Abstract
Since the influence of carbon dioxide (CO2) on the dynamic interfacial tension of crude oil and aqueous solutions at the elevated temperatures and pressures is an important issue, this investigation is aimed to measure the IFT of crude oil/water, CO2, and carbonated water. The measured values reveal that the IFTs of crude oil/CO2 are decreased through the first interval, while a gradual and continuous reduction in IFT is obtained at the second interval. Although both intercepts and slopes of the first interval lines are more than the second interval for all the studied temperatures, they show a similar trend as a function of temperature. The used vanishing IFT method measurement reveals that the minimum miscibility pressure (MMP), first contact miscibility pressure, and intersection point (where the slopes of the linear curves change) of two linear equations have a linear increasing trend as temperature changes. However, this trend was not observed for the aqueous solution saturated with CO2 (i.e., carbonated water) which can be related to the difference between liquid- and gas-phase behavior as well as the formation of carbonic acid in the carbonated water process. IFT values reduced from 18.6 mN/m (@ pH = 8) to 0.3 mN/m (@ pH = 14) with an increase in pH of the aqueous solution, while miscibility conditions can be obtained for CO2 cases at pressures higher than the MMP points. Considerably higher IFT values were measured in the presence of water and carbonated water in the range of 9.1–17.5 mN/m and 12.2–17.4 mN/m, respectively. The most important feature of the CW process can be the swelling factor of crude oil due to the fact that the oil swelling of up to 40% was achieved at elevated temperature (75 °C) and pressure (4500 psi) after only1600 s.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Enhanced oil recovery (EOR) methods such as CO2 injection have attracted a lot of attention in the oil industries. CO2 injection is one of the most fundamental and reliable EOR methods, regardless of the oil type (Al-Jarba and Al-Anazi 2009; Bougre and Gamadi 2021; Lashkarbolooki et al. 2016b). Resecting the unique features of this method, several CO2-based flooding processes were proposed and examined (Lashkarbolooki and Ayatollahi 2018c; Mahinpey et al. 2007; Rostami et al. 2017; Sasaki et al. 2013; Zhao et al. 2011; Zhu et al. 2021; Zihao et al. 2011). It is possible to inject the CO2 in the miscible or immiscible conditions regarding thermodynamic conditions and crude oil properties. Besides, it is mentioned that CO2 is mostly immiscible fluid with the crude oil at the first contact, while it can be miscible through the second contiguity with the crude oil which is called multi-contact miscibility (MMP) (Alomair and Garrouch 2016; Choubineh et al. 2019; Green and Willhite 1998; Wang et al. 2010).
At the MMP point, CO2 can be dissolved in crude oil through a multi-contact conditions (Green and Willhite 1998; Holm and Josendal 1974; Lashkarbolooki and Ayatollahi 2018c). Therefore, it is essential to determine the MMP to propose the most efficient procedure for the CO2 flooding process. Unfortunately, direct injection of CO2 has several drawbacks including gravity segregation, high mobility, low sweep efficiency, gas fingering, and quick CO2 breakthrough making it an untrustworthy EOR method in some cases (Gogoi and Gogoi 2019; Lashkarbolooki et al. 2017; Riazi 2011). As a way out, carbonated water (CW) injection was proposed not only for EOR purposes but also even for underground CO2 reservations (Ahmadi et al. 2016; Bisweswar et al. 2020; De Nevers 1966; Duguid et al. 2011; Honarvar et al. 2017; Lashkarbolooki et al. 2017; Riazi 2011; Walsh et al. 2014). In this process, CO2 will diffuse from the aqueous phase into the oleic phase consequently leading to a desired swelling and viscosity reduction (Alizadeh et al. 2014; Foroozesh et al. 2016). Furthermore, CW is considered an efficient method since it can capture CO2 underground through the CO2 sequestration phenomenon which can reduce greenhouse gas emissions which is a global concern (Mosavat and Torabi 2013; Wang et al. 2015).
