Abstract
Abstract
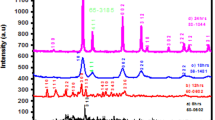
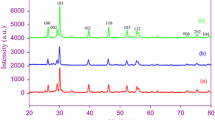
A facile sol–gel technique was employed to synthesize lanthanum oxide nanoparticles (hereafter La2O3 NPs) using micro-sized La2O3 powders, 20% nitric acid, and high-molecular weight polyethylene glycol (PEG) as raw materials. The synthesized La2O3 NPs were calcined at 750, 900, and 1000 °C in air for 2 h. The calcined products were characterised using numerous experimental techniques, namely X-ray diffraction (XRD), scanning electron microscopy (SEM), energy-dispersive X-ray spectroscopy (EDXS), thermogravimetric analysis (TGA), differential scanning calorimetry (DSC), Fourier-transform infrared (FTIR) spectroscopy, and photoluminescence (PL) spectroscopy. The experimental results indicated that the calcination temperatures have remarkable effects on the crystallinity, particle size, and lattice strains of the La2O3 NPs. The XRD patterns confirmed the hexagonal phase of the La2O3 NPs with lattice constants: a = b = 0.3973, nm and c = 0.6129 nm. The average crystallite size of the La2O3 NPs estimated by electron miscroscopy was in good agreement with the XRD results. The degree of crystallinity, and the average crystallite size of the NPs were increased, while the lattice strains were decreased with the calcination temperatures. The photoluminescence spectra of nanoparticles illustrated a strong emission band at the vicinity of 364 nm, which is typically known to be the green band for La2O3 NPs.
Graphical abstract










Similar content being viewed by others
References
P. Huang, J. Li, S. Zhang, C. Chen, Y. Han, N. Liu, Y. Xiao, H. Wang, M. Zhang, Q. Yu, Y. Liu, W. Wang, Effects of lanthanum, cerium, and neodymium on the nuclei and mitochondria of hepatocytes: Accumulation and oxidative damage. Environ. Toxicol. Pharmacol. 31, 25–32 (2011)
Z.K. Bolaghi, S.M. Masoudpanah, M. Hasheminiasari, Photocatalytic properties of ZnO powders synthesized by conventional and microwave-assisted solution combustion method. J. Sol-Gel. Sci. Technol. 86, 711–718 (2018)
M.H. Oghaz, R.S. Razavi, M. Barekat, M. Naderi, S. Malekzadeh, M. Rezazadeh, Synthesis and characterization of Y2O3 nanoparticles by sol–gel process for transparent ceramics applications. J. Sol-Gel. Sci. Technol. 78, 682–691 (2016)
Y. Xin, Y. Qi, X. Ma, Z. Wang, Z. Zhang, S. Zhang, Rare-earth (Nd, Sm, Eu, Gd and Y) enhanced CeO2 solid solution nanorods prepared by co-precipitation without surfactants. Mater. Lett. 64, 2659–2662 (2010)
G. Oskam, Metal oxide nanoparticles: synthesis, characterization and application. J. Sol-Gel Sci Techn. 37, 161–164 (2006)
A.H. Lu, E.L. Salabas, F. Schüth, Magnetic nanoparticles: Synthesis, protection, functionalization, and application, Angew. Chem. Int. Ed. 46, 1222–1244 (2007)
J. Das, V.S. Moholkar, S. Chakma, Structural, magnetic and optical properties of sonochemically synthesized Zr-ferrite nanoparticles. Powder Technol. 328, 1–6 (2018)
C. Aubery, C. Solans, S. Prevost, M. Gradzielski, M. Sanchez-Dominguez, Microemulsions as reaction media for the synthesis of mixed oxide nanoparticles: Relationships between microemulsion structure, reactivity, and nanoparticle characteristics. Langmuir 29, 1779–1789 (2013)
N.C. Zheng, Z. Wang, J.Y. Long, L.J. Kong, D.Y. Chen, Z.Q. Liu, Shape-dependent adsorption of CeO2 nanostructures for superior organic dye removal. J. Colloid Interface Sci. 525, 225–233 (2018)
H. Abdulhamid, E. Fridell, M. Skoglundh, Influence of the type of reducing agent (H2, CO, C3 H6 and C3 H8) on the reduction of stored NOX in a Pt/BaO/Al2O3 model catalyst, 2010:161–168 (2004 )
S.F. Hasany, I. Ahmed, A. Rehman, Systematic review of the preparation techniques of iron oxide magnetic nanoparticles. Nanosci. Nanotechnol. 2, 148–158 (2012)
