Abstract
In this study, nano-crystalline lead sulfide (PbS) and lead oxide (PbO) were synthesized using hassle-free and cost-effective chemical route. Lead oxalate (PbC2O4) precursor was thermally decomposed to obtain the nano-crystalline PbO, while PbS nanoparticles were synthesized by microwave irradiation on a mixture of PbC2O4 precursor and sodium thiosulfate. Resulting materials were characterized by X-ray diffraction (XRD), scanning electron microscopy (SEM), Fourier transform infrared spectroscopy, ultraviolet–visible (UV–Vis) spectrophotometry, differential scanning calorimetry (DSC), and thermogravimetric analysis (TGA). XRD confirmed the tetragonal structure for PbO and face-centered cubic for PbS with average crystallite sizes varying from 20 to 30 nm for both materials. From UV–Vis spectra, direct band gap energies were calculated to be 2.51 and 2.23 eV for PbO and PbS, respectively. Various decomposition stages during heat treatment of PbO and PbS, as revealed by TGA/DSC, are discussed in detail.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
In recent times, semiconducting nanomaterials have attracted lot of attention due to their commendable physical and chemical properties which can be tuned by adjusting their shape, size, and surface morphology (Chan 1998; Chang et al. 2004; Milliron et al. 2004; Murray et al. 1993). These properties depend directly on the crystal structure of the material which can be controlled by manipulating thermodynamic and kinetic processes involved in the crystal growth. Therefore, studies relating to the crystal structure and properties are essential to realize potential applications these materials have to offer. Lead-based compounds demonstrate interesting chemical and physical characteristics and are widely used in many applications such as FE transistor, photoresistors, diode, lasers, optical detectors, hybrid organic solar cells, and imaging-labeling applications (Xiao et al. 2013; Patil et al. 2006; Ikram et al. 2014, 2016; So et al. 2017). Lead oxide, which is one of the basic lead compounds, exists in different stoichiometric forms depending on the lead-to-oxygen ratio, e.g., PbO, PbO2, Pb2O3, Pb3O4, and Pb9O12. Among these, PbO, a direct band gap semiconductor having two polymorphs α-PbO and β-PbO with band gap energies of 2.2 and 2.5 eV, respectively, has attracted a greater focus due its exceptional mechanical, electronic, and optical properties (Karami and Alipour 2009; Lafront et al. 2010; Martos et al. 2001; Xi et al. 2004). Another important lead compound is PbS. It is a subject of growing attention due to its exclusive properties associated with size quantization. Bulk PbS has cubic crystal structure and a small direct band gap of 0.41 eV at 300 K and a large Bohr radius (around 18 nm). A blend of these extraordinary properties makes PbS a very promising candidate for electroluminescent devices (Wang et al. 1987). Recent approaches for the synthesis of PbO and PbS nanostructures include sonochemical methods, thermal decomposition, self-organization (Leontidis 2003), hydrothermal synthesis (Li et al. 2009), homogeneous hydrolysis (Li et al. 2007), electro deposition (Sharon et al. 1997), and microwave heating (Ding et al. 2003; Sun et al. 2009). Utilization of PbO and PbS nanostructures for potential applications is strongly subjected to their thermal stability in air and inert atmosphere. Structural phase transitions, oxidation, and recrystallization occur rapidly in metal sulfides upon small increments of temperature (Nafees et al. 2011, 2012, 2015; Qadri et al. 2003; Sadovnikov et al. 2009, 2011; Sadovnikov and Rempel 2009). Therefore, a better understanding of oxidation temperature/resistance and thermal stability of PbO and PbS is crucial for realizing potential applications these materials promise. Still, sufficient literature on these issues is not available.
In this work, we report the simple and cost-effective aqueous medium-based chemical method to synthesize PbO and PbS using a single precursor. Thermal stability and various transitions/reactions occurring in nanostructured PbO and PbS during heat treatment in air and inert atmosphere are also discussed in detail.
Experimental work
Materials
Analytical grade pure lead (II) chloride (PbCl2), sodium thiosulfate Na2S2O3, and oxalic acid (H2C2O4) were purchased from Unichem Laboratories Ltd. Absolute ethanol and distilled water were used to wash the products. All chemicals were used without any further purification.
