Abstract
Bacterial cellulose has been used in the food industry for applications such as low-calorie desserts, salads, and fabricated foods. It has also been used in the paper manufacturing industry to enhance paper strength, the electronics industry in acoustic diaphragms for audio speakers, the pharmaceutical industry as filtration membranes, and in the medical field as wound dressing and artificial skin material. In this study, different types of plastic composite support (PCS) were implemented separately within a fermentation medium in order to enhance bacterial cellulose (BC) production by Acetobacter xylinum. The optimal composition of nutritious compounds in PCS was chosen based on the amount of BC produced. The selected PCS was implemented within a bioreactor to examine the effects on BC production in a batch fermentation. The produced BC was analyzed using X-ray diffraction (XRD), field emission scanning electron microscopy (FESEM), thermogravimetric analysis (TGA), and dynamic mechanical analysis (DMA). Among thirteen types of PCS, the type SFYR+ was selected as solid support for BC production by A. xylinum in a batch biofilm reactor due to its high nitrogen content, moderate nitrogen leaching rate, and sufficient biomass attached on PCS. The PCS biofilm reactor yielded BC production (7.05 g/L) that was 2.5-fold greater than the control (2.82 g/L). The XRD results indicated that the PCS-grown BC exhibited higher crystallinity (93%) and similar crystal size (5.2 nm) to the control. FESEM results showed the attachment of A. xylinum on PCS, producing an interweaving BC product. TGA results demonstrated that PCS-grown BC had about 95% water retention ability, which was lower than BC produced within suspended-cell reactor. PCS-grown BC also exhibited higher Tmax compared to the control. Finally, DMA results showed that BC from the PCS biofilm reactor increased its mechanical property values, i.e., stress at break and Young's modulus when compared to the control BC. The results clearly demonstrated that implementation of PCS within agitated fermentation enhanced BC production and improved its mechanical properties and thermal stability.
Similar content being viewed by others
Introduction
Cellulose is the most abundant macromolecule on earth [1] and most cellulose is produced by vascular plants. A substitute to reduce the demand from plants is the production of cellulose using a microbial system [1, 2]. Bacterial cellulose (BC) has been used in the food industry for applications such as low-calorie desserts, salads, and fabricated food, in the paper manufacturing industry to enhance paper strength, in acoustic diaphragms for audio speakers, and in the pharmaceutical industry as a filtration membrane, wound dressing and artificial skin [3, 4].
BC produced by Acetobacter xylinum in static cultures is initially extruded from the cell surface as microfibers and entangle together to form ribbons, which then intertwine to form a dense, gelatinous pellicle at the air/liquid interface. Traditional static culture has been used for BC production, which produces pellicles on the surface of fermentation broth. The pellicle grows downward since cells that are entrapped into the pellicle become inactive or die from lack of oxygen [5].
Several cultivation improvements have been presented to enhance BC production; Yoshino et al. [6] developed a cylindrical silicone membrane vessel that provided oxygen from the bottom, resulting in a two-fold improvement in BC production. Serafica et al. [7] made bacterial cellulose in a rotating disk bioreactor that consists of a cylindrical trough with inoculated medium into which are dipped flat, circular disks mounted on a rotating central shaft. A rotating disk bioreactor is more efficient and reduces the time of a run to about 3.5 days instead of the usual 12–20 days. Hornung et al. [8] developed a novel reactor where both glucose and oxygen were fed directly to the BC-producing cells. These modified production processes were explored to increase the oxygen rich surface area to reactor volume ratio, which improves BC production.
An alternative method for BC production is submerged fermentation. Several strains of BC-producing bacteria had been screened for aerated and agitated cultivation [9, 10]. Instead of a cellulose pellicle, small pellets of BC were produced. These BC pellets exhibit a lower degree of polymerization, crystallinity, and Young's modulus than that produced under static cultivation. The less-organized form of BC may have resulted from shear stress during agitation [11].
High biomass density also proved to be beneficial for BC production in many cases [12, 13]. High density of biomass can be achieved by several ways such as cell immobilization, cell-recycle reactor, and hollow-fiber reactors. Cell-recycle reactors and hollow-fiber reactors have their limitations due to the high capital and operational cost, as well as the potential risk of membrane fouling and/or contamination during fermentation. Biofilm reactors, on the other hand, provide a substitute of high-biomass density systems with lower capital cost. Biofilm reactors have demonstrated a very high volumetric productivity of submerged fermentation, especially the continuous cultures when compared with suspended-cell cultures due to the high cell density maintained in the reactor [14].
