F, Anderson W, Moo-Young M (2008) Ethanol fermentation technologies from sugar and starch feedstocks. Biotechnol Adv 26(1):89–105
Bailey MJ, Biely P, Poutanen K (1992) Interlaboratory testing of methods for assay of xylanase activity. J Biotechnol 23(3):257–270
Blibech M, Ellouz Ghorbel R, Chaari F, Dammak I, Bhiri F, Neifar M, Ellouz Chaabouni S (2011) Improved mannanase production from Penicillium occitanis by fed-batch fermentation using acacia seeds. ISRN Microbiol. doi:10.5402/2011/938347
Chauhan PS, Puri N, Sharma P, Gupta N (2012) Mannanases: microbial sources, production, properties and potential biotechnological applications. Appl Microbiol Biotechnol 93(5):1817–1830
Cheng K-C, Catchmark JM, Demirci A (2009) Enhanced production of bacterial cellulose by using a biofilm reactor and its material property analysis. J Biol Eng 3(1):12
Coban HB, Demirci A (2016) Enhancement and modeling of microparticle-added Rhizopus oryzae lactic acid production. Bioprocess Biosyst Eng 39(2):323–330
Coban HB, Demirci A, Turhan I (2015a) Enhanced Aspergillus ficuum phytase production in fed-batch and continuous fermentations in the presence of talcum microparticles. Bioprocess Biosyst Eng 38(8):1431–1436
Coban HB, Demirci A, Turhan I (2015b) Microparticle-enhanced Aspergillus ficuum phytase production and evaluation of fungal morphology in submerged fermentation. Bioprocess Biosyst Eng 38(6):1075–1080
de Vries R (2003) Regulation of Aspergillus genes encoding plant cell wall polysaccharide-degrading enzymes; relevance for industrial production. Appl Microbiol Biotechnol 61(1):10–20
Demirci A, Pometto A III, Ho KG (1997) Ethanol production by Saccharomyces cerevisiae in biofilm reactors. J Ind Microbiol Biotechnol 19(4):299–304
Driouch H, Hänsch R, Wucherpfennig T, Krull R, Wittmann C (2012) Improved enzyme production by bio-pellets of Aspergillus niger: targeted morphology engineering using titanate microparticles. Biotechnol Bioeng 109(2):462–471
Duruksu G, Ozturk B, Biely P, Bakir U, Ogel ZB (2009) Cloning, expression and characterization of endo-β-1,4-mannanase from Aspergillus fumigatus in Aspergillus sojae and Pichia pastoris. Biotechnol Prog 25(1):271–276
El-Naggar MY, El-Aassar S, Youssef AS, El-Sersy NA, Beltagy E (2006) Extracellular β-mannanase production by the immobilization of the locally isolated Aspergillus niger. Int J Agric Biol 8:57–62
Ercan D, Demirci A (2013) Production of human lysozyme in biofilm reactor and optimization of growth parameters of Kluyveromyces lactis K7. Appl Microbiol Biotechnol 97(14):6211–6221
Ercan D, Demirci A (2014) Enhanced human lysozyme production in biofilm reactor by Kluyveromyces lactis K7. Biochem Eng J 92:2–8
Ercan D, Demirci A (2015a) Effects of fed-batch and continuous fermentations on human lysozyme production by Kluyveromyces lactis K7 in biofilm reactors. Bioprocess Biosyst Eng 38(12):2461–2468
Ercan D, Demirci A (2015b) Enhanced human lysozyme production by Kluyveromyces lactis K7 in biofilm reactor coupled with online recovery system. Biochem Eng J 98:68–74
Germec M, Turhan I, Karhan M, Demirci A (2015) Ethanol production via repeated-batch fermentation from carob pod extract by using Saccharomyces cerevisiae in biofilm reactor. Fuel 161:304–311
Germec M, Turhan I, Demirci A, Karhan M (2016) Effect of media sterilization and enrichment on ethanol production from carob extract in a biofilm reactor. Energy Sources Part A Recovery Util Environ Effects 38(21):3268–3272
Großwindhager C, Sachslehner A, Nidetzky B, Haltrich D (1999) Endo-β-1, 4-d-mannanase is efficiently produced by Sclerotium (Athelia) rolfsii under derepressed conditions. J Biotechnol 67(2):189–203
Ho K, Pometto AL, Hinz PN, Dickson JS, Demirci A (1997) Ingredient selection for plastic composite supports for l-(+)-lactic acid biofilm fermentation by Lactobacillus casei subsp. rhamnosus. Appl Environ Microbiol 63(7):2516–2523
Kaup BA, Ehrich K, Pescheck M, Schrader J (2008) Microparticle-enhanced cultivation of filamentous microorganisms: increased chloroperoxidase formation by Caldariomyces fumago as an example. Biotechnol Bioeng 99(3):491–498
Lee KH, Choi IS, Kim Y-G, Yang D-J, Bae H-J (2011) Enhanced production of bioethanol and ultrastructural characteristics of reused Saccharomyces cerevisiae immobilized calcium alginate beads. Bioresour Technol 102(17):8191–8198
