Abstract
In this paper, ion-selective electrodes sensitive to copper(II) ions are presented, in which new composite, synthesized from copper(II) oxide nanoparticles (CuONPs) and multi-walled carbon nanotubes (MWCNTs), was used as a solid contact. For comparison, electrodes obtained using separate components of the nanocomposite, i.e., CuONPs and MWCNTs, as well as unmodified electrodes, were also studied. The tested nanomaterials have been applied in two ways: as an intermediate layer placed between the ion-sensitive membrane and the internal electrode, and as an additional component of the ion-selective membrane mixture. To investigate the influence of the electrode’s structure modification, the selected analytical parameters obtained by potentiometric measurements (slope, linearity range, detection limit, potential stability, and reversibility) and electrochemical impedance spectroscopy measurements (membrane resistance and charge transfer resistance as well as double layer capacitance) were determined and compared. It was found that the use of all nanomaterials improves the properties of the electrodes, with the effect being the strongest for electrodes modified with the CuO-MWCNTs nanocomposite. The nanocomposite-based electrodes, both those with an intermediate layer and those with a nanocomposite-modified membrane, showed a Nernstian slope of the characteristic, a wider working range and a lower detection limit compared to unmodified electrodes. Moreover, application of all nanomaterials, especially nanocomposite resulted in improvement of both, stability and reversibility of the sensor potential. Modification of the electrodes did not make them sensitive to changing external measurement conditions (lighting, presence of gasses, redox potential). The electrode with the best parameters (based on nanocomposite) was successfully used to determine the Cu2+ ions content in tap water and mineral water, obtaining satisfactory results.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Ion-selective electrodes with solid contact (SCISEs) have been gaining popularity for 30 years now. For the first time, an additional material (the so-called solid contact) to improve the analytical and electrical parameters of the electrodes, was used in 1992 by Cadogan et al. (1992). For this purpose, a polypyrrole placed as an intermediate layer before putting the ion-selective membrane on the GC (glassy carbon) electrode was used. The introduction of an additional modification was necessary after the removal of the liquid contact in the construction of classical electrodes, which was connected with a significant deterioration in the stability and reproducibility of the sensor potential after direct application of the ion-selective membrane on the electrode surface (coated wire electrodes, 1971) (Cattrall and Freiser 1971). In the case of SCISEs, two types of mechanisms with ion–electron transduction were experimentally confirmed, enabling the conversion of the input signal (ion activity) to the output signal (electric potential): redox capacitance mechanism (Veder et al. 2013) and electrical double layer capacitance mechanism (Cuartero et al. 2016). Materials characterized by electronic and ionic conductivity, high volumetric capacity, chemically stable, and hydrophobic properties can be used as a solid contact to prevent the formation of an undesirable water layer between the electrode material and the layer of ion-selective membrane (Fibbioli et al. 2000; Yin and Qin 2013). The use of solid contact greatly facilitates the processes of charge transfer between the solid electrode material and the ion-selective membrane, which results in lowering the membrane resistance and charge transfer resistance and increasing the double layer capacitance (Bobacka 1999). In addition to the improvement of typical analytical parameters, such as the slope and linearity of the electrode characteristic curve and the lowering of the detection limit, as well as a significant improvement in the stability, reversibility, and reproducibility of the electrode potential, the use of solid contact also affects the typically mechanical and economic aspects. SCISEs are easier to use, store, and transport compared to classical electrodes and it is possible to significantly reduce their size, which also reduces the amount of materials and chemical compounds necessary for their preparation (Bobacka et al. 2008; Cuartero and Crespo 2018). There are two possibilities of modifying ISEs using solid contact: the previously mentioned application of an intermediate layer and the direct addition of a solid contact material to the membrane mixture (single-piece electrodes), which makes the sensor preparation process even simpler and faster (Bobacka et al. 1995). From the viewpoint of the determination of natural samples, especially in the in situ environment, it is very important to study the stability of the potential of the electrodes (Hu et al. 2016) and their resistance to changes in external conditions, among others lighting (Lindfors 2009), the presence of gasses in the solution (Vázquez et al. 2002), changes in the redox potential or pH of the sample. Due to the use of ISEs in many areas of human life, e.g., in the food (Birinci et al. 2016; Fan et al. 2017) and pharmaceutical industry (Abd El-Rahman and Salem 2015), clinical diagnostics (Koncki 2007; Parrilla et al. 2019) and agriculture and environmental monitoring (De Marco et al. 2007; Crespo 2017), there are still efforts to obtain materials that, when used as a solid contact in SCISEs, will enable obtaining sensors with very good parameters, operating effectively for a long time without losing their initial properties.
Since then, other compounds from the group of conductive polymers (Bobacka et al. 1999; Vázquez et al. 2002; Sutter et al. 2004; Kisiel et al. 2005; Han et al. 2008; Ansari and Mosayebzadeh 2014; Huang et al. 2015; Zhang et al. 2021), carbon nanomaterials (Crespo et al. 2008; Fouskaki and Chaniotakis 2008; Parra et al. 2009; Fierke et al. 2010; Ping et al. 2011, 2012; Najafi et al. 2011; Li et al. 2012; Paczosa-Bator 2012; Liang et al. 2015; Hassan et al. 2019), nanoparticles of metals and metal oxides (Khun et al. 2013; Paczosa-Bator et al. 2018; Liu et al. 2020; Yin et al. 2020; Lenar et al. 2022), and various types of nanocomposites (Rzewuska et al. 2008; O’Neil et al. 2011; Boeva and Lindfors 2016; Piȩk et al. 2016; Ghosh et al. 2017; Li et al. 2017; Topcu et al. 2018; Kałuza et al. 2019) were used as solid contact in ion-selective electrodes. Particularly noteworthy here are composites, which are made of at least two different components to improve the parameters of individual components and/or to obtain new properties of the created material. Most often, nanocomposites are obtained using carbon nanomaterials and polymers, but other compounds can also be used (Coleman et al. 2006).