It has been thoroughly reported that as CO2 dissolves in water, the interfacial tension (IFT) of crude oil/water decreases (i.e., the IFT values of CW/crude oil were lower than that obtained for crude oil/water), which means IFT reduction can be considered as an important mechanism for CW flooding. Conversely, the results reported by Lashkarbolooki et al. (2017) demonstrated that the type of crude oil has an undeniable impact on the IFT of crude oil/CW. Their observations supported the idea that the movement and partitioning of natural active agents in crude oil and their interfacial orientation can be disordered by CO2 diffusion leading to higher IFT values, especially for the acidic crude oil.
Among the different effective thermodynamic parameters, the temperature has a considerable effect on both IFT of crude oil/carbonated water and MMP (Gu et al. 2013; Lashkarbolooki et al. 2019a). Besides, natural surface-active components existing in crude oil can introduce different dissociations at different pH values since carbonated water pH significantly reduces even at low CO2 content compared to water (Crawford et al. 1963; Lashkarbolooki et al. 2017). So, knowing the IFT behavior at different pH values is an important parameter that must be carefully examined especially through carbonated water and alkaline flooding processes. Performing a comprehensive literature review enlightens that IFT of crude oil has a profound effect on the fluid properties and several contradicting results were reported for IFT variation such as (a) IFT decreases as a function of pH because of dissociation of acidic components (Cratin 1993; Keleşoğlu et al. 2011), (b) dissociation of basic compounds at low pH values leading IFT reduction (Peters 1931) and (c) IFT decreases at low and high pH values due to ionization of both basic and acidic components and the highest IFT value was observed for neutral pH (Bai et al. 2010; Buckley 1996).
Because several contradicting results regarding IFT of crude oil and CO2, water and CW systems as functions of pressure and temperature have been reported (Escrochi et al. 2013; Jaeger and Pietsch 2009; Jaeger and Eggers 2012; Wang et al. 2010); therefore, the current work is concentrated on the evaluation of DIFT of crude oil in the presence of different solutions. In this way, the IFT of crude oil/CO2 was investigated and MMP values at three different temperatures including 25 °C, 50 °C, and 75 °C were determined. After the evaluation of the impact of pH on the IFT of crude oil, DIFT and equilibrium IFTs (EIFT) between the crude oil phase and aqueous solutions (water and carbonated phases) at two different isobars of 500 psi and 4500 psi were investigated to reveal the effect of pH and CO2 on the IFT of crude oil/carbonated water. In the last stage, the adsorption time of studied cases was evaluated by an empirical adsorption decay model to clarify the effect of CO2 on the interfacial properties of crude oil.
Materials and methods
Materials
The required CO2 with a purity of better than 99% was used to prepare the carbonated water. Besides, the crude oil sample with 32 API° was kindly supplied by National Iranian South Oil Co. (NISOC Co.) from one of the southern Iranian oil reservoirs. Moreover, different aqueous solutions with different pH values were prepared (using 1 M HCl and 1 M NaOH solutions) to investigate the effects of pH and the activity of basic and acidic components of crude oil.
IFT apparatus
Among the different possible methods for measuring the MMP point, vanishing interfacial tension (VIT) is one of the recent techniques proposed for measuring the MMP (Wang et al. 2010). In this method, IFT between two different phases is measured as a function of pressure and then extrapolated to the zero IFT value which is corresponded to the MMP where there is no differentiation between two liquids (Ahmad et al. 2016; Lashkarbolooki and Ayatollahi 2018c).
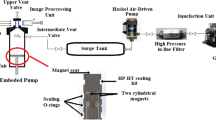
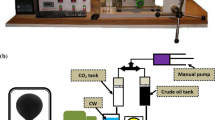
In the current investigation, a high pressure- high temperature pendant drop IFT measurement equipment was used (Fanavri Atiyeh Pouyandegan Exir Co., Arak, Iran). This device (see Fig. 1) consists of a mechanical section and an image processing section that provides the capability of IFT measurement for the operator. In detail, the equipment is comprised of a visual cell equipped with two HP sapphire sight glasses