Abstract
Ni–Cr, Ni–SiC and novel Ni–SiC–Cr composite coatings were electrodeposited on pure copper samples from a Watt’s nickel electroplating bath containing chromium and silicon carbide nanoparticles with an average particle size of 50 nm. The coatings composition, crystalline structure and surface morphology were studied by X-ray diffractometry, energy-dispersive X-ray spectroscopy and field emission scanning electron microscopy. The results indicate that Ni–SiC–Cr hybrid composite films with an acceptable homogeneity having 8.1 and 5.4 % vol. SiC and Cr nanoparticles were developed successfully. The incorporated SiC and Cr particles in Ni–SiC–Cr composite layer effectively increased the microhardness and wear resistance due to grain refining strengthening, changing the columnar grain structure of nickel matrix to a granular structure and crystalline preferred growth direction from (100) to the close-packed (111).
Similar content being viewed by others
Introduction
Co-electrodeposition of metal matrix composites (MMCs) consisting of dispersed micron and submicron size particles of oxides, carbides, borides, metals and some other organic and inorganic materials is a flexible and low cost method of composite coating. These novel composite films have shown enhanced mechanical, tribological, anti-corrosion and anti-oxidation properties compared to the pure metal coatings.
Considering the enormous applications of conventional nickel electroplating in industry, nickel-base composite films have received considerable attention in order to improve the rather poor mechanical properties of the nickel layers. It has been shown that implantation of particles such as Al, Cr, SiC, Al2O3, SiO2, TiO2 and Cr2O3 reinforces the electroplated nickel layer and improves the mechanical properties such as microhardness and wear resistance by forming a composite structure. These toughened nickel composite coatings can be used as suitable protective films for industrial parts subjected to severe thermal and mechanical working conditions, such as turbine blades and slurry pump impellers (Gheorghihs et al. 2006; Feng et al. 2007; Zhang et al. 2008; Purwadaria 2008; Thiemig and Bund 2008; Jung and Natter 2009; Gül et al. 2009; Xu et al. 2009).
Recently, the co-electrodeposition of chromium and silicon carbide nanoparticles with metal nickel has been studied by several researchers owing to their special characteristics. The Ni–SiC nanocomposites exhibit an interesting combination of hardness, wear resistance, ductility and low hot-oxidation rate (Lim et al. 1997; Garcia et al. 2001; Zhou et al. 2005; Zanella et al. 2009).
The electrodeposited Ni–Cr nanocomposite films have an ability to form a continues protective compact Cr2O3 thermally grown oxide (TGO) scale during oxidation and shows a superior oxidation resistance along with improved hardness and wear resistance. (Suryanarayana and Koch 2000; Jung and Natter 2009; Lin and Huang 2004; Zhang et al. 2004; Pavlatou et al. 2006; Zhao et al. 2009; Zhou et al. 2010).
In this research, a novel Ni–SiC–Cr hybrid nanocomposite film was prepared by electroplating technique to achieve a combination of superior mechanical and chemical properties of the Ni–SiC and Ni–Cr mono-composites. The crystalline structure, surface morphology and mechanical properties of the hybrid Ni–SiC–Cr MMC film were investigated and compared to the pure nickel film and mono Ni–SiC and Ni–Cr composite coatings.
Experimental aspects
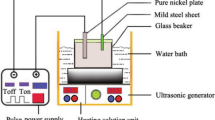
The Ni–SiC, Ni–Cr and Ni–SiC–Cr MMC composites and pure nickel films were electroplated by applying a constant current in a Watts plating solution. The nanosilicon carbide and chromium powders of 99.8 % purity as supplied by the manufacturer with a 50-nm mean size (Qinhuangdao TaijiRing Nano-Products Company, China) were introduced into the electrolyte without any further treatment. The electroplating bath was warmed up to 45 °C and mixed prior to the electroplating process by a magnetic stirrer at 600 rpm for 4 h and using an ultrasonic cell (JAC ultrasonic 2010) for 30 min. The chemical composition and electroplating parameters are shown in Table 1. The pH of the electrolyte was regulated at 4 ± 0.2 by adding 0.1 mol HCl and 0.05 mol NaOH solutions based on the fluctuation in pH. The electrolyte was mechanically stirred constantly at 350 rpm using a magnetic stirrer to prevent the suspended particles settling down during plating courses.