Our team has already described, as a solid contact, inter alia: doped polyaniline nanofibers (PANINFs-Cl and PANINFs-NO3) (Pietrzak et al. 2021), nanocomposites: [MWCNTs:THTDPCl (Pietrzak and Wardak 2021), MWCNTs:PANINFs-Cl (Pietrzak et al. 2022b), MWCNTs:BMImPF6 (Wardak et al. 2021), and metal oxide nanoparticles (ZnO, CuO, Fe2O3) (Pietrzak et al. 2022a). Due to the satisfactory results obtained after the use of an intermediate layer of nanoparticles, it was decided to extend the research to nanocomposite based of them. This time, a nanocomposite containing CuO nanoparticles and multi-walled carbon nanotubes (CuONPs:MWCNTs) was used as a solid contact in ion-selective electrodes sensitive to copper ions. Both components of the composite are hydrophobic, have excellent electrochemical properties and a high surface-to-volume ratio. Taking into account that composite materials can show even better properties than the components from which they are built, it seems that the nanocomposite obtained from them is a good candidate for solid contact material and gives hope for obtaining electrodes characterized by good analytical parameters. To the best of our knowledge, such a composite has not been studied as an electroactive material in ISEs so far. In this paper, extensive comparative studies were carried out, including: unmodified electrodes and those modified with CuONPs and MWCNTs layers, as well as electrodes in which the nanocomposite was placed both as an intermediate layer placed between the inner electrode and the ion-selective membrane and also as an additional membrane component. Basic analytical parameters were estimated and compared for all received sensors. Furthermore, successive investigation of the influence of changeable conditions on the operation of the sensors (the effect of the presence of light and oxygen or changes pH and redox potential of the sample solution) was carried out. Whereas, the electrical parameters of the electrodes which values could have changed after the sensors modifications were determined using electrochemical impedance spectroscopy method (EIS).
Materials and methods
Apparatus
The potentiometric measurements were done using a galvanic cell consisting of two kinds of electrodes: the tested ion-selective electrodes and a silver/silver chloride reference electrode with double junction system (Metrohm 6.0750.100) using a 16-channel data acquisition system (Lawson Labs. Inc., USA) connected to a computer with appropriate software for data collection. The electromotive force (EMF) measurements were made in mixed solutions using a magnetic stirrer. The Elmetron CX-741 multifunction meter coupled with ORION 81–72 glass electrode was used in pH measurements. All measurements were made at room temperature.
Electrochemical impedance spectroscopy measurements were carried out for a three-electrode system in which the tested ion-selective electrode was the working electrode, Ag/AgCl (Metrohm 6.0733.100)—reference electrode, and glassy carbon rod (Metrohm)—auxiliary electrode. All measurements were made in a Cu(NO3)2 solution of concentration 1 × 10–2 mol L–1. The impedance spectra were recorded in the frequency range 0.1–100 kHz at the open circuit potential with an amplitude 10 mV using an electrochemical analyzer AUTOLAB (Eco Chemie, Netherlands) with NOVA 2.1 software. All measurements were made at room temperature.
Reagents
CuONPs (particie size < 50 nm) (Merck), MWCNTs (length: 3–6 um, outer diameter 10 nm ± 1 nm, inner diameter 4.5 nm ± 0.5 nm) (Sigma-Aldrich), 2-nitrophenyl octylether (NPOE) (Fluka), poly(vinylchloride) high molecular weight (PVC) (Sigma Aldrich), N,N,N′,N′-tetracyclohexyl-2,2′-thiodiacetamide [copper(II) ionophore IV] (Fluka), potassium tetrakis (p-chlorophenyl) borate (KTpClB) (Fluka), tetrahydrofuran (THF) (Chempur).
Other reagents were purchased from Fluka.
All aqueous solutions were prepared with salts of the highest purity available (pure pro analysis) using freshly deionized water.
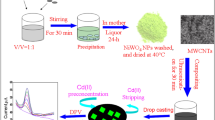
Preparation of CuONPs–MWCNTs nanocomposite
CuONPs–MWCNTs nanocomposite denoted herein after as NC was obtained by mixing 10 mg of CuONPs with 10 mg of MWCNTs, adding 10 mL of THF and thorough homogenization of the suspension in an ultrasonic bath for 1 h. The vessel was then opened and left in a fume hood until the solvent evaporated. Dry NC was used as a component of the ion-sensitive membrane and as an intermediate layer in the form of a suspension of 3 mg NC in 1 mL of THF.
Preparation of the ion-selective membrane
To prepare every ion-selective membrane mixture, all their components were weighed out on an analytical balance according to the previously calculated mass values, and then were mixed with a membrane organic solvent (THF) and homogenized for half an hour in an ultrasonic water bath until a homogeneous mixtures were obtained. 1 mL of solvent was added per 0.1 g of membrane mixture. Four types of membrane mixtures were prepared: basic mixture [1% coper(II) ionophore IV, 0.5% KTpClPB, 33% PVC, 65.5% NPOE] and mixtures containing 1% addition of appropriate nanomaterial or nanocomposite; CuONPs containing mixture [1% CuONPs, 1% coper(II) ionophore IV, 0.5% KTpClPB, 33% PVC, 64.5% NPOE], MWCNTs containing mixture [1% MWCNTs, 1% coper(II) ionophore IV, 0.5% KTpClPB, 33% PVC, 64.5% NPOE], and CuONPs–MWCNTs nanocomposite (NC) containing mixture [1% NC, 1% copper(II) ionophore IV, 0.5% KTpClPB, 33% PVC, 64.5% NPOE].
