Abstract
The aggregation of CuS nanoparticles during synthesis by a hassle-free aqueous route under microwave irradiation gave remarkable spherical shape, utilizing Cu(CH3COO)2·H2O as the source of copper and Na2S2O3·5H2O, as sulfur source; these materials were used without assistance of any surfactant or template. An X-ray powder diffraction pattern proved that the product is hexagonal CuS phase. The morphologies of the product were observed by scanning electron microscopy. Thermal behavior, different solid state, and chemical conversion in CuS with respect to temperature were studied by DSC/TGA, which confirmed the thermal oxidation of CuS and its conversion into CuO then to Cu2O.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
For the past few years, researchers have been showing extensive concentration on the study of the appropriate control over the size and shape of nanoparticles. Nanomaterials show illustrious and exceptional chemical, optical, catalytic, magnetic, and electronic properties depending on the size of the nanoparticles (Shipway et al. 2000; Wong et al. 2002; Qingyi et al. 2002; Brust and Kiely 2002). Copper sulfide exist in various stoichiometric products including covellite (CuS), djurleite (Cu1.95S), digenite (Cu9S5), anilite (Cu7S4), and chalcocite (Cu2S) (Wuled Lenggoro et al. 1998). Copper sulfides are a predominantly attractive group of metal sulfides due to their use in widespread applications in fields such as semiconductors electrochemistry cells, IR detectors, sensors, and catalysts and solar energy conversion (Thongtem et al. 2007; Rodriguez et al. 2000; Chunyan et al. 2006; Kiely et al. 1998; Anuar et al. 2002; Nair and Nair 1989; Parkin 1996; Nascu et al. 1997). However, copper sulfide has multifarious crystal chemistry due to its capability to form a variety of stoichiometric copper sulfide compounds. Jumble phase copper sulfides are generally synthesized in many synthetic means (Yang and Xiang 2005; Zou et al. 2007; Tan et al. 2005; Zhu et al. 2007; Wang et al. 2007; Zhang et al. 2004; Nafees et al. 2011). Ni et al. (2004) reported the formation of CuS nanotubes using aqueous phase technique with different starting materials of diameter ranging from 350 to 1,000 nm. While we adopted an aqueous sol method to synthesize definite morphology of CuS nanomaterial with spherical structure of average diameter of 850 nm under microwave irradiation.
Experimental details
In our experiment, all chemicals were analytically pure and used without additional purification. Na2S2O3·5H2O was used as sulfur source, which provides the sulfur ion for the reaction and similarly Cu(CH3COO)2·H2O as copper sources, which provides the copper ion for the reaction. Solutions were prepared for copper and sulfur sources for required molarities. Then, solution of copper source was added to solution of sulfur source drop wise under sturdy stirring condition. The mixed solution was then treated under microwave irradiation (2.45 GHz) at 160 and 320 W for 30 and 15 min by a domestic microwave oven. After the treatment of solution, CuS (black ppt) was formed which were collected, these CuS (black ppt) was washed by distilled water and ethanol several times and dried at 600 °C in air. Effects of experimental conditions on synthesis of CuS are given in Table 1.
Chemical reactions involve can be describe as
Copper source decompose into Cu+2 in their aqueous solution as
For copper source and sodium thiosulfate system the reactions can be written as
Now CuS nanoparticles aggregated to spherical shape
Microwave irradiation played a vital role in the aggregation process; to get an appropriate morphology of CuS, power of microwave and time of irradiation should be adopted carefully, erroneous combination of microwave power and time may knock down the required results.
Characterization of the product
Powder X-ray diffraction (XRD) data were recorded and collected on the XRD model MPD X’PERT PRO of PANalytical Company Ltd., Holland using Cu Kα as characteristic radiation (λ = 0.15418 nm) with θ–θ configuration. The measurements were made in 2θ ranging from 20° to 70°. Analysis was mainly done by the software X’Pert HighScore of the same company. Scanning electron microscopy (SEM) images were taken on a scanning electron microscope (JEOL JSM-6480). Thermal oxidation, phase changes in copper sulfide, a differential scanning calorimeter (DSC) and thermal gravimetric analysis (TGA) were performed by SDT Q600 of TA Instrument in oxygen and argon environment.
Results and discussion
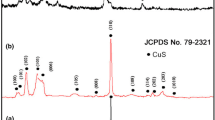
Figure 1 gives the XRD patterns for the products. Samples were well crystallized. All diffraction peaks can be indexed as the hexagonal CuS by comparison with data from JCPDS file no. 00-001-1281 with lattice constants a = 3.8020 Å, b = 3.8020 Å and c = 16.4300 Å, no characteristic peaks of any other impurity was observed. Crystallite size calculated by Scherrer formula is 15 nm.