Biofilms grow on the solid support when microorganisms attach, and are a natural form of cell immobilization [15]. Plastic composite support (PCS) is an extrusion product of a mixture between polyprolylene and nutritious compounds [16]. Polypropylene acts as a matrix and integrates agricultural mixtures, such as ground soybean hulls, soybean flour, and microbial nutrients such as bovine albumin, red blood cells, yeast extract, and peptone, as well as mineral salts. As a result, PCS not only provides an ideal physical structure for biofilm formation, but also releases nutrients slowly during fermentation.
Many studies showed that using PCS biofilm reactors can enhance production of ethanol, organic acid, and bacteriocin [14, 17–26]. Therefore, a PCS biofilm reactor system was evaluated for BC production by A. xylinum, which is suitable for submerged fermentation [27].
The goal of this study was to evaluate effects of PCS implementation on bacterial cellulose production by A. xylinum, which was divided into two specific objectives: (1) to evaluate BC production in a PCS biofilm reactor and compare to one produced from the conventional suspended-cell culture, and (2) to analyze the material properties of the produced BC, including degree of crystallinity and crystal size by X-ray diffraction (XRD), thermogravimetric analysis (TGA) to determine its water content and thermal decomposition behavior, scanning electron microscopy analysis (SEM) for determining the structure of BC, and dynamic mechanical analysis (DMA) for determining tensile strength of BC.
Materials and methods
Microorganism
The bacterial strain used in this study was Acetobacter xylinum (ATCC 700178) obtained from the American Type Culture Collection (Rockville, MD), which was grown in CSL-Fru medium with agitation as a BC producer. The cell suspension of A. xylinum was stored at -80°C in a 20% glycerol solution. One milliliter of cell suspension stored at -80°C was added to 100 ml of CSL-Fru medium in a 500 ml flask and statically cultivated at 30°C for 3 days. The cellulose pellicle formed on the surface of the broth was homogenized using a homogenizer (model 7011S, Waring Co., Torrington, CT) at 10,000 rpm for 1 min and filtered through a sterile gauze to remove BC. Ten ml of the filtrate containing the cell suspension was added to 90 ml of CSL-Fru medium and cultivated at 30°C and 200 rpm for 24 h and used as an inoculum.
Media
All the chemicals used were of analytical grade and commercially available unless specified description. For BC production, corn steep liquor with fructose (CSL-Fru) medium was slightly modified as previously described [27], and contained the following constituents per liter: 50 g of fructose, 20 ml of CSL (corn steep liquor, Nihon Starch Industry, Kagoshima, Japan), 1.0 g of KH2PO4, 0.25 g of MgSO4. 7H2O, 3.3 g of (NH)2SO4, 3.6 mg of FeSO4. 7H2O, 1.5 mg of CaC12. 2H2O, 2.4 mg of Na2MoO2. 2H2O, 1.7 mg of ZnSO2. 7H2O, 1.4 mg of MnSO4. 5H2O, 0.05 mg of CuSO4. 5H2O, 2.0 mg of inositol, 0.4 mg of nicotinic acid, 0.4 mg of pyridoxine. HCl, 0.4 mg of thiamine. HCl, 0.2 mg of pantothenic acid Ca salt, 0.2 mg of riboflavin, 0.2 mg of pamino-benzoic acid, 0.002 mg of folic acid, and 0.002 mg of biotin. Agar (Becton, Dickinson Co., Sparks, MD), carboxymethylcellulose (Fluka Co., Buchs SG, Switzerland), microcrystalline cellulose (FMC Co., Newark, DE), and sodium alginate (Sigma, St. Louis, MO) were used as additives at various concentrations.
Plastic composite support
Thirteen types of PCS tubes (Table 1) were manufactured in the Center for Crops Utilization Research (CCUR) at Iowa State University using a twin-screw corotating Brabender PL2000 extruder (model CTSE-V; C.W. Brabender Instruments, Inc., South Hackensack, NJ) as described by Ho et al. [24]. Polypropylene and other ingredients of PCS were mixed together before being extruded at 13 rpm through a medium pipe die with barrel temperatures of 200, 220, and 200°C and a die temperature of 165°C. To randomize manufacturing effects, 500 g of the mixture was extruded twice for each blend in a random order to ensure reproducibility, producing 1 kg of PCS from each blend. The resulting tubes had a wall thickness of 2.5 mm and an outer diameter of 10.5 mm. For bioreactor trials, these PCS tubes were cut into 6.0-cm length with both ends cut at a 45° angle to allow better flow of medium inside the tubes. For testing and selection of PCS in test tube fermentations, the PCS tubes were cut into small disks with a thickness of 3 mm using a band saw.