Preparation of lead oxalate precursor
Separate solutions of desired molarity were prepared by dissolving PbCl2 and H2C2O4 in distilled water under vigorous stirring at room temperature then these solutions were mixed dropwise under the same condition. White precipitate of lead oxalate (PbC2O4) was produced which was collected and washed with absolute ethanol and distilled water several times to remove the traces of impurities. As prepared PbC2O4 was dried by aging for 7 h at 60 °C.
Preparation of lead oxide
Dry PbC2O4 was heated for 3 h at 425 °C in a muffle furnace with the ramp rate of 20 °C min−1. After cooling naturally to room temperature (RT), red-colored PbO was formed and collected for characterization.
Chemical reactions involved in the synthesis are:
Preparation of lead sulfide
For the synthesis of PbS nano-crystalline material, solution of required molarity was prepared by dissolving Na2S2O3 in distilled water before the addition of as prepared PbC2O4 precursor under stirring.
After 2 h of stirring, resulting solution was heated/irradiated in microwave oven (2.45 GHz, 1000 W) for 40 min with 20% power. Upon heating, the black precipitate was formed then filtered and washed with distilled water and absolute ethanol before being dried at room temperature.
The reaction between PbC2O4 and Na2S2O3 involves multiple steps similar to those reported for CuS synthesis (Nafees et al. 2012, 2015). At first, S2O3 2− ions from Na2S2O3 react with water molecules to form H2S gas which, upon reacting with Pb2+ ions from PbC2O4, results in the formation of PbS nanoparticles, further aggregated to the nano-crystalline material. A simplified reaction scheme is presented in Fig. 1.
Characterization
Structural and phase information on PbO and PbS nanoparticles were obtained using PANalytical X’Pert PRO X-ray diffractometer with Cu Kα radiation (λ = 0.15418 nm). X-ray diffraction (XRD) patterns were recorded in the 2θ range of 20–75 using a step size of 0.05° s−1. Morphology and microstructure of the synthesized materials were probed using field emission scanning electron microscope (SEM, JOEL JSM-6480). Differential scanning calorimeter (DSC) and thermogravimetric analysis (TGA) were performed by TGA, SDT Q600 (TA Instrument) in controlled environment to observe the mass loss/increment, thermal phase transitions, and thermal decomposition/oxidation in PbS nanomaterial. Thermal analysis was carried out using TA Universal Analysis 2000. Fourier transform infrared (FTIR) spectra were recorded from 4000 to 400 cm−1 using a PerkinElmer spectrum two universal attenuated total reflectance Fourier transform infrared (UATR–FTIR) spectrometer. Ultraviolet–visible (UV–Vis) spectra were recorded using Genesys 10 s UV–Vis spectrophotometer.
Results and discussion
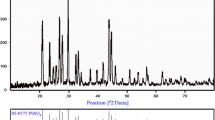
XRD patterns of nano-crystalline PbO, PbS, and PbC2O4 are represented in Fig. 2. It can be observed that all synthesized products demonstrate high crystallinity and match well with reported patterns as mentioned in JCPDS card numbers (PbC2O4, 00-014-0803; PbO, 01-085-1414; PbS, 03-065-2935). XRD pattern of PbO (Fig. 2c) is indexed as tetragonal, a very small peak present at 30.25 (2θ) shows the presence of orthorhombic phase. Long heating time required to decompose PbC2O4 precursor results in the phase change and a corresponding appearance of the additional peak. PbS crystallizes in face-centered cubic crystal system with the presence of no other phases. Average crystallite size for PbO and PbS was found to be around 20–30 nm as calculated by Scherer’s formula. Optical absorbance and band gap energies for PbO and PbS are shown in Fig. 3. Both the PbO and PbS absorb in ultraviolet and visible regions with respective band gap energies of 2.51 and 2.23.
The optical absorption with band gap energies of PbO and PbS are shown in Fig. 3a, b, respectively. The PbO absorption was in UV as well as in visible region while PbS absorption was maximum around 375 nm (Fig. 3a) and their corresponding band gap energies were calculated using a Tauc plot, shows (αhν)2 vs hν for the nanoparticles. The intersection of the straight line with x-axis indicates that the calculated band gap energy (E g) for PbO and PbS was 2.95 and 2.15 eV, respectively.