Morphological characterization by SEM imaging shows the growth of CuS nanoparticle, their aggregation to form spherical shape. From Fig. 2a, b the aggregation of CuS nanoparticles into spheres can be seen, some non-aggregated particles are also indentified from SEM; size of the spheres were calculated in the range of 794 nm to 1.47 μm from SEM images. Figure 3a, b shows the CuS nanoparticles of disorganized morphology due to haphazard aggregation.
Power and time for microwave irradiation played very significant role in aggregation process; we used microwave irradiation (2.45 GHz, 800 W) at 160 W (20 %) for 30 min by a domestic microwave oven for the formation of spherical morphology of CuS nanomaterial. Aggregation is assumed to be restricted only by the limited transportation of particles on to one another; hence, we could not get the exact morphology, just the non-aggregated nanoparticle when we apply microwave irradiation for less time (15 min) as shown in SEM images Fig. 4a, b. By increasing time or power of operating microwave oven aggregation kinetics could be increased and structure size will be changed; at low power microwave irradiation, nanoparticles aggregation would not start properly due to lesser number of collisions between CuS nanoparticles. In this situation when nanoparticles are not able to collide well with each other, time of irradiation cannot contribute greatly. On the other hand, when appropriate power of microwave irradiation is used, more time and high power make aggregation process fast, morphology of product can be changed. To get definite morphology of product, an apt combination is required.
Thermal behavior of CuS nanomaterials
It was well known that thermal decomposition of CuS in free air is a process of several steps, and depending on experimental circumstances (temperature, environment). In this process different copper sulfides (Cu1.8S and/or Cu2S), copper oxides (Cu2O and CuO), copper sulfates (Cu2SO4, CuSO4), copper oxy-sulfates (CuO·CuSO4) were formed (Godočíková et al. 2006; Simonescu et al. 2007). The thermal stability and decomposition curves (DSC, TGA) of the copper sulfides (CuS) nanospheres are depicted in (Fig. 5). We treated the sample prepared from room temperature to 1,175 °C in oxygen atmosphere at the ramp rate of 10 °C/min, TGA curve shows CuS nanospheres experienced a mass loss of 6.41 % between 250 and 300 °C, which is due to the fractional decomposition of CuS to Cu2S. 40 % of total material (CuS) was converted to Cu2S at that stage due to formation of sulfur dioxide It was trailed by of increment of 61.5 % which corresponds to the conversation from CuS to CuSO4 and Cu2S to CuO·CuSO4; the experimental and theoretical values (61 %) have very close agreement. The oxidation reactions are exothermic in nature indicated by sharp peak in the DSC curve. Further, copper sulfate and copper oxy-sulfate were decomposed into CuO with a large mass loss (70.9 %) starting at 750 °C, the remaining mass was 84.01 % at that stage which is very close to theoretical one (83.25 %). The reaction was endothermic as shown by the DSC curve from 960 °C. There was another thermal conversion and TGA/DSC graph shows endothermic mass loss of 13.9 % which was caused by conversion of CuO to Cu2O. Theoretical amount of residue should be 74.8 % but we got very close to a residual amount of 70.1 %. Different phase changes in CuS during DSC/TGA are discussed in Table 2.
The chemical equations involve during DSC/TGA can be describe as:
When DSC/TGA of CuS nanospheres was done in inert atmosphere (argon flow, 100 ml/s), CuS nanospheres showed great stability under inert atmospheres, from 50 to 1,000 °C. There was only 0.7 % mass loss and no other characteristic transition in mass and heat flow was observed (Fig. 6).
It is clear from the DSC/TGA graph of CuS nanospheres that thermal oxidation of CuS produces CuO above 750 °C; to confirm the formation of CuO, we treated CuS nanomaterial at 800 °C for 1 h in open air atmosphere.
The composition and phase purity of CuO nanocrystals were examined by powder XRD, which confirmed good crystallinity and peaks can be indexed to the monoclinic CuO (JCPDS card no. 00-48-1548) having cell parameters a = 4.688 Å and c = 5.132 Å (in Fig. 7). CuO was fabricated by thermal oxidation of CuS nanomaterial; Fig. 8a, b shows the SEM images of fabricated CuO nanocrystal with random morphology and wide size distribution.
Conclusion
CuS nanomaterial with spherical structures have been prepared in a simple aqueous sol system, employing Cu (CH3COO)·2H2O as copper sources and Na2S2O3·5H2O as sulfur ion source, under microwave irradiation. Experimental results showed that a great deal of spheres of average diameter of 900 nm was obtained; the thermal decomposition of copper sulfide (CuS) is complex in nature. The associated solid-state transformations and phase changes depend upon preparation of the sulfides. Thermal decomposition of CuS gives Cu2S then converted to CuSO4 which was decomposed into CuO. In inert atmosphere no phase change was observed, CuS nanomaterial showed great stability.