Preparation of ion-selective electrodes
A glassy carbon electrodes (GCEs) of 0.3 cm diameter were used as inner electrodes. Before applied membrane mixture, they were polished carefully with 5000 grit sandpaper and then with wetted alumina powder (0.3 µm grain diameter). Later the electrodes were rinsed thoroughly with distilled water, immersed in water in an ultrasonic bath, and rinsed again with distilled water to remove residues of alumina powder. To remove organic residues, the electrodes were immersed additionally in THF and allowed to dry in a stand. For all types of nanomaterials, two types of electrodes were made: electrodes in which nanomaterial (30 µL of 3 mg mL–1 nanomaterial suspension in THF) was used as intermediate layer (GCE/CuONPs/ISM, GCE/MWCNTs/ISM and GCE/NC/ISM) and electrodes with 1.0% nanomaterial in the ion-selective membrane (GCE/(ISM + CuONPs); GCE/(ISM + MWCNTS); and GCE/(ISM + NC). For comparison, unmodified electrodes containing only the basic mixture without nanomaterial—GCE/ISM was also prepared. After drying the surface of the electrodes, appropriate membrane mixtures were spotted on them (three times per 30 µL with an interval of 30 min). Then electrodes were allowed to dry overnight and the next day immersed in a conditioning solution (1 × 10–3 mol L–1 solution of Cu(NO3)2). All electrodes were stored in dark place immersed in the conditioning solution in separate containers.
Results and discussion
In this work, application of new NC material obtained from CuONPs and MWCNTs as solid contact in Cu2+-selective electrodes based on PVC membrane is investigated. The NC was used in two ways: (I) placed on internal electrode surface and covered by drop casting with membrane mixture and (II) directly dispersed in the ion-selective membrane to form one-piece SCISE. The properties of NC-based electrodes were compared with parameters of electrodes based on CuONPs and MWCNTs, respectively. In addition, unmodified electrode without nanomaterial was also investigated.
Potentiometric response
To study the effect of application of additional material in the electrode construction, the calibration curves of prepared electrodes were determined. The measurements were done in the Cu(NO3)2 solutions in the concentration range of 1 × 10–8–1 × 10–1 mol L–1 and repeated twice a week during 2 months. On the basis of these measurements, the parameters of the electrodes were determined, among others the detection limit, the slope and range of linearity of the characteristics, and changes in these parameters over time. The values of individual parameters determined 1 week and 8 weeks after the preparation of the electrodes are summarized in Table 1. In addition, to estimate the stability of the response of the sensors over longer period of time, the average values of the slope of the characteristic and E0 potential (taken from regression equation of the linear section of the calibration curves) and the corresponding values of the standard deviation from all calibrations were determined (Table 1). Exemplary of calibration curves of the electrodes determined 1, 2, 4 and 8 weeks after preparation for GCE/ISM, GCE/CuONPs/ISM, GCE/MWCNTs/ISM, GCE/NC/ISM, and GCE/(ISM + NC) is presented in Fig. 1, where it can be seen that the electrodes prepared with all types of nanomaterials showed improved properties compared to the unmodified electrode. Around one order of magnitude lower detection limit and a wider measuring range was noticed for the electrodes prepared with CuONPs and MWCNTs (both types of construction), whereas nanocomposite-based electrodes showed an even lower detection limit of 2.2 × 10–8 and 1.5 × 10–8 mol L–1 for electrode GCE/NC/ISM and GCE/(ISM + NC), respectively. An additional advantage of the modified electrodes is their very good stability over time, which is manifested by an almost constant slope of the electrode characteristics over time and small changes in the E0 potential. In this case, the smallest differences in the determined S and E0 values were observed for the nanocomposite-modified electrodes (the smallest SD values for both slope and E0 potential) (Table 1). This is a very valuable property of the electrodes, as it allows them to be used in measurements without the need for recalibration and guarantees the correct determination of the results. The positive change in the electrode response is due to the modification of the electrode with CuONPs, MWCNTS, and NC. These materials have the valuable properties mentioned in the introduction, thanks to which they reduce the membrane resistance, while increasing its hydrophobicity, which results in improved electrode parameters. It seems that NC exhibits a synergistic effect compared to the single components as the NC-based electrodes showed better performance parameters than those based on CuONPs or MWCNTs. Improved copper sensing using carbon paste electrode modified with CuONPs and chemically modified MWCNTS was also reported by Ghaedi et al. (2015).
Short-term stability of the potential
Instability of the potential is a well-known disadvantage of simple coated wire electrode without solid contact electroactive material. To check the effectiveness of the nanomaterials in improving the potential stability of the obtained electrodes, their potential was measured in a solution of 0.001 mol L–1 for 3.5 h. The course of electrode potential change during this experiment is shown in Fig. 2. The potential drift expressed as ΔE/Δt was determined from the results of these measurements. As it was expected, a significant improvement in potential stability of electrodes was obtained as a result of their modification with nanomaterial. Unmodified GCE/ISM showed big potential drift of 1021 µV min–1 during the first 30 min and 182 µV min–1 during the next 3 h whereas modified electrodes had much lower potential drift. It was 26.8, 29.5, and 7.4 µV min–1 for GCE/CuONPs/ISM, GCE/MWCNTs/ISM, and GCE/NC/ISM, respectively, and 13.1, 14.3, and 2.2 µV min–1 for GCE/(ISM + CuONPs), GCE/ISM + MWCNTs), and GCE/(ISM + NC), respectively. Comparing these values, it is easy to see that the electrodes with the nanomaterial in the membrane showed a smaller potential drift than those in which it was placed as an intermediate layer between the membrane and the inner electrode. The best potential stability among all tested electrodes was obtained for the electrode GCE/(ISM + NC).