Flask Fermentations for Selection of PCS
Thirteen types of PCS with different compositions were evaluated for both biofilm formation and BC production using flask fermentation with three replicates. For each replicate, 3 g of PCS disks was sterilized dry in 150 mL flask for 1 h at 121°C. Fifty mL of sterilized medium with 5% (w/v) fructose as a carbon source was added aseptically to the sterile PCS before being incubated at 30°C overnight in order to hydrate the PCS. The medium was then aseptically decanted, and fresh sterile medium was added with 1% (v/v) inoculum of 24-h A. xylinum before incubating at 30°C for 120 h. Each replicate was determined the biomass on the PCS, BC production, and residual fructose. The best PCS type was selected according to the biofilm formation on PCS (g-biomass/g-PCS), BC production (g/mL), nitrogen content (% w/w), and nitrogen leaching rate from PCS (% w/w of total nitrogen after the 120-h fermentation). A control experiment without PCS rings was also performed simultaneously. The results on nitrogen content and nitrogen leaching rate were obtained from the study of Ho et al. [22].
BC Fermentation in the Bioreactor
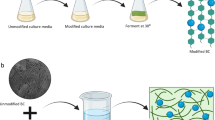
BC fermentations were conducted in a 1.25-L Bioflo 3000 fermentor (New Brunswick Scientific, Edison, NJ) at 30°C with a working volume of 1 L and the agitation rate of 100 rpm. For the biofilm reactor, 12 PCS tubes were bound to the agitator shaft in a gridlike fashion, with 6 rows of two parallel tubes (Figure 1). The reactor vessel with PCS was autoclaved with water at 121°C for 45 min. Fructose and nitrogenous components with mineral salts were autoclaved separately and added to the reactor aseptically after draining the water from the reactor as recommended by Ho et al. [24]. After inoculation with a 24-h culture of A. xylinum (10% v/v), 120-h batch fermentation was carried out. The initial pH was set at 5.0, but pH was not controlled during fermentation. After each run, the biomass on the PCS, BC production, and residual fructose were evaluated. Suspended-cell fermentation as control (i.e. without PCS) was conducted using the same protocol except that the reactor containing medium without PCS was autoclaved and inoculated with 24-h culture of A. xylinum (10% v/v) at the beginning of each batch. Each treatment was duplicated.
Measurement of biomass and bacterial cellulose
At the end of the 5-day incubation, BC on the PCS shaft was detached using a knife and combined with culture broth and then centrifuged at 3300 × g for 20 min (Super T-21, Sorvall Co., Norwich, CT). For biomass, the precipitated pellicle was added to 90 ml of buffer (0.1 M potassium acetate-acetate buffer (pH 5.0) and 10 ml of 20% cellulase (Celluclast, Novo Nordisk A/S, Denmark) and incubated at 50°C with shaking at 100 rpm for 1 h to hydrolyze BC [27]. Then, the solution was centrifuged at 3,300 × g for 20 min. The precipitate was dried in an oven at 80°C overnight and then weighed to determine biomass. For BC determination, the precipitated BC pellicle was treated with 0.1 N NaOH solution at 80°C for 30 min to remove the bacterial cells and medium components [28]. This NaOH treatment was repeated three times and then, the solution was centrifuged at 3,300 × g for 20 min. The obtained cellulose was dried in an oven at 80°C overnight and then weighed.
Fructose
Fructose concentration was determined using a Waters high-performance liquid chromatography unit equipped with column heater, autosampler, computer controller, and a refractive index detector (Waters, Franklin, MA). Components were separated on a Bio-Rad Aminex HPX-87H column (300 mm × 7.8 mm) (Bio-Rad, Richmond, CA) with 0.012 N sulfuric acid as a mobile phase at a flow rate of 0.8 ml/min with an injection volume of 20 μl and a column temperature of 65°C. Before injection, the samples were centrifuged in a microcentrifuge at 2,000 × g for 5 min (Model C-1200, National Labnet Co., Woodbridge, NJ) and filtered through 0.22 μm filters (13 mm diameter disk filters, Millipore, Bedford, MA) to remove suspended solid particles.