Figure 4 shows the energy dispersive X-ray spectroscopy (EDX) results of as prepared PbS and PbO. Figure 4a indicates that PbS sample contains Pb, S, Na, and O; the presence of Na and O corresponds to Na2S2O3 which remained unreactive during synthesis. EDX spectrum of PbO consists of peaks corresponding to Pb and O. Absence of additional peaks confirms the high purity of the sample.
Surface morphology and microstructure of PbS and PbO were probed by field emission scanning electron microscopy (FESEM) images of PbO and PbS as shown in Fig. 5. It is clear that PbS nanoparticles exist in cubic geometry with significant agglomeration as shown in Fig. 5a, b. The increase in particle size occurs mainly due to aggregation of nanoparticles depending on reaction parameters like reaction time, microwave power, and concentration of precursors. Particle size and shape can be controlled by tuning these parameters.
Figure 5c, d reveals the presence of very fine PbO nanoparticles. Amalgamation of these nanoparticles leads to the formation of porous structures with very narrow pore size in the 40–100 nm range.
Fourier transform infrared (FTIR) spectra of PbO and PbS nanoparticles are shown in Fig. 6. The board peak around 3400 cm−1 corresponds to (O–H) stretching vibrations originating due to small amount of ethanol used in washing the samples. A sharp peak at 1705 cm−1, in both graphs is assigned to vibrations of the carbonyl group (C=O), while another peak at 1395 cm−1 is recognized as stretching vibration of carboxyl group (C–O). Peaks lying in the 300–900 cm−1 are identified as metal–oxygen (M–O) stretching vibrations. Although the FTIR spectra of PbO and PbS are identical, a well-defined peak around 600 cm−1 in PbS spectra is assigned to (S–S) stretching vibrations (Bakshi et al. 2007; Senvaitiene et al. 2007; Timar and Lucăcel-Ciceo 2008).
Differential scanning calorimetry and thermogravimetric analysis (DSC/TGA) curves of PbC2O4 precursor recorded in air are shown in Fig. 7. Sample was heated from room temperature to 500 °C with the ramp rate of 10 °C min−1. No significant mass loss was observed up to 325 °C indicating that sample was free from moisture and solvent molecules. A notable mass loss (26%) occurs between 350 and 400 °C due to decomposition of Pb2C2O4 to PbO with a corresponding endothermic peak appearing at 390 °C.
For PbO, DSC/TGA curves were recorded from room temperature (RT) to 1175 °C in air with the ramp rate of 10 °C min−1. A mass loss of almost 2% occurs up to 500 °C as shown in Fig. 7. This initial mass loss involves multiple transitions occurring during the heating process (Nafees et al. 2011, 2012). As observed from differential thermogravimetric (DTGA) curve, dehydration takes place first resulting in the removal of water of crystallization around 100 °C while a hump between 200 and 250 °C corresponds to endothermic nature of transition confirmed by DSC curve. The inorganic compound PbO contains minute quantities of PbO2; thermal decomposition from PbO2 to PbO occurs in many steps from 290 to 650 °C accompanied by endothermic mass losses in designated temperatures. Decomposition of PbO2 to PbO proceeds as follows (Greenwood and Earnshaw 1997; White and Roy 1964):
A sharp endothermic peak observed in DSC curve at 880 °C indicates melting of PbO without the involvement of any mass loss as supported by TGA and DTGA curves. Around 1100 °C, another endothermic mass loss was observed most probably due to partial evaporation of very small PbO nanoparticles. A small percentage of mass loss (2.7%) from RT to 1175 °C indicates excellent thermal stability of PbO nanoparticles.
Figure 8 shows the DSC/TGA analysis of PbS at temperature range 25–1000 °C with the ramp rate of 10 °C min−1 in argon and air atmosphere. TGA curve indicates an initial mass loss of about 20% from 50 to 300 °C. This is confirmed by DTGA curve which reveals three peaks corresponding to vaporization of water and ethanol contents, removal of water of crystallization and liberation of sulfur at 140, 215, and 290 °C, respectively. The second mass loss from 600 to 900 °C is attributed to evaporation of sulfur and production of metallic lead Pb with associated endothermic and DTGA peaks appearing at 810 and 880 °C.