pH range
Measurements of the potential of constructed sensors were also performed in solutions of various pH, with the main Cu(II) ion concentration equal to 1 × 10–4 mol L–1 over the pH range 2.0–8.0. The pH was adjusted using HNO3 or NaOH solutions. The pH range at which the potential was constant did not depend essentially on the presence of nanomaterials and was 4.0–6.0 for GCE/(ISM + NC) and GCE/(ISM + MWCNTs). In the case of other modified electrodes as well as unmodified one, it was 4.5–6.0. Narrow pH range is connected with properties of ionophore used for membrane preparation which is H+ sensitive (Szigeti et al. 2005).
Selectivity
The selectivity is one of the most important analytical parameters of ion-selective electrodes. Knowledge of this parameter is necessary for obtaining correct results of analysis. The values of selectivity coefficients were determined using the separate solutions method (SSM) (Bakker et al. 2000). The application of tested nanomaterials did not significantly impact the selectivity of the sensors. Nevertheless, in the case of electrodes with membrane modified by nanomaterials, a slight improvement in selectivity was observed. The results obtained for unmodified electrode GCE/ISM and nanocomposite-based electrode GCE/(ISM + NC) are presented in Table 2. It can be seen that the greatest improvement in the values of selectivity coefficients was noted for the coefficients determined in relation to lead and cadmium ions. This is extremely important as these are the most interfering ions for copper selective electrodes, not only for the electrodes described here, but also for others described in the literature and commercially offered.
Resistance to the changes in measurements conditions
The sensitivity of the electrodes to changes in the measurement conditions can cause potential instability and is a source of measurement errors. It is crucial to ensure the correctness of the obtained results. It is commonly known that electrodes based on conductive polymers are often sensitive to light (Lindfors 2009), the presence of gasses in the solution (Vázquez et al. 2002), and the presence of CO2 and O2 (Vázquez et al. 2002) while electrodes based on carbon nanomaterials can exhibit redox sensitivity (Mousavi et al. 2011). Therefore, when introducing a new material as a solid contact, it is necessary to check the potentiometric behavior of the electrodes under different conditions.
Light sensitivity
The research on the influence of light on the electrode potential was performed in 1 × 10–3 mol L–1 Cu(NO3)2 solution, the measuring system (electrodes and sample solution) was alternately illuminated with sunlight every 10 min and shielded with a light-tight screen. Time-dependent potential traces recorded for electrodes containing nanomaterial in the membrane in different light conditions are shown in Fig. 3, where it can be seen that the indications of the modified electrodes were constant irrespective of the amount of light, while the unmodified electrode showed potential changes during the measurement. This was rather not related to its sensitivity to light, but to its poor potential stability, as shown in Fig. 2. In the case of electrodes with an intermediate layer, no changes in potential were recorded in response to changing light conditions.
Sensitivity to the presence of O2 and CO2
The sensitivity of the electrodes to the presence of O2 and CO2 in the solution was checked by measuring their potential in a Cu(NO3)2 solution (concentration of 1 × 10–3 mol L–1), which was left in the air, and in a solution of the same concentration through which N2 was passed for 10 min and the N2 atmosphere was maintained over the sample during the measurement. The results of these measurements obtained for electrodes with a modified membrane and for an unmodified electrode are shown in Fig. 4. All modified electrodes (also those with an intermediate layer of nanomaterial not shown in the Fig. 4) worked properly, regardless of change of gasses content in the solution. The measured potential did not change significantly, what gives us information that tested electrodes are not sensitive to the presence of gasses.
Redox sensitivity
Potentiometric behavior of tested electrodes in the solution with different redox potential was tested in 1 × 10–3 mol L–1 CuSO4 solutions containing a redox couple of ions Fe2+ and Fe3+, where [Fe2+]/[Fe3+] ratio was 10, 5, 1, 0.5, and 0.1. In Fig. 5, the dependence of the electrode potential as a function of the redox potential of the sample solution is presented where it can be seen that change of the redox potential does not have impact on the electrode potential, which was almost constant in all solutions. Only for the electrode without any nanomaterial, slight differences in indications were noted. Resistance of tested electrodes to the changes in measurement conditions is a beneficial effect that allows the obtained sensors to be used in environmental samples usually containing dissolved gasses (O2 and CO2) and differing in redox potential.