Substrate samples with dimensions of 25 mm × 10 mm × 1 mm were cut from a commercial pure copper plate and all samples were polished with silicon carbide papers up to grade 2000 grit, cleaned in 0.1 mol NaOH solution and activated in 5 % hydrochloric acid at room temperature. The anode was a pure nickel (99.98 %) plate with an area of 10 cm2. The temperature of the electrolyte was maintained at 45 °C by an automatic temperature controller (PLT, Scientific Company—accuracy ±1 °C). The plating was conducted at a current density of 10 A/dm2 for 20 min using a DC power supply (EKK-AJ5D) with ±0.05 A current accuracy. After the co-deposition process, the samples were washed with distilled water and then cleaned ultrasonically for 15 min to remove any loose particles from the surface.
The microstructure and morphology of the coatings’ surfaces and cross sections were studied by field emission scanning electron microscopy (FESEM, Nova Nano SEM, 2300). The amount of incorporated particles in the nickel matrix layer was evaluated by micrographs image analysis and using an energy-dispersive X-ray diffraction analyser (EDS) attached to the FESEM machine. The crystalline structure and grain size of the nanopowders, the pure nickel layer and the composite coatings were determined by X-ray diffractometry (Cu, Kα) at room temperature and by applying the Scherrer formula using the (220) reflection. The X-ray scan rate was 0.15° min−1 over a 2θ range of 10° to 90°.
The hardness of the pure nickel and composite films were determined using a Vickers microhardness measuring device (WOLPERIW-401 MVD). The test was performed under a 100 g load and the corresponding final values were determined as the average of a minimum of five measurements. The wear tests were performed at room temperature by measuring the weight lost during grinding of the samples on 2000 grit silicon carbide waterproof paper. The mass loss of the samples to an accuracy of 0.1 mg was monitored to compare the wear loss of pure nickel and composite electroplated samples. The wear tests were repeated three times on each type of samples and for different sliding distances to minimize error and data scattering.
Results and discussion
Characterization of the nanoparticles
Figure 1a and b show XRD patterns of the Cr and SiC nanoparticles which are matched with hexagonal silicon carbide and cubic chromium reference peaks. The average particle sizes of the Cr and SiC powders were calculated to be 61 and 37 nm, respectively, using three main peaks of the X-ray diffraction patterns in the Scherrer equation disregarding the effect of the microstrains. The SEM observations also showed that as-received powders are semi-spherical with a diameter between 50 and 75 nm. Hence, the nominal size of 50 nm given by producer can be accepted as the average value.
Using the characteristic peak broadening of the co-deposited SiC and Cr particles in the composite layer (Fig. 1d), the embedded Cr and SiC mean particle sizes were estimated at about 64 and 73 nm, respectively. These results and also the SEM micrographs (Fig. 2) showed no noticeable particles agglomeration in the formed composites.
Particles content of the nanocomposite coatings
The amount of particles incorporation during nickel deposition is affected by number of variables such as electrolyte composition and electroplating controlling parameters, particles physical and chemical properties, mixing hydraulic forces and probable interaction between the particles.
The XRD patterns (Fig. 1) and SEM micrographs (Fig. 2) and EDX analysis (Fig. 3) portray a successful formation of Ni–SiC, Ni–Cr and Ni–SiC–Cr composite layers with an almost homogeneous dispersion of nanoparticles and average 36, 36 and 37 µm thickness, respectively. The volume percentage of the implanted nanoparticles are shown in Table 2. The SiC showed a higher deposition rate compared to the chromium particles in the mono and hybrid composite layers. This behavior can be understood in terms of deposition mechanism. The surface energy and molecular polarization in the electrical field of the plating cell initiates a type of ions physical adsorption on the surface of neutral particles. The adsorbed ions around the particles create a driving force for moving towards the cathode surface. Simply, if the concentration polarization near to the cathode is considered to be negligible, combinations of electrical and hydraulic forces define the probability of particle adsorption. The SiC showed a higher deposition rate could be attributed to the higher surface energy and polarity. The particle content of the Ni–SiC–Cr was less the total co-deposited particles in the Ni–Cr and Ni–SiC layers due to probable interaction between the particles in the electrolyte and over the cathode surface.