X-ray diffraction
To determine the crystallinity of the produced BC, the X-ray diffraction (XRD) patterns of the samples were collected on a Scintag PAD V theta-2-theta diffractometer (Scintag, Cupertino, CA) using a copper x-ray source. Scans were collected at 2 deg per minute from 5–70 degree 2θ. Samples of BC were lyophilized overnight at 0.133 mbar first by using a lab-scale freeze dryer (Model Freezoom 2.5 L, Labconco co., Kansas, MO) and pressed into a thin and flat layer (~1.0 mm thickness) for analysis. MDI Jade 8 software (Materials Data, Inc., Livermore, CA) was used to process the diffraction pattern and to calculate the crystallinity of BC. The degree of crystallinity was taken as Cr I = (I200 - Iam)/I200, where I200 is the overall intensity of the peak at 2θ about 22.9° and Iam is the intensity of the baseline at 2θ about 18° [29]. The crystal size was calculated by the Scherrer equation [30]:

where β is the breadth of the peak of a specific phase (hkl), K is a constant that varies with the method of taking the breadth (0.89<K<1), λ is the wavelength of incident x-rays, θ is the center angle of the peak, and L is the crystallite length (size).
Field emission scanning electron microscopy (FESEM)
Both BC samples before and after removal of cells were lyophilized overnight at 0.133 mbar and then coated with thin Platinum film around 5 nm. A LEO 1530 field emission scanning electron microscope (Leo Co., Oberkochen, German) operating at 2 kV was used for examination of the samples. An imaging magnification of approximately 27,000, 7,000, and 250 times was used for BC, bacteria, and PCS rings, respectively.
Thermogravimetric analysis (TGA)
The dynamic weight loss tests were conducted on a thermogravimetric analyzer (TGA) machine (model Q500, TA instruments-Water LLC, New Castle, DE). For water content determination of cellulose, all sample tests were conducted in a N2 purge (40 ml/min) over a temperature range 30–650°C at an increase rate of 10°C/min. BC samples were placed on the absorbent wipers to eliminate excess water. For thermal decomposition behavior test, cellulose samples were dried at 80°C and tests were then conducted in a N2 purge (40 ml/min) over a temperature range 80–650°C at an increase rate of 10°C/min. The initial weight at 80°C was set as 100%.
Mechanical properties of bacterial cellulose
The mechanical properties of BC were performed by a dynamic mechanic analyzer (DMA) (model Q800, TA instrument-Water LLC, New Castle, DE). BC from agitation cultivation after removal of cells was lyophilized first and pressed into a flat piece at 2,500 psi using a press. The BC samples were then cut to make 20 × 5 mm plates for testing. Sample were mounted between upper (fix) and lower (movable) clamps, and two ends were fixed to avoid slip. Force was applied to the lower clamp to pull the sample in tension. Experiments were run at 0.1 N/min force. Tests were performed at an environmental temperature of 35°C. Stress (σ) was calculated by F/A where A is the area measured as width × thickness of sample and F is force in Newtons (N). Strain (ε) was calculated by ΔL/L o where ΔL is exerted extension from starting point L o . Young's modulus was calculated by Stress/Strain in the linear region.
Statistical analysis
All treatments were replicated at least three times. The significant difference of the results was evaluated using the Generalized Linear Model (GLM; with p < 0.05) and Tukey's honestly significant differences (HSD) multiple comparison module of the MINITAB Statistical Software package (Release 13.30; State College, PA, USA)
Results and Discussion
PCS selection
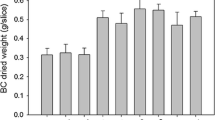
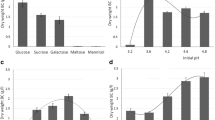
BC production (g/l) and biomass formation (g/g-PCS) in flask fermentation from three replicates of each PCS types are presented in Figures 2 and 3. When biomass vs. BC was plotted (not shown), the result showed no specific relationship between biomass attached on PCS and BC production as the correlation coefficients between these values were extremely low: R-squared values of 0.01 for biomass versus BC production. This pattern was also observed in the PCS biofilm fermentation of succinic acid and nisin [24, 26]. Comparable levels of biomass were attached on each PCS, ranging from 1.1 to 3.3 g/g-PCS. In an attempt to select an optimal PCS for BC production, the means of BC produced (g/l) were ranked as follow according to the results from Tukey's multiple comparisons: SY+ > SFR, SFYR+, SR+, SFYBR > S, SB+, SFY, SYB+ > SFYB+ > SFYB and the ranking of biomass produced (g/g) on PCS was: SFYR+, SYB+, SFYB > SF+, SR+, SY+ > S, SB+, SFB, SFR, SFY, SFYB+, SFYBR. Overall, after considering other factors such as nitrogen content per g of PCS, nitrogen leaching rate, and amounts of biomass attached on PCS, SFYR+ was selected as solid support for BC production by A. xylinum in a batch biofilm reactor due to its high nitrogen content, moderate nitrogen leaching rate [22–24], and high biomass attachment (Figure 2).