Electrochemical impedance spectroscopy
To determine and compare electric properties of the tested sensor which impact on electrode response, the electrochemical impedance spectroscopy measurements were performed. EIS is a very sensitive technique useful for studying electrode processes and interfaces. With regard to SCISEs, this technique enables the estimation of the membrane resistance and the tracking of processes at the membrane/electron conductor interface (Suni 2008). The obtained impedance spectra for all tested electrodes are shown in Fig. 6, where it can be seen plots with a shape typical for ISEs with a polymeric membrane. All spectra consisted of two parts: a high-frequency semicircle which is attributed to the bulk resistance (Rb) and geometric capacitance (Cg) of the ion-selective membrane (Horvai et al. 1986), and low-frequency partial semicircle connected with the double layer capacitance, charge transfer resistance at the interface between polymeric membrane and the inner GCE electrode (Bobacka 1999). Although all impedance spectra have similar shape, they differ essentially in size depending on the type of nanomaterial used to modify the electrode and the method of its application. The bulk membrane resistance (Rb) determined from high-frequency semicircle diameter was the biggest for unmodified GCE/ISM and was 308 kΩ. As a result of introducing an intermediate layer of nanomaterial between the membrane and the inner electrode, a decrease in the membrane resistance was observed to the value 233, 194, and 167 kΩ, for GCE/CuONPs/ISM, GCE/MWCNTs/ISM, and GCE/NC/ISM, respectively. Significantly greater resistance changes were noted for the electrodes, where the nanomaterial was dispersed in the membrane. For these electrodes, the determined Rb values were 179, 40.9, and 27.0 kΩ, for GCE/(ISM + CuONPs), GCE/(ISM + MWCNTs), and GCE/(ISM + NC), respectively. Comparing the membrane resistance of electrodes obtained on the basis of the same material, it can be seen that, in each case, the resistance of the modified membrane is significantly lower than the resistance of the membrane in the electrodes with an intermediate layer. This is understandable given the good electrical conductivity of MWCNTs and the semiconductor properties of CuONPs. Dispersing them throughout the membrane phase gives better results than the contact between the membrane and the nanomaterial layer, which only partially penetrates the membrane. It should be emphasized that the best effect in reducing the membrane resistance was obtained with the use of NC. This can be explained by the CuONPs surrounding the MWCNTs to form a three-dimensional structure. This reduces the aggregation of both nanomaterials and increases the contact surface of the membrane phase with the nanocomposite.
The low-frequency part of the impedance spectrum was used to estimate the low-frequency capacitance Clf of the electrodes, which is inversely proportional to the charge transfer resistance and provides us with information about diffusion processes and the easiness of charge transport between the polymer membrane and the inner electrode, in this case GCE. The low-frequency capacitance Clf was determined from the low-frequency limit using the following dependence Clf = − 1/(2лfZ″), where f = 0.1 Hz (Mousavi et al. 2011; Wardak 2015). As it was expected, Clf value was the smallest for unmodified GCE/ISM and was equal to 1.20 µF. It was increased after covering inner electrode with intermediate nanomaterial layer as evidenced by the Clf values 5.5, 18.4, and 53.7 obtained for electrodes GCE/CuONPs/ISM, GCE/MWCNTs/ISM, and GCE/NC/ISM, respectively. An even greater increase in low-frequency capacitance was obtained by modifying the membrane with the addition of nanomaterial. Clf values obtained for electrodes GCE/(ISM + CuONPs), GCE/(ISM + MWCNTs), and GCE/(ISM + NC) were 8.4, 45.9, and 92.5 µF.
Summarizing the EIS results, it can be concluded that the best material for improving the electrical parameters of the sensors from the materials tested in the work turned out to be NC, which caused the greatest reduction of the membrane resistance and more effective increase of the capacitance Clf.
Better results were obtained by utilizing this material as a component directly added to the ion-selective membrane. The improvement of the electrical parameters of the electrodes results in better analytical parameters, which is confirmed by the results of potentiometric measurements.
Analytical application
Copper is a trace element and a micronutrient essential for the functioning of the body which in increased doses can be harmful to health and in extreme cases even cause death. Due to the constantly increasing share of copper in water transport installations in individual countries, as well as in the European Union, standards are set that allow the maximum concentration of copper ions in water transported through copper pipes. The standards define a maximum concentration of copper ions in drinking water of 2 mg L−1.
In the case of an improperly installed installation or when the water is acidic, corrosion occurs and copper is washed out of the installation. This can cause not only a significant exceedance of the norm, even several times, and with prolonged exposure to high doses, it can cause adverse health effects (WHO 2008).
Considering the very good analytical parameters of the developed GCE/(ISM + NC) electrode and its excellent stability of indications over time, it seems to be a good device for monitoring copper content in drinking water. This has been demonstrated by its use for the determination of copper in tap water (from a copper installation) and mineral water. To verify the correctness of the determination results, recovery tests were also performed.
Water samples were analyzed directly after collection using the standard addition method without sample preparation (addition only 0.01 mol L–1 of NaNO3 solution and checking the pH if it was within the optimal pH range of the electrode). For samples with pH ≥ 5.5, the pH was adjusted to pH = 5.0 using 0.1 mol L−1 HNO3 solution. At this pH, according to speciation calculations (Inczedy 1979), 97% of copper occurs in its free, uncomplexed form which can be detected with ion-selective electrode. The obtained results are summarized in Table 3, where it can be noticed that good recovery results were obtained in all cases which confirms that the proposed GCE/(ISM + NC) electrode can be used for monitoring of copper content in drinking water. It is a good alternative for other more complicated and expensive method.
Conclusion
The article describes the use of a new CuONPs–MWCNTs nanocomposite as a solid contact in Cu2+-SCISEs. The obtained sensors were characterized by a good slope of calibration curve (30.1 mV decade–1), a low detection limit (1.5 × 10–8 mol L–1), and a very good potential stability. The parameters of the CuONPs–MWCNTs nanocomposite-based electrodes were better in comparison to the unmodified electrode and to the electrodes based on CuONPs and MWCNTs. GCE/(ISM + NC) worked flawlessly in the pH range of 4.0–6.0 and was resistant to changes in external measurement conditions (light, gasses, redox potential). Answering the question which way of using the nanocomposite is better, it can be stated that it is more effective to disperse the nanomaterial throughout the membrane.