Almost identical behavior was observed in air atmosphere from 25 to 280 °C. In DSC curve, a peak around 290 °C corresponds to the exothermic reaction of PbS with oxygen yielding SO2 and PbO as confirmed by small peak at 310 °C in DTGA curve. TGA graph shows mass gain from 450 to 700 °C which is attributed to exothermic reaction at 460 °C producing PbSO4 and PbO. This is also confirmed by DSC and TGA curves. Furthermore, PbSO4 decomposes from 750 to 1000 °C to Pb. This can be represented in terms of chemical reactions as follows:
Conclusions
In this study, cost-effective and low-temperature synthesis of single Pb2C2O4 precursor was reported, which is used to synthesize PbO nano-crystalline material by heating PbC2O4 at 425 °C and PbS nanostructured material was produced by microwave irradiation (2.45 GHz, 1000 W, 40 min) on aqueous solution prepared with Na2S2O3 and PbC2O4 precursor. This synthesis process demands effective and proper washing to remove byproducts.
The formation of PbO and PbS was confirmed by XRD, which reveals high crystalline nature of both materials with a crystallite size of 20–30 nm. Optical band gap was found to be 2.15 and 2.95 eV for PbS and PbO, respectively, using UV–Vis measurements. EDX data vet the presence of Pb, S, and O in the synthesized samples. Surface morphology of PbS nanoparticles was found to be cubic with many particles of sizes less than 100 nm, while PbO features highly porous surface with very small pore sizes varying from 40 to 100 nm. PbS demonstrates excellent thermal behavior with complete decomposition involving many steps; by heating PbS in air, system absorbs heat thereby releasing SO2 with the production of PbO followed by exothermic conversion to PbSO4 and metallic Pb at higher temperatures from 700 to 1000 °C, simultaneously endothermic decomposition of lead sulfate takes place. No mass increment was detected in argon atmosphere due to the absence of O2. It was noted that small PbS particles react at low temperature compared with the large size. The proposed research can be used for the cost-effective synthesis of PbS, PbO, and related materials.
References
Bakshi MS, Thakur P, Sachar S, Kaur G, Banipal TS, Possmayer F, Petersen NO (2007) Aqueous phase surfactant selective shape controlled synthesis of lead sulfide nanocrystals. J Phys Chem C 111:18087–18098
Chan WC (1998) Quantum dot bioconjugates for ultrasensitive nonisotopic detection. Science 281:2016–2018
Chang T-WF, Musikhin S, Bakueva L, Levina L, Hines MA, Cyr PW, Sargent EH (2004) Efficient excitation transfer from polymer to nanocrystals. Appl Phys Lett 84:4295
Ding T, Zhang J-R, Long S, Zhu J-J (2003) Synthesis of HgS and PbS nanocrystals in a polyol solvent by microwave heating. Microelectron Eng 66:46–52
Greenwood NN, Earnshaw A (1997) Chemistry of the elements, 2nd edn. Reed Educational and Professional, Oxford
Ikram M, Murray R, Hussain A, Ali S, Shah SI (2014) Hybrid organic solar cells using both ZnO and PCBM as electron acceptor materials. Mater Sci Eng B 189:64–69
Ikram M, Murray R, Imran M, Ali S, Shah SI (2016) Enhanced performance of P3HT/(PCBM:ZnO:TiO2) blend based hybrid organic solar cells. Mater Res Bull 75:35–40
Karami H, Alipour M (2009) Investigation of organic expanders effects on the electrochemical behaviors of new synthesized nanostructured lead dioxide and commercial positive plates of lead-acid batteries. J Power Sourc 191:653–661
Lafront A-M, Zhang W, Ghali E, Houlachi G (2010) Electrochemical noise studies of the corrosion behaviour of lead anodes during zinc electrowinning maintenance. Electrochim Acta 55:6665–6675
Leontidis E (2003) Composite nanotubes formed by self-assembly of PbS nanoparticles. Nano Lett 3:569–572
Li C, Shi G, Xu H, Guang S, Yin R, Song Y (2007) Nonlinear optical properties of the PbS nanorods synthesized via surfactant-assisted hydrolysis. Mater Lett 61:1809–1811