BC production by A. xylinum in the PCS biofilm reactor
A. xylinum was grown in CSL-Fru medium with the selected PCS in a biofilm reactor. Fermentation in the PCS biofilm reactor yielded 7.05 g/l BC, which is 2.5-fold greater than BC production in the suspended-cell reactor (2.82 g/l). This result was probably due to the higher biomass density accumulated on PCS and the cell-density-dependent BC production relation as previously described [9, 12].
BC structure and physical properties
Instead of forming small chunks within cultivation broth, BC produced in the PCS biofilm reactor attached on the PCS shaft. Figure 1(B) shows the phenomenon of BC produced on the PCS due to the accumulation of biomass on it. The morphological structures of BC fibers from lyophilized BC samples with and without removal of cells were analyzed by FESEM. Figure 4(B) shows that A. xylinum as indicated with an arrow in the figure are producing an extracellular cellulose, where as twisting ribbon of cellulose and forming BC on PCS surface is shown in Figure 4(B). PCS-grown BC detached from PCS after removal of cells was also analyzed. Figure 4(C) showed that the BC sample retained its interweaving structure of fibers. The width of BC fibers had a broader range than control, around 15~40 nm, which could influence BC properties such as water-holding capacity, thermal stability, and mechanical strength.
XRD patterns obtained from our BC samples demonstrated three main characteristic peaks standing for crystal plane <-110>, <110>, and <200> (Figure 5), which showed that the cellulose exhibited primarily the I-β pattern [31]. The crystalline index of PCS-grown BC was higher than BC harvested from the agitated reactor (Table 2). The crystallinity of BC increased slightly from 85% to 93% when PCS was used as a solid support in the medium, which may arise as a result of longer polymerization of BC when compared to control BC from suspended-cell fermentation. The crystal size of <200> crystal plane remained the same around 5.2 nm.
Thermogravimetric analysis
To determine water retention and information on thermal decomposition behavior of PCS-grown BC, thermogravimetric analysis (TGA) was performed on BC samples at the end of cultivation with and without PCS present. The TGA thermograms of BC samples are shown in Figure 6. PCS-grown BC exhibited around 95% water retention ability, which is lower than BC from the suspended-cell reactor (98%) (Figure 6A), but higher than a BC pellicle from static culture (73%) as reported earlier by Seifert et al. [32]. The results are probably due to incorporation of some water-insoluble particles of PCS, that alter the morphology of the BC structure (Figure 4C). For thermal decomposition behavior of BC samples, dried BC samples were used. Figure 6B showed two distinct steps for weight loss of BC samples, indicating the possibility of two types of decomposition. Yang and Chen [33] illustrated that the initial weight loss at lower temperature ranging from 200°C to 360°C is attributed to the removal of small molecular fragments such as hydroxyl and methylhydroxyl groups. The second weight loss ranging from 360°C to 600°C showed the degradation of polymeric chains and the six-member cyclic structure, pyran. Since the thermal degradation behavior is affected by some structure parameters such as molecular weight, crystallinity, and orientation [34], the more sharp decrease in weight of PCS-grown BC at both stages could be due to its higher crystallinity, degree of polymerization and compact interweaving structure. The maximum decomposition temperature (Tmax), known as a criterion of thermal decomposition position, was calculated from different TGA curves. Each peak on the Tmax pattern represents the steepest slope of weight loss (%/°C) for each step during decomposition. The PCS-grown BC displayed two peaks (Figure 7), one at 265°C and another at 445°C. In the cases of PCS-grown BC, the Tmax of first peak increased by about 30°C, which indicated PCS-grown BC possessed higher thermal stability and began to degrade at higher temperature.