E.K. Goharshadi, S.H. Sajjadi, R. Mehrkhah, P. Nancarrow, Sonochemical synthesis and measurement of optical properties of zinc sulfide quantum dots. Chem. Eng. J. 209, 113–117 (2012)
E.K. Goharshadi, H. Azizi-Toupkanloo, Silver colloid nanoparticles: ultrasound-assisted synthesis, electrical and rheological properties. Powder Technol. 237, 97–101 (2013)
Y. Gao, Y. Masuda, K. Koumoto, Micropatterning of lanthanum-based oxide thin film on self-assembled monolayers. J. Colloid Interface Sci. 274, 392–397 (2004)
L. Zhang, L. Zhou, Q.X. Li, H. Liang, H. Qin, S. Masutani, B. Yoza, Toxicity of lanthanum oxide nanoparticles to the fungus Moniliella wahieum Y12T isolated from biodiesel. Chemosphere 199, 495–501 (2018)
J. Kang, Y. Kim, D.W. Cho, Y. Sohn, Synthesis and physicochemical properties of La(OH)3 and La2O3 nanostructures. Mater. Sci. Semicon. Proces. 40, 737–743 (2015)
S. Khanjani, A. Morsali, Synthesis and characterization of lanthanum oxide nanoparticles from thermolysis of nanostructured supramolecular compound. J. Mol. Liq. 153, 129–132 (2010)
J. Liu, G. Wang, L. Lu, Y. Guo, L. Yang, Facile shape-controlled synthesis of lanthanum oxide with different hierarchical micro/nanostructures for antibacterial activity based on phosphate removal. RSC Adv. 7, 40965–40972 (2017)
C.C. Li, M.J. Li, Y.P. Huang, Dispersion of aluminum-doped zinc oxide nanopowder with high solid content in ethylene glycol. Powder Technol. 327, 1–8 (2018)
A.V. Murugan, S.C. Navale, V. Ravi, Synthesis of nanocrystalline La2O3 powder at 100 °C. Mater. Lett. 60, 848–849 (2006)
A. Bahari, A. Anasari, Z. Rahmani, Low temperature synthesis of La2O3 and CrO2 by Sol—Gel process. J. Engi. Technol. Res. 3, 203–208 (2011)
S. Jafari Nejad, H. Abolghasemi, M.A. Moosavian, A. Golzary, M.G. Maragheh, Fractional factorial design for the optimization of hydrothermal synthesis of lanthanum oxide nanoparticles under supercritical water condition. J. Supercrit. Fluids 52, 292–297 (2010)
J. Sheng, S. Zhang, S. Lv, W. Sun, Surfactant-assisted synthesis and characterization of lanthanum oxide nanostructures. J. Mater. Sci. 42, 9565–9571 (2007)
M. Salavati-Niasari, G. Hosseinzadeh, F. Davar, Synthesis of lanthanum hydroxide and lanthanum oxide nanoparticles by sonochemical method. J. Alloys Compd. 509, 4098–4103 (2011)
M. Ranjbar, M. Yousefi, Synthesis and characterization of lanthanum oxide nanoparticles from thermolysis of nano-sized lanthanum(III) supramolecule as a novel precursor. J. Inorg. Organomet. Polym Mater. 24, 652–655 (2014)
S. Wang, Y. Zhao, J.Chen,R. Xu, L. Luo, S. Zhong, Self-assembled 3D La(OH)3 and La2O3 nanostructures: fast microwave synthesis and characterization. Superlattices Microstruct. 47, 597–605 (2010)
B. Tang, J. Ge, C. Wu, L. Zhuo, J. Niu, Z. Chen, Z. Shi, Y. Dong, Sol–solvothermal synthesis and microwave evolution of La(OH)3 nanorods to La2O3 nanorods This. Nanotechnol. 15, 1273–1276 (2004)
M.F. Vignolo, S. Duhalde, M. Bormioli, G. Quintana, Structural and electrical properties of lanthanum oxide thin films deposited by laser ablation. Appl. Surf. Sci. 197, 522–526 (2002)
M. Ghiasi, A. Malekzadeh, Synthesis, characterization and photocatalytic properties of lanthanum oxy-carbonate, lanthanum oxide and lanthanum hydroxide nanoparticles. Superlattices Microstruct. 77, 295–304 (2015)
Q.L. Zhang, Z.J. Ji, J. Zhou, X.C. Zhao, X.Z. Lan, Preparation of lanthanum oxide nanoparticles by chemical precipitation method. Mater. Sci. Forum 724, 233–236 (2012)
M.A. Farrukh, F. Imran, S. Ali, M.K. Rahmanand, I.I. Naqvi, Micelle assisted synthesis of La2O3 nanoparticles and their applications in photodegradation of bromophenol blue. Russian J. Appl. Chem. 88, 1523–1527 (2015)