For electrodes with the addition of nanomaterials directly to the membrane mixture, significantly lower values of membrane resistance and higher values of low-frequency capacitance were obtained than for electrodes with intermediate layers of solid contact. Due to this, these electrodes were characterized by better potential stability. The electrode with the best parameters (GCE/(ICM + NC) was successfully used to determine the content of Cu2+ ions in tap water and mineral water, obtaining satisfactory results.
Availability of data and materials
The data presented in this study are available on request from the corresponding author.
Code availability
Not applicable.
References
Abd El-Rahman MK, Salem MY (2015) Ion selective electrode (in-line analyzer) versus UV-spectroscopy (at-line analyzer); which strategy offers more opportunities for real time monitoring of the degradation kinetics of pyridostigmine bromide. Sens Actuators B Chem 220:255–262. https://doi.org/10.1016/j.snb.2015.05.092
Ansari R, Mosayebzadeh Z (2014) Construction of a new solid-state U(VI) ion-selective electrode based on polypyrrole conducting polymer. J Radioanal Nucl Chem 299:1597–1605. https://doi.org/10.1007/s10967-013-2836-9
Bakker E, Pretsch E, Bühlmann P (2000) Selectivity of potentiometric ion sensors. Anal Chem 72:1127–1133. https://doi.org/10.1021/ac991146n
Birinci A, Eren H, Coldur F et al (2016) Rapid determination of trace level copper in tea infusion samples by solid contact ion selective electrode. J Food Drug Anal 24:485–492. https://doi.org/10.1016/j.jfda.2016.02.012
Bobacka J (1999) Potential stability of all-solid-state ion-selective electrodes using conducting polymers as ion-to-electron transducers. Anal Chem 71:4932–4937. https://doi.org/10.1021/ac990497z
Bobacka J, Lindfors T, McCarrick M et al (1995) Single-piece all-solid-state ion-selective electrode. Anal Chem 67:3819–3823
Bobacka J, Ivaska A, Lewenstam A (1999) Plasticizer-free all-solid-state potassium-selective electrode based on poly(3-octylthiophene) and valinomycin. Anal Chim Acta 385:195–202. https://doi.org/10.1016/S0003-2670(98)00667-9
Bobacka J, Ivaska A, Lewenstam A (2008) Potentiometric ion sensors. Chem Rev 108:329–351. https://doi.org/10.1021/cr068100w
Boeva ZA, Lindfors T (2016) Few-layer graphene and polyaniline composite as ion-to-electron transducer in silicone rubber solid-contact ion-selective electrodes. Sens Actuators B Chem 224:624–631. https://doi.org/10.1016/j.snb.2015.10.054
Cadogan A, Gao Z, Lewenstam A et al (1992) All-solid-state sodium-selective electrode based on a calixarene ionophore in a poly(vinyl chloride) membrane with a polypyrrole solid contact. Anal Chem 64:2496–2501. https://doi.org/10.1021/ac00045a007
Cattrall RW, Freiser H (1971) Coated wire ion selective electrodes. Anal Chem 43:1905–1906. https://doi.org/10.1021/ac60307a032
Coleman JN, Khan U, Blau WJ, Gun’ko YK (2006) Small but strong: a review of the mechanical properties of carbon nanotube–polymer composites. Carbon 44:1624–1652. https://doi.org/10.1016/j.carbon.2006.02.038
Crespo GA (2017) Recent advances in ion-selective membrane electrodes for in situ environmental water analysis. Electrochim Acta 245:1023–1034. https://doi.org/10.1016/j.electacta.2017.05.159
Crespo GA, Macho S, Rius FX (2008) Ion-selective electrodes using carbon nanotubes as ion-to-electron transducers. Anal Chem 80:1316–1322. https://doi.org/10.1021/ac071156l
Cuartero M, Crespo GA (2018) All-solid-state potentiometric sensors: a new wave for in situ aquatic research. Curr Opin Electrochem 10:98–106. https://doi.org/10.1016/j.coelec.2018.04.004
Cuartero M, Bishop J, Walker R et al (2016) Evidence of double layer/capacitive charging in carbon nanomaterial-based solid contact polymeric ion-selective electrodes. Chem Commun 52:9703–9706. https://doi.org/10.1039/c6cc04876e
De Marco R, Clarke G, Pejcic B (2007) Ion-selective electrode potentiometry in environmental analysis. Electroanalysis 19:1987–2001. https://doi.org/10.1002/elan.200703916
Fan Y, Xu C, Wang R et al (2017) Determination of copper(II) ion in food using an ionic liquids-carbon nanotubes-based ion-selective electrode. J Food Compos Anal 62:63–68. https://doi.org/10.1016/j.jfca.2017.05.003
Fibbioli M, Morf WE, Badertscher M et al (2000) Potential drifts of solid-contacted ion-selective electrodes due to zero-current ion fluxes through the sensor membrane. Electroanalysis 12:1286–1292. https://doi.org/10.1002/1521-4109(200011)12:16%3c1286::AID-ELAN1286%3e3.0.CO;2-Q
Fierke MA, Lai CZ, Bühlmann P, Stein A (2010) Effects of architecture and surface chemistry of three-dimensionally ordered macroporous carbon solid contacts on performance of ion-selective electrodes. Anal Chem 82:680–688. https://doi.org/10.1021/ac902222n
Fouskaki M, Chaniotakis N (2008) Fullerene-based electrochemical buffer layer for ion-selective electrodes. Analyst 133:1072–1075. https://doi.org/10.1039/b719759d