References
Anuar K, Zainal Z, Hussein MZ, Saravanan N, Haslina I (2002) Cathodic electrodeposition of Cu2S thin film for solar energy conversion. Sol Energy Mater Sol Cells 73:351
Brust M, Kiely CJ (2002) Some recent advances in nanostructure preparation from gold and silver particles: a short topical review. Colloids Surf A 202:175
Godočíková E, Baláž P, Criado JM, Real C, Gock E (2006) Thermal behaviour of mechanochemically synthesized nanocrystalline CuS. Thermochim Acta 440:19–22
Kiely CJ, Fink J, Brust M, Bethell D, Schiffrin DJ (1998) Spontaneous ordering of bimodal ensembles of nanoscopic gold clusters. Nature 396:444
Lu Q, Gao F, Zhao D (2002) One-step synthesis and assembly of copper sulfide nanoparticles to nanowires, nanotubes, and nanovesicles by a simple organic amine-assisted hydrothermal process. Nano Lett 2:725
Nafees M, Ali S, Rasheed K, Idrees S (2011) The novel and economical way to synthesize CuS nanomaterial of different morphologies by aqueous medium employing microwaves irradiation. Appl Nanosci. doi:10.1007/s13204-011-0050-z
Nair MTS, Nair PK (1989) Chemical bath deposition of CuxS thin films and their prospective large area applications. Semicond Sci Technol 4:191
Nascu C, Pop I, Ionescu V, Indrea E, Bratu I (1997) Spray pyrolysis deposition of CuS thin films. Mater Lett 32:73–77
Ni Y, Liu H, Wang F, Yin G, Hong J, Ma X, Xu Z (2004) Self-assembly of CuS nanoparticles to solid, hollow, spherical and tubular structures in a simple aqueous-phase reaction. Appl Phys A 79:2007–2011
Parkin IP (1996) Solid state metathesis reaction for metal borides, silicides, pnictides and chalcogenides: ionic or elemental pathways. Chem Soc Rev 25:199
Rodriguez JA, Jirsak T, Dvorak J, Sambasivan S, Fischer D (2000) Reaction of NO2 with Zn and ZnO: photoemission, XANES, and density functional studies on the formation of NO3. J Phys Chem B 104:319
Shipway AN, Katz E, Willner I (2000) Nanoparticle arrays on surfaces for electronic, optical, and sensor applications. Chem Phys Chem 1:18
Simonescu CM, Teodorescu VS, Carp O, Patron L, Capatina C (2007) Thermal behaviour of CuS (covellite) obtained from copper–thiosulfate system. J Therm Anal Calorim 88(1):71–76
Tan C, Zhu Y, Lu Ran, Xue P, Bao C, Liu X, Fei Z, Zhao Y (2005) Synthesis of copper sulfide nanotube in the hydrogel system. Mater Chem Phys 91:44–47
Thongtem T, Phuruangrat A, Thongtem S (2007) Synthesis and analysis of CuS with different morphologies using cyclic microwave irradiation. J Mater Sci 42:9316–9323
Wang Q, Li J-X, Li G-D, Cao X-J, Wang K-J, Chen J-S (2007) Formation of CuS nanotube arrays from CuCl nanorods through a gas–solid reaction route. J Cryst Growth 299:386–392
Wong MS, Cha JN, Choi K-S, Deming TJ, Stucky GD (2002) Assembly of nanoparticles into hollow spheres using block copolypeptides. Nano Lett 2:583
Wu C, Yu S-H, Chen S, Liu G, Liu B (2006) Large scale synthesis of uniform CuS nanotubes in ethylene glycol by a sacrificial templating method under mild conditions. J Mater Chem 16:3326–3331
Wuled Lenggoro I, Kang YC, Komiya T, Okuyama K, Tohge N (1998) Formation of submicron copper sulfide particles using spray pyrolysis method. Jpn J Appl Phys 37:L288–L290
Yang YJ, Xiang JW (2005) Template-free synthesis of CuS nanorods with a simple aqueous reaction at ambient conditions. Appl Phys A 81:1351–1353
Zhang YC, Hu XY, Qiao T (2004) Shape-controlled synthesis of CuS nanocrystallites via a facile hydrothermal route. Solid State Commun 132:779–782
Zhu Y, Guo X, Jin J, Shen Y, Guo X, Ding W (2007) Controllable synthesis of CuS nanotubes and nanobelts using lyotropic liquid crystal templates. J Mater Sci 42:1042–1045
Zou J, Zhang J, Zhang B, Zhao P, Xu X, Chen J, Huang K (2007) Synthesis and characterization of copper sulfide nanocrystal with three-dimensional flower-shape. J Mater Sci 42:9181–9186
Acknowledgments
The authors acknowledge Higher Education Commission, Pakistan, for financial support through “Indigenous Ph.D. Fellowship Program (5000 Fellowships)”.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 2.0 International License (https://creativecommons.org/licenses/by/2.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
About this article
Cite this article
Nafees, M., Ali, S., Idrees, S. et al. A simple microwave assists aqueous route to synthesis CuS nanoparticles and further aggregation to spherical shape. Appl Nanosci 3, 119–124 (2013). https://doi.org/10.1007/s13204-012-0113-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13204-012-0113-9