Mechanical properties
BC produced in either agitated or air-lifted culture is believed to lose its high polymerization property which results in low mechanical strength [35] due to the formation of small pellets. As a result, DMA analysis was performed to determine if there was any improvement in mechanical strength of PCS-grown BC as compared to control BC. Both types of BC were investigated: PCS-grown BC from a PCS biofilm reactor, and control from agitated culture. Figure 8 shows a typical stress-strain response of the PCS-grown BC (A) and control (B) samples. The extension of our testers showed a linear-elastic behavior. PCS-grown BC exhibited a longer elastic deformation around 1.4%, where a yield point was seen, which was followed by irreversible deformation. On the other hand, control BC testers showed only 0.8% elongation.
Figure 9 shows the mean value (μ) and standard deviation (σ) of the tested samples' tensile properties (n = 5). Tensile strength (σmax), deformation at break (εmax (%)) and Young's modulus (E (MPa)) were measured. BC tested from the PCS biofilm reactor showed a significant increase in both stress at break and Young's modulus when compared to BC from agitated culture. The stress at break increased from 2.8 to 34.2 MPa (PCS-grown BC). Young's modulus increased from 286 to 2,401 MPa (PCS-grown BC). The strain at break also increased from 0.8 to 1.4%. The Young's modulus showed that mechanical strength of PCS-grown BC increased 840% when compared to control BC. We hypothesize that this improvement is due to an increase in glucose polymerization of the cellulose produced, or due to the incorporation of PCS components.
Conclusion
Based on the results of this study, BC production by A. xylinum can be enhanced using a PCS biofilm reactor system. The material properties of BC are changed when grown in the presence of the PCS. BC produced in a biofilm reactor grew primarily on the PCS supports and is mainly in cellulose I-β pattern. Among thirteen types of PCS, PCS blended with dried soybean hulls, defatted soybean flour, yeast extract, dried bovine red blood cells, and mineral salts (SFYR+) was selected for biofilm BC fermentation. The PCS biofilm reactor yielded the highest BC (7.05 g/l) which is 2.5 fold compared to control (2.82 g/l). The XRD studies indicated that crystallinity of PCS-grown BC is about 93%, which is higher than control, and a crystal size of 5.2 nm in <200> crystal plane. FESEM image provided the evidence that A. xylinum can grow and produce BC on the PCS. TGA results showed that PCS-grown BC possessed 95% water content and demonstrated higher thermal stability (~30°C) when compared to control BC. BC harvested from the biofilm reactor also exhibited higher mechanical strength than BC produced from agitated culture. The PCS-grown BC exhibited higher stress at break, Young's modulus, and strain at break than one produced from agitated culture.
In conclusion, PCS biofilm reactor system successfully demonstrated several advantages for BC production, such as enhanced production yield, higher thermal stability, and stronger mechanical strength. Based on these results, the development of a semi-continuous and continuous fermentation process for producing BC will be the next challenge. Further study on determining other properties of BC produced using PCS, such as gas-penetration ability, metal-ion chelating capacity, and crystallization characteristics are needed.
References
Brown RM: Cellulose Structure and biosynthesis: What is in store for the 21th century? J Poly Sci: Part A: Polymer Chem 2004, 42: 487-495. 10.1002/pola.10877
Lynd LR, Weimer PJ, van Zyl WH, Pretorius IS: Microbial cellulose utilization: fundamentals and biotechnology. Microbiol Mol Biol Rev 2002,66(3):506-77. 10.1128/MMBR.66.3.506-577.2002
Eming S, Smola H, Kreig T: Treatment of chronic wounds: state of the art and future concepts. Cells Tissues Organs 2002, 172: 105-117. 10.1159/000065611
Fontana JD, de Souza AM, Fontana CK, Torriani IL, Moreschi JC, Gallotti BJ, de Souza SJ, Narcisco GP, Bichara JA, Farah LFX: Acetobacter Cellulose Pellicle as a Temporary Skin Substitute. Appl Biochem Biotech 1990, 24/25: 253-263. 10.1007/BF02920250
Borzani W, Desouza SJ: Mechanism of the film thickness increasing during the bacterial production of cellulose on non-agitated liquid-media. Biotech Lett 1995,17(11):1271-1272. 10.1007/BF00128400
Yoshino T, Asakura T, Toda K: Cellulose Production by Acetobacter pasteurianus on silicon Membrane. J Ferment Bioeng 1996,81(1):32-36. 10.1016/0922-338X(96)83116-3
Serafica G, Mormino R, Bungay H: Inclusion of solid particle in bacterial cellulose. Appl Microbiol Biotechnol 2002, 58: 756-760. 10.1007/s00253-002-0978-8
Hornung M, Ludwig M, Schmauder HP: Optimizing the production of bacterial cellulose in surface culture: A novel aerosol bioreactor working on a fed batch principle (Part 3). Eng Life Sci 2007, 7: 35-41. 10.1002/elsc.200620164
Ishikawa A, Matioka M, Tsuchida T, Yoshinaga F: Increasing of bacterial cellulose by sulfaguanidine-resistant mutants derived from Acetobacter xylinum subsp. sucrofermentants BPR 2001. Biosci Biotechnol Biochem 1995, 59: 2259-2262.