Ghaedi M, Jaberi SYS, Hajati S et al (2015) CuO nanoparticles intermixed with chemically modified multiwalled carbon nanotubes as a novel electrode for Cu2+ ion determination. IEEE Sens J 15:2882–2890. https://doi.org/10.1109/jsen.2014.2374773
Ghosh T, Chung HJ, Rieger J (2017) All-solid-state sodium-selective electrode with a solid contact of chitosan/prussian blue nanocomposite. Sensors (switzerland) 17:2536. https://doi.org/10.3390/s17112536
Han WS, Lee YH, Jung KJ et al (2008) Potassium ion-selective polyaniline solid-contact electrodes based on 4′,4″(5″)-di-tert-butyldibenzo-18-crown-6-ether ionophore. J Anal Chem 63:987–993. https://doi.org/10.1134/S1061934808100110
Hassan SSM, Eldin AG, Amr AEGE et al (2019) Improved solid-contact nitrate ion selective electrodes based on multi-walled carbon nanotubes (MWCNTs) as an ion-to-electron transducer. Sensors 19:3891. https://doi.org/10.3390/s19183891
Horvai G, GráF E, TóTh K et al (1986) Plasticized poly(vinyl chloride) properties and characteristics of valinomycin electrodes. 1. High-frequency resistances and dielectric properties. Anal Chem 58:2735–2740. https://doi.org/10.1021/ac00126a034
Hu J, Stein A, Bühlmann P (2016) Rational design of all-solid-state ion-selective electrodes and reference electrodes. Trends Anal Chem 76:102–114. https://doi.org/10.1016/j.trac.2015.11.004
Huang Y, Li J, Yin T et al (2015) A novel all-solid-state ammonium electrode with polyaniline and copolymer of aniline/2,5-dimethoxyaniline as transducers. J Electroanal Chem 741:87–92. https://doi.org/10.1016/j.jelechem.2014.12.041
Inczedy J (1979) Complexation equilibria in aqueous solutions. PWN, Warsaw
Kałuza D, Jaworska E, Mazur M et al (2019) Multiwalled carbon nanotubes-poly(3-octylthiophene-2,5-diyl) nanocomposite transducer for ion-selective electrodes: Raman spectroscopy insight into the transducer/membrane interface. Anal Chem 91:9010–9017. https://doi.org/10.1021/acs.analchem.9b01286
Khun K, Ibupoto ZH, Willander M (2013) Urea assisted synthesis of flower like CuO nanostructures and their chemical sensing application for the determination of cadmium ions. Electroanalysis 25:1425–1432. https://doi.org/10.1002/elan.201200660
Kisiel A, Marcisz H, Michalska A, Maksymiuk K (2005) All-solid-state reference electrodes based on conducting polymers. Analyst 130:1655–1662. https://doi.org/10.1039/b510868c
Koncki R (2007) Recent developments in potentiometric biosensors for biomedical analysis. Anal Chim Acta 599:7–15. https://doi.org/10.1016/j.aca.2007.08.003
Lenar N, Piech R, Paczosa-Bator B (2022) Hydrous cerium dioxide-based materials as solid-contact layers in potassium-selective electrodes. Membranes 12:349. https://doi.org/10.3390/membranes12040349
Li F, Ye J, Zhou M et al (2012) All-solid-state potassium-selective electrode using graphene as the solid contact. Analyst 137:618–623. https://doi.org/10.1039/c1an15705a
Li J, Yin T, Qin W (2017) An effective solid contact for an all-solid-state polymeric membrane Cd2+-selective electrode: three-dimensional porous graphene-mesoporous platinum nanoparticle composite. Sens Actuators B Chem 239:438–446. https://doi.org/10.1016/j.snb.2016.08.008
Liang R, Yin T, Qin W (2015) A simple approach for fabricating solid-contact ion-selective electrodes using nanomaterials as transducers. Anal Chim Acta 853:291–296. https://doi.org/10.1016/j.aca.2014.10.033
Lindfors T (2009) Light sensitivity and potential stability of electrically conducting polymers commonly used in solid contact ion-selective electrodes. J Solid State Electrochem 13:77–89. https://doi.org/10.1007/s10008-008-0561-z
Liu Y, Liu Y, Yan R et al (2020) Bimetallic AuCu nanoparticles coupled with multi-walled carbon nanotubes as ion-to-electron transducers in solid-contact potentiometric sensors. Electrochim Acta 331:135370. https://doi.org/10.1016/j.electacta.2019.135370
Mousavi Z, Teter A, Lewenstam A et al (2011) Comparison of multi-walled carbon nanotubes and poly(3-octylthiophene) as ion-to-electron transducers in all-solid-state potassium ion-selective electrodes. Electroanalysis 23:1352–1358. https://doi.org/10.1002/elan.201000747
Najafi M, Maleki L, Rafati AA (2011) Novel surfactant selective electrochemical sensors based on single walled carbon nanotubes. J Mol Liq 159:226–229. https://doi.org/10.1016/j.molliq.2011.01.013
O’Neil GD, Buiculescu R, Kounaves SP, Chaniotakis NA (2011) Carbon-nanofiber-based nanocomposite membrane as a highly stable solid-state junction for reference electrodes. Anal Chem 83:5749–5753. https://doi.org/10.1021/ac201072u
Paczosa-Bator B (2012) All-solid-state selective electrodes using carbon black. Talanta 93:424–427. https://doi.org/10.1016/j.talanta.2012.02.013
Paczosa-Bator B, Piech R, Wardak C, Cabaj L (2018) Application of graphene supporting platinum nanoparticles layer in electrochemical sensors with potentiometric and voltammetric detection. Ionics 24:2455–2464. https://doi.org/10.1007/s11581-017-2356-7
Parra EJ, Crespo GA, Riu J et al (2009) Ion-selective electrodes using multi-walled carbon nanotubes as ion-to-electron transducers for the detection of perchlorate. Analyst 134:1905–1910. https://doi.org/10.1039/b908224g