Coatings microstructure
The pure nickel exhibited a columnar structure (Fig. 2), oriented in the (110) direction. The Ni–SiC composite also had a columnar grain structure (Fig. 2a) with high density of surface porosity, oriented in the (100) direction. On the other hand, Ni–Cr and Ni–SiC–Cr composite coatings characterized by fine grained granular structure (Fig. 2c) and preferred crystalline growth direction of close-compact (111).
A general observation was that the co-deposition of chromium and silicon carbide nanoparticles resulted in refinement of the nickel matrix grains. The mean grain sizes of the electroplated pure nickel and nickel matrix of the composite layers were determined using the Scherrer formula as shown in Table 2. Clearly, the composite coating had an average grain sizes smaller than that of the pure nickel films which were electrodeposited under the same conditions. This grain refinement could be attributed to the role of the dispersed particles on nickel layer nucleation rate and crystal growth.
Microhardness and wear resistance
The microhardness of pure nickel and nickel composite coatings are shown in Table 2. The results showed that adding dispersed particles to the nickel layer increased the microhardness significantly. This improvement is mainly related to Hall–Petch hardening effect (grain refinement-strengthening) and incorporation of the nanoparticles (dispersion-strengthening) which inhabit the plastic flow of the metal matrix by blocking the free sliding of the dislocations. Furthermore, a portion of enhanced hardness owing to the particles influences on the morphology and crystalline structure of coatings. The Ni–SiC–Cr showed higher hardness compared to the pure Ni, Ni–SiC and Ni–Cr coatings which is due to synergetic effects of grain refining and dispersive strengthening whereas Ni–SiC–Cr has higher particles content, fine granular grain structure and a texture in close-compact and hard (111) direction.
The weight lost after sliding test of nickel and nickel nanocomposite coatings is shown in Fig. 4. As it evident from this graph, generally the wear of the films decreased by adding dispersed particles to the nickel layer. The Ni–SiC–Cr hybrid composite coating showed a minimum wear weight loss which is in accordance with Archard’s law. The most common form of the Archard wear equation is shown below (Eq. 1). Here W is the wear volume lost, K0 is the dimensionless wear coefficient and K is the dimensional wear coefficient, H is the hardness, F is the normal force and S is the sliding distance.
The Fig. 5 shows the dimensional wear coefficient factor of the tested samples using Eqs. (1) and (2) for 100-m sliding distance which confirms Ni–SiC–Cr nano composite superior wear resistance. It is suggested that combining nickel-base ductility and excellent wear resistance and toughness of the silicon carbide with good mechanical properties of the chromium particles causes good load-bearing capacity and delaminating resistance. The projected hard particles on the coating surface increase the sliding wear resistance. Moreover, semi-sphere-shaped nanoparticles release from the composite surface after some period of time and it may change the sliding friction into rolling friction and produces a kind of self-lubricating capability.
Conclusion
The pure nickel and Ni–SiC, Ni–Cr, Ni–SiC–Cr nanocomposite coatings were prepared by means of electrodeposition technique. The characteristics of the coatings were investigated comparatively by scanning electron microscopy, EDS, XRD, microhardness and wear tests. The results indicate that the formed novel Ni–SiC–Cr hybrid composite consisted of homogeneous and compact embedded nanoparticles with 8.1 and 5.4 % vol. of SiC and Cr, respectively. The SiC particles did not change the columnar structure of the nickel matrix whereas the incorporation of Cr particles in Ni–SiC–Cr and Ni–Cr coatings led to the formation of a granular grain structure. The preferred grain growth crystalline direction of the nickel matrix changed from (110) in to (100) in Ni–SiC and close-compact (111) in Ni–Cr and Ni–SiC–Cr nanocomposite. The co-deposition of nanosilicon carbide and chromium particles with nickel effectively reduced the size of nickel crystals due to particles influences on the nickel nucleation rate and crystal growth. The composite coatings showed improved microhardness and wear resistance compared to the pure nickel film as the result of change in crystalline structure and surface morphology, grain refinement and dispersion-strengthening. The Ni–SiC–Cr showed superior tribological properties which can be explained in terms of its lower wear coefficient, higher hardness and particle content, fine crystalline structure in hard (111) crystalline growth direction.