Li F, Huang X, Kong T, Liu X, Qin Q, Li Z (2009) Synthesis and characterization of PbS crystals via a solvothermal route. J Alloys Compd 485:554–560
Martos M, Morales J, Sánchez L, Ayouchi R, Leinen D, Martin F, Barrado JRR (2001) Electrochemical properties of lead oxide films obtained by spray pyrolysis as negative electrodes for lithium secondary batteries. Electrochim Acta 46:2939–2948
Milliron DJ, Hughes SM, Cui Y, Manna L, Li J, Wang L-W, Alivisatos AP (2004) Colloidal nanocrystal heterostructures with linear and branched topology. Nature 430:190–195
Murray CB, Norris DJ, Bawendi MG (1993) Synthesis and characterization of nearly monodisperse CdE (E = sulfur, selenium, tellurium) semiconductor nanocrystallites. J Am Chem Soc 115:8706–8715
Nafees M, Ali S, Rasheed K, Idrees S (2011) The novel and economical way to synthesize CuS nanomaterial of different morphologies by aqueous medium employing microwaves irradiation. Appl Nanosci 2:157–162
Nafees M, Ali S, Idrees S, Rashid K, Shafique MA (2012) A simple microwave assists aqueous route to synthesis CuS nanoparticles and further aggregation to spherical shape. Appl Nanosci 3:119–124
Nafees M, Ikram M, Ali S (2015) Thermal behavior and decomposition of copper sulfide nanomaterial synthesized by aqueous sol method. Dig J Nanomater Biostruct 10:635–641
Patil RS, Pathan HM, Gujar TP, Lokhande CD (2006) Characterization of chemically deposited nanocrystalline PbS thin films. J Mater Sci 41:5723–5725
Qadri SB, Singh A, Yousuf M (2003) Structural stability of PbS films as a function of temperature. Thin Solid Films 431–432:506–510
Sadovnikov SI, Rempel AA (2009) Nonstoichiometric distribution of sulfur atoms in lead sulfide structure. Dokl Phys Chem 428:167–171
Sadovnikov SI, Gusev AI, Rempel AA (2009) New crystalline phase in thin lead sulfide films. JETP Lett 89:238–243
Sadovnikov SI, Kozhevnikova NS, Rempel AA (2011) Oxidation of nanocrystalline lead sulfide in air. Russ J Inorg Chem 56:1864–1869
Senvaitiene A, Smirnova J, Beganskiene J, Kareiva A (2007) XRD and FTIR characterisation of lead oxide-based pigments and glazes. Acta Chim Slov 54:185
Sharon M, Ramaiah KS, Kumar M, Neumann-Spallart M, Levy-Clement C (1997) Electrodeposition of lead sulphide in acidic medium. J Electroanal Chem 436:49–52
So B, Heo J, Liu C, Ju S, Han W-T (2017) Formation of channels containing lead sulfide quantum dots using continuous-wave laser for active planar waveguides in glasses. Opt Mater Express 7:281–285
Sun J-Q, Shen X-P, Guo L-J, Chen K-M, Liu Q (2009) Microwave-assisted synthesis of flower-like PbS crystals. Phys E Low Dimens Syst Nanostruct 41:1527–1532
Timar V, Lucăcel-Ciceo R (2008) Structural studies of iron doped 3B2O3·0.7PbO·0.3Ag2O glasses by FT-IR and Raman spectroscopies. Semicond Phys Quantum Electron Optoelectron 11:221–225
Wang Y, Suna A, Mahler W, Kasowski R (1987) PbS in polymers. From molecules to bulk solids. J Chem Phys 87:7315
White WB, Roy R (1964) Phase relations in the system lead-oxygen. J Am Ceram Soc 47:242–249
Xi G, Peng Y, Xu L, Zhang M, Yu W, Qian Y (2004) Selected-control synthesis of PbO2 submicrometer-sized hollow spheres and Pb3O4 microtubes. Inorg Chem Commun 7:607–610
Xiao G, Wang Y, Ning J, Wei Y, Liu B, Yu WW, Zou G, Zou B (2013) Recent advances in IV–VI semiconductor nanocrystals: synthesis, mechanism, and applications. RSC Adv 3:8104–8130
Acknowledgements
The authors acknowledge Higher Education Commission, Pakistan, for financial support through “Indigenous Ph.D. Fellowship Program (5000 Fellowships)”.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Nafees, M., Ikram, M. & Ali, S. Thermal stability of lead sulfide and lead oxide nano-crystalline materials. Appl Nanosci 7, 399–406 (2017). https://doi.org/10.1007/s13204-017-0578-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13204-017-0578-7