Parrilla M, Cuartero M, Crespo GA (2019) Wearable potentiometric ion sensors. Trends Anal Chem 110:303–320. https://doi.org/10.1016/j.trac.2018.11.024
Piȩk M, Piech R, Paczosa-Bator B (2016) All-solid-state nitrate selective electrode with graphene/tetrathiafulvalene nanocomposite as high redox and double layer capacitance solid contact. Electrochim Acta 210:407–414. https://doi.org/10.1016/j.electacta.2016.05.170
Pietrzak K, Wardak C, Malinowski S (2021) Application of polyaniline nanofibers for the construction of nitrate all-solid-state ion-selective electrodes. Appl Nanosci. https://doi.org/10.1007/s13204-021-02228-1
Pietrzak K, Wardak C (2021) Comparative study of nitrate all solid state ion-selective electrode based on multiwalled carbon nanotubes-ionic liquid nanocomposite. Sens Actuators B Chem 348:130720. https://doi.org/10.1016/j.snb.2021.130720
Pietrzak K, Krstulović N, Blažeka D et al (2022a) Metal oxide nanoparticles as solid contact in ion-selective electrodes sensitive to potassium ions. Talanta 243:123335. https://doi.org/10.1016/j.talanta.2022.123335
Pietrzak K, Morawska K, Malinowski S, Wardak C (2022b) Chloride ion-selective electrode with solid-contact based on polyaniline nanofibers and multiwalled carbon nanotubes nanocomposite. Membranes 12:1
Ping J, Wang Y, Wu J, Ying Y (2011) Development of an all-solid-state potassium ion-selective electrode using graphene as the solid-contact transducer. Electrochem Commun 13:1529–1532. https://doi.org/10.1016/j.elecom.2011.10.018
Ping J, Wang Y, Ying Y, Wu J (2012) Application of electrochemically reduced graphene oxide on screen-printed ion-selective electrode. Anal Chem 84:3473–3479. https://doi.org/10.1021/ac203480z
Rzewuska A, Wojciechowski M, Bulska E et al (2008) Composite polyacrylate-poly(3,4-ethylenedioxythiophene) membranes for improved all-solid-state ion-selective sensors. Anal Chem 80:321–327. https://doi.org/10.1021/ac070866o
Suni II (2008) Impedance methods for electrochemical sensors using nanomaterials. Trends Anal Chem 27:604–611. https://doi.org/10.1016/j.trac.2008.03.012
Sutter J, Radu A, Peper S et al (2004) Solid-contact polymeric membrane electrodes with detection limits in the subnanomolar range. Anal Chim Acta 523:53–59. https://doi.org/10.1016/j.aca.2004.07.016
Szigeti Z, Bitter I, Toth K et al (2005) A novel polymeric membrane electrode for the potentiometric analysis of Cu2+ in drinking water. Anal Chim Acta 532:129–136. https://doi.org/10.1016/j.aca.2004.10.061
Topcu C, Lacin G, Yilmaz V et al (2018) Electrochemical determination of copper(II) in water samples using a novel ion-selective electrode based on a graphite oxide—imprinted polymer composite. Anal Lett 51:1890–1910. https://doi.org/10.1080/00032719.2017.1395035
Vázquez M, Bobacka J, Ivaska A, Lewenstam A (2002) Influence of oxygen and carbon dioxide on the electrochemical stability of poly(3,4-ethylenedioxythiophene) used as ion-to-electron transducer in all-solid-state ion-selective electrodes. Sens Actuators B Chem 82:7–13. https://doi.org/10.1016/S0925-4005(01)00983-2
Veder JP, De Marco R, Patel K et al (2013) Evidence for a surface confined ion-to-electron transduction reaction in solid-contact ion-selective electrodes based on poly(3-octylthiophene). Anal Chem 85:10495–10502. https://doi.org/10.1021/ac4024999
Wardak C (2015) Solid contact cadmium ion-selective electrode based on ionic liquid and carbon nanotubes. Sens Actuators B Chem 209:131–137
Wardak C, Pietrzak K, Grabarczyk M (2021) Ionic liquid-multiwalled carbon nanotubes nanocomposite based all solid state ion-selective electrode for the determination of copper in water samples. Water 13:2869. https://doi.org/10.3390/w13202869
WHO (2008) Copper in: guidelines for drinking-water quality, vol 3. WWHO Press, Genewa, pp 335–336
Yin T, Qin W (2013) Applications of nanomaterials in potentiometric sensors. Trends Anal Chem 51:79–86. https://doi.org/10.1016/j.trac.2013.06.009
Yin T, Han T, Li C et al (2020) Real-time monitoring of the dissolution of silver nanoparticles by using a solid-contact Ag+-selective electrode. Anal Chim Acta 1101:50–57. https://doi.org/10.1016/j.aca.2019.12.022
Zhang Y, Tao Y, Wang K et al (2021) Two kinds of polyaniline fiber photo sensor with interdigital electrode and flexible hydrogel. J Appl Polym Sci 138:1–12. https://doi.org/10.1002/app.50628
Acknowledgements
We acknowledge support from the Polish Ministry of Science and Higher Education.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
On behalf of all authors, the corresponding author states that there is no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Wardak, C., Pietrzak, K. & Morawska, K. Nanocomposite of copper oxide nanoparticles and multi-walled carbon nanotubes as a solid contact of a copper-sensitive ion-selective electrode: intermediate layer or membrane component–comparative studies. Appl Nanosci 13, 7017–7028 (2023). https://doi.org/10.1007/s13204-023-02846-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13204-023-02846-x