C. Wang, Y. Yang, Z. Zhang, F. Liao, J. Ju, Z. Shi, J. Lin, Y. Li, F. Huang, Synthesis of nano-structured La2O3/La2O2CO3:Eu phosphors from arc-discharged graphene-contained composites. Mater. Lett. 134, 176–179 (2014)
Y. Xiao, Z. Feng, X. Huang, L. Huang, Z. Long, Q. Wang, Y. Hou, Synthesis of lanthanum oxide nanosheets by a green carbonation process. Chin. Sci. Bull. 59, 1864–1867 (2014)
M. Moothedan, K.B. Sherly, Synthesis, characterization and sorption studies of nano lanthanum oxide. J. Water Process Engi. 9, 29–37 (2016)
E.K. Goharshadi, T. Mahvelati, M. Yazdanbakhsh, Influence of preparation methods of microwave, sol–gel, and hydrothermal on structural and optical properties of lanthania nanoparticles. J. Iran. Chem. Soc. 13, 65–72 (2016)
X. Wang, M. Wang, H. Song, B. Ding, A simple sol-gel technique for preparing lanthanum oxide nanopowders. Mater. Lett. 60, 2261–2265 (2006)
M.S. Niasaria, G. Hosseinzadeh, F. Davar, Synthesis of lanthanum carbonate nanoparticles via sonochemical method for preparation of lanthanum hydroxide and lanthanum oxide nanoparticles. J. Alloys Compd. 509, 134–140 (2011)
Q. Zhou, H. Zhang, F. Chang, H. Li, H. Pan, W. Xue, D.Y. Hu, S. Yang, Nano La2O3 as a heterogeneous catalyst for biodiesel synthesis by transesterification of Jatropha curcas L. oil. J. Indust. Eng. Chem. 31, 385–392 (2015)
H. Kabir, S.H. Nandyala, M.M. Rahman, M.A. Kabir, Z. Pikramenou, M. Laver, A. Stamboulis, Polyethylene glycol assisted facile sol-gel synthesis of lanthanum oxide nanoparticles: Structural characterizations and photoluminescence studies. Ceram. Int. 45, 424–431 (2019)
G.Z. Jia, Y.F. Wang, J.H. Yao, Fabrication and strain investigation of ZnO nanorods on Si composing sol-gel and chemical bath deposition method. J. Phys. Chem. Solids 73, 495–498 (2012)
C. Hu, H. Liu, W. Dong, Y. Zhang, G. Bao, C. Lao, Z.L. Wang, La(OH)3 and La2O3 nanobelts—synthesis and physical properties. Adv. Mater 19, 470–474 (2007)
B.D. Cullity, Elements of X-ray diffraction, Second edn. (Addison-Wesley Company, Boston, 1978)
R. Jenkins, R.L. Snyder, Chemical Analysis: Introduction to ray Powder Diffractometry (Wiley, New York, 1996)
R. John, R. Rajakumari, Synthesis and characterization of rare earth ion doped nano ZnO. Nano-Micro Lett 4, 65–72 (2012)
F. Ozutok, B. Demirselcuk, E. Sarica, S. Turkyilmaz, V. Bilgin, Study of ultrasonically sprayed ZnO films: thermal annealing effect. Acta Phys. Polonica. A 121, 53–55 (2012)
D. Dickson, G. Liu, C. Li, G. Tachiev, Y. Cai, Dispersion and stability of bare hematite nanoparticles: Effect of dispersion tools, nanoparticle concentration, humic acid and ionic strength. Sci. Total Environ. 419, 170–177 (2012)
N. Zhang, R. Yi, L. Zhou, G. Gao, R. Shi, G. Qiu, X. Liu, Lanthanide hydroxide nanorods and their thermal decomposition to lanthanide oxide nanorods. Mater. Chem. Phys. 114, 160–167 (2009)
G. Wang, Y. Zhou, D.G. Evans, Y. Lin, Preparation of highly dispersed nano-La2O3 particles using modified carbon black as an agglomeration inhibitor. Ind. Eng. Chem. Res. 51, 14692–14699 (2012)
Acknowledgements
The author (H. Kabir) acknowledges gratefully the financial support of Bangladesh Government under the Bangabandhu Fellowship. He also would like to thank Jahangirnagar University, Bangladesh, for providing the required study leave to carry out this work at the Biomaterials Group in the School of Metallurgy and Materials, University of Birmingham, UK. M Mahbubur is also grateful to Jahangirnagar University and Murdoch University for providing logistic supports.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Kabir, H., Nandyala, S.H., Rahman, M.M. et al. Influence of calcination on the sol–gel synthesis of lanthanum oxide nanoparticles. Appl. Phys. A 124, 820 (2018). https://doi.org/10.1007/s00339-018-2246-5
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00339-018-2246-5