Toyosaki H, Naritomi T, Seto A, Matsuoka M, Tsuchida T, Yoshinaga F: Screen of bacterial cellulose-producing Acetobacter strains suitable for agitated culture. Biosci Biotechnol Biochem 1995, 59: 1498-1502.
Watanabe K, Hori Y, Tabuchi M, Morinaga Y, Yoshinaga F, Horii F, Sugiyama J, Okano T: Structural features of bacterial cellulose vary depending on the cultural conditions. Proc Cellulose Society of Japan, Kyoto, Japan 1994, 45-50.
Marx-Figini M, Pion BG: Kinetic investigations on biosynthesis of cellulose by Acetobacter xylinum . Biochim Biophys Acta 1974, 338: 382-393.
Marx-Figini M: The control of molecular weight and molecular-weight distribution in the biogenesis of cellulose. In Cellulose and other natural polymer systems: Biogenesis, structure, and degradation. Edited by: Brown RM. New York: Plenum Press; 1982:243-271.
Cotton JC, Pometto AL III, Gvocdenovic-Jeremic J: Continuous lactic acid fermentation using a plastic composite support biofilm reactor. Appl Microbio Biotechno 2001, 57: 626-630. 10.1007/s002530100820
Characklis WG, Wilderer PA: Structure and Function of Biofilms. Wiley, New York; 1989:6-9.
Pometto AL III, Demirci A, Johnson KE: Immobilization of microorganisms on a support made of synthetic polymer and plant material. U.S. Patent No. 5595893 1997.
Demirci A, Pometto AL III, Ho KLG: Ethanol production by Saccharomyces cere Visiae in biofilm reactors. J Ind Microbiol 1997, 19: 299-304. 10.1038/sj.jim.2900464
Velazquez AC, Pometto AL III, Ho KLG, Demirci A: Evaluation of plastic-composite supports in repeated fed-batch biofilm lactic acid fermentation by Lactobacillus casei . Appl Microbiol Biotechnol 2001, 55: 434-441. 10.1007/s002530000530
Demirci A, Pometto AL III, Ho KLG: Continuous ethanol production in biofilm reactors containing plastic composite rings and disks. In Proceedings of theSecond Biomass Conference of the Americas: Energy, Environment, Agricultural and Industry, Portland, OR, 21–24 Aug 1995. U.S. Department of Energy, National Renewable Energy Laboratory: Golden, CO; 1995.
Kunduru RM, Pometto AL III: Evaluation of plastic composite supports for enhanced ethanol production in biofilm reactors. J Ind Microbiol 1996, 16: 241-248. 10.1007/BF01570028
Kunduru RM, Pometto AL III: Continuous ethanol production by Zymomonas mobilis and Saccharomyces cere visiae in biofilm reactors. J Ind Microbiol 1996, 16: 249-256. 10.1007/BF01570029
Ho KLG, Pometto AL III, Hinz PN: Ingredient selection for plastic composite supports for L-(+)-lactic acid biofilm fermentation by Lactobacillus casei subsp. Rhamnosus . Appl Environ Microbiol 1997, 63: 2516-2523.
Ho KLG, Pometto AL III, Hinz PN, Demirci A: Nutrient leaching and end product accumulation in plastic composite supports for L-(+)-lactic acid biofilm fermentation. Appl Environ Microbiol 1997, 63: 2524-2532.