Liu CT, Erh MH, Lin SP, Lo KY, Chen KI, Cheng KC (2016a) Enrichment of two isoflavone aglycones in black soymilk by Rhizopus oligosporus NTU 5 in a plastic composite support bioreactor. J Sci Food Agric 96(11):3779–3786
Liu JM, Yu TC, Lin SP, Hsu RJ, Hsu KD, Cheng KC (2016b) Evaluation of kojic acid production in a repeated-batch PCS biofilm reactor. J Biotechnol 218:41–48
Mabrouk ME, El Ahwany AM (2008) Production of 946-mannanase by Bacillus amyloliquifaciens 10A1 cultured on potato peels. Afr J Biotechnol. doi:10.4314/ajb.v7i8.58631
Miller GL (1959) Use of dinitrosalicylic acid reagent for determination of reducing sugar. Anal Chem 31(3):426–428
Oziyci HR, Tetik N, Turhan I, Yatmaz E, Ucgun K, Akgul H, Gubbuk H, Karhan M (2014) Mineral composition of pods and seeds of wild and grafted carob (Ceratonia siliqua L.) fruits. Sci Hortic 167:149–152
Ozturk B, Cekmecelioglu D, Ogel ZB (2010) Optimal conditions for enhanced β-mannanase production by recombinant Aspergillus sojae. J Mol Catal B Enzym 64(3):135–139
Puchart V, Vršanská M, Svoboda P, Pohl J, Ögel ZB, Biely P (2004) Purification and characterization of two forms of endo-β-1, 4-mannanase from a thermotolerant fungus, Aspergillus fumigatus IMI 385708 (formerly Thermomyces lanuginosus IMI 158749). Biochim Biophys Acta (BBA) Gen Subj 1674(3):239–250
Roth R, Moodley V, van Zyl P (2009) Heterologous expression and optimized production of an Aspergillus aculeatus endo-1, 4-β-mannanase in Yarrowia lipolytica. Mol Biotechnol 43(2):112–120
Shulter M, Kargi F (2000) Bioprocess engineering basic concept. Parentice-Hall of India Pvt Ltd, New Delhi
Singh R, Kumar M, Mittal A, Mehta PK (2016) Microbial enzymes: industrial progress in 21st century. 3 Biotech 6(2):174
Soni H, Rawat HK, Pletschke BI, Kango N (2016) Purification and characterization of β-mannanase from Aspergillus terreus and its applicability in depolymerization of mannans and saccharification of lignocellulosic biomass. 3 Biotech 6(2):1–11
Turhan I, Bialka KL, Demirci A, Karhan M (2010) Ethanol production from carob extract by using Saccharomyces cerevisiae. Bioresour Technol 101(14):5290–5296
Vijayalaxmi S, Prakash P, Jayalakshmi S, Mulimani V, Sreeramulu K (2013) Production of extremely alkaliphilic, halotolerent, detergent, and thermostable mannanase by the free and immobilized cells of Bacillus halodurans PPKS-2. Purification and characterization. Appl Biochem Biotechnol 171(2):382–395
Walisko R, Krull R, Schrader J, Wittmann C (2012) Microparticle based morphology engineering of filamentous microorganisms for industrial bio-production. Biotechnol Lett 34(11):1975–1982
Wang J, Shao Z, Hong Y, Li C, Fu X, Liu Z (2010) A novel β-mannanase from Pantoea agglomerans A021: gene cloning, expression, purification and characterization. World J Microbiol Biotechnol 26(10):1777–1784
Yang J, Jiao R-H, Yao L-Y, Han W-B, Lu Y-H, Tan R-X (2016) Control of fungal morphology for improved production of a novel antimicrobial alkaloid by marine-derived fungus Curvularia sp. IFB-Z10 under submerged fermentation. Process Biochem 51(2):185–194
Yatmaz E, Karahalil E, Germec M, Ilgin M, Turhan I (2016) Controlling filamentous fungi morphology with microparticles to enhanced β-mannanase production. Bioprocess Biosyst Eng. doi:10.1007/s00449-016-1615-8
Zheng J, Zhao W, Guo N, Lin F, Tian J, Wu L, Zhou H (2012) Development of an industrial medium and a novel fed-batch strategy for high-level expression of recombinant β-mananase by Pichia pastoris. Bioresour Technol 118:257–264
Acknowledgements
This study was supported by the Scientific and Technological Research Council of Turkey (TUBITAK) foundation (Grant No. #112O167).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
All the authors in this study mutually agree for submitting our manuscript to 3 Biotech and declare that they have no conflict of interest in the publication.
Rights and permissions
About this article
Cite this article
Germec, M., Yatmaz, E., Karahalil, E. et al. Effect of different fermentation strategies on β-mannanase production in fed-batch bioreactor system. 3 Biotech 7, 77 (2017). https://doi.org/10.1007/s13205-017-0694-9
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s13205-017-0694-9