References
Feng Q, Li T et al (2007) Preparation of nanostructured Ni/Al2O3 composite coatings in high magnetic field. Surf Coat Technol 201(14):6247–6252
Garcia I, Fransaer J et al (2001) Electrodeposition and sliding wear resistance of nickel composite coatings containing micron and submicron SiC particles. Surf Coat Technol 148(2–3):171–178
Gheorghihs C, Carac G et al (2006) Preparation and structural characterization of nickel/ alumina nano-particles composite coatings. J Optoelectron Adv Mater 8(3):1234–1237
Gül H, KilIç F et al (2009) Characteristics of electro-co-deposited Ni-Al2O3 nano-particle reinforced metal matrix composite (MMC) coatings. Wear 267(5–8):976–990
Jung A, Natter H (2009) Nanocrystalline alumina dispersed in nanocrystalline nickel:enhanced mechanical properties. J Mater Sci 44:2725–2735
Lim H, Park S et al (1997) The effect of particle size of alumina dispersions on the oxidation resistance of Ni-Cr alloys. Oxid Met 48(5):391–415
Lin CS, Huang KC (2004) Codeposition and microstructure of nickel-SiC composite coating electrodeposited from sulphamate bath. J Appl Electrochem 34(10):1013–1019
Pavlatou EA, Stroumbouli M et al (2006) Hardening effect induced by incorporation of SiC particles in nickel electrodeposits. J Appl Electrochem 36(4):385–394
Suryanarayana C, Koch CC (2000) Nanocrystalline materials: current research and future directions. Hyperfine Interact 130:5–44
Thiemig D, Bund A (2008) Characterization of electrodeposited Ni-TiO2 nanocomposite coatings. Surf Coat Technol 202(13):2976–2984
Xu R-d, Wang J-l et al (2009) High-temperature oxidation behavior of CeO2-SiO2/Ni-W-P composites. Trans Nonferr Metals Soc China 19(5):1190–1195
Zanella C, Lekka M et al (2009) Influence of the particle size on the mechanical and electrochemical behaviour of micro- and nano-nickel matrix composite coatings. J Appl Electrochem 39(1):31–38
Zhang Y, Peng X et al (2004) Development and oxidation at 800 °C of a novel electrodeposited Ni-Cr nanocomposite film. Mater Lett 58(6):1134–1138
Zhang H, Song YW et al (2008) Electrodeposited Ni/Al2O3 composite coating on NdFeB permanent magnets. Key Eng Mater 373–374:232–235
Zhao G-g, Zhou Y-b et al (2009) Sliding wear behaviors of electrodeposited Ni composite coatings containing micrometer and nanometer Cr particles. Trans Nonferr Metals Soc China 19(2):319–323
Zhou Y, Peng X et al (2005) Size effect of Al particles on the oxidation of electrodeposited Ni–Al composite coatings. Oxid Met 64(3):169–183
Zhou Y-b, Zhao G-g et al (2010) Fabrication and wear properties of co-deposited Ni-Cr nanocomposite coatings. Trans Nonferr Metals Soc China 20(1):104–109
Acknowledgments
This research has been supported by Advanced Materials and Nanotechnology Laboratory, Institute of Advanced Technology, Universiti Putra Malaysia, 43400 UPM Serdang, Selangor, Malaysia.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 2.0 International License (https://creativecommons.org/licenses/by/2.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
About this article
Cite this article
Masoudi, M., Hashim, M., Kamari, H.M. et al. Fabrication and characterization of Ni–SiC–Cr nanocomposite coatings. Appl Nanosci 3, 357–362 (2013). https://doi.org/10.1007/s13204-012-0136-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13204-012-0136-2