Ho KLG, Pometto AL III, Hinz PN: Optimization of L-(+)-lactic acid production by ring and disc plastic composite supports through repeated-batch biofilm fermentation. Appl Environ Microbiol 1997, 63: 2533-2542.
Urbance SE, Pometto AL III, DiSpirito AA, Demirci A: Medium evaluation and plastic composite support ingredient selection for biofilm formation and succinic acid production by Actinobacillus succinogenes . Food Biotechnol 2003, 17: 53-65. 10.1081/FBT-120019984
Pongtharangkul T, Demirci A: Evaluation of culture medium for nisin production in a repeated-batch biofilm reactor. Biotechnol Prog 2006, 22: 217-224. 10.1021/bp050295q
Kouda T, Yano H, Yoshinaga F: Effect of agitator configuration on bacterial cellulose productivity in aerated and agitated culture. J Ferment Bioeng 1997, 83: 371-376. 10.1016/S0922-338X(97)80144-4
Hwang JW, Yang YK, Hwang JK, Pyun YR, Kim YS: Effects of pH and dissolved oxygen on cellulose production by Acetobacter xylinum BRC5 in agitated culture. J Biosci Bioeng 1995,88(2):183-188. 10.1016/S1389-1723(99)80199-6
Mihranyan A, Llagostera AP, Karmhag R, Strømme M, Ek R: Moisture sorption by cellulose powders of varying crystallinity. Int J Pharm 2004, 269: 433-442. 10.1016/j.ijpharm.2003.09.030
Zhang LN, Xi Q, Mo ZS, Jin XG: Current researching methods on polymer physics. Wuhan College Press 2003, 194-195.
Czaja W, Romanovicz D, Brown RM: Structural investigations of microbial cellulose produced in stationary and agitated culture. Cellulose 2004, 11: 403-411. 10.1023/B:CELL.0000046412.11983.61
Yang CM, Chen CY: Synthesis, characterization and properties of polyanilines containing transition metal ions. Synth Met 2005, 153: 133-136. 10.1016/j.synthmet.2005.07.136
Seifert M, Hesse S, Kabrelian V, Klemm D: Controlling the water content of never dried and reswollen bacterial cellulose by the addition of water-soluble polymers to the culture medium. J Poly Sci: Part A: Polymer Chemistry 2003, 42: 463-470. 10.1002/pola.10862
Um IC, Ki CS, Kweon HY, Lee KG, Ihm DW, Park YH: Wet spinning of silk polymer II. Effect of drawing on the structure characteristics and properties of filament. Int J Biol Macromol 2004, 34: 107-119. 10.1016/j.ijbiomac.2004.03.011
Benziman M, Haigler C, Brown RM, Alan White, Cooper K: Cellulose biogenesis: polymerization and crystallization are coupled process in Acetobacter xylinum . Proc Natl Acad Sci USA 1980,77(11):6678-6682. 10.1073/pnas.77.11.6678
Acknowledgements
The authors thank Dr. Anthony L. Pometto III from Iowa State University for kindly supplying the PCS tubes used in this research. Funding for this project was supported in part by a seed grant from the College of Agricultural Sciences at the Pennsylvania State University and the Pennsylvania Agricultural Experiment station. The authors are very grateful to Nichole Wonderling from the Materials Research Institute of the Pennsylvania State University for her assistance with x-ray diffraction measurements. Thanks also to Dr. Xuelian Zhang and Yufen Zeng at the Department of Forest Resources for their help for DMA and TGA analysis.
Author information
Authors and Affiliations
Corresponding author
Additional information
Competing interests
The authors declare that they have no competing interests.
Authors' contributions
KC carried out the whole experiment including fermentation and material property analysis. JC conceived of the study, and participated its design and coordination. AD conceived of the study, and participated its design and coordination. All authors read and approved the final manuscript.
Authors’ original submitted files for images
Below are the links to the authors’ original submitted files for images.
Rights and permissions
Open Access This article is published under license to BioMed Central Ltd. This is an Open Access article is distributed under the terms of the Creative Commons Attribution License ( https://creativecommons.org/licenses/by/2.0 ), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
About this article
Cite this article
Cheng, KC., Catchmark, J.M. & Demirci, A. Enhanced production of bacterial cellulose by using a biofilm reactor and its material property analysis. J Biol Eng 3, 12 (2009). https://doi.org/10.1186/1754-1611-3-12
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/1754-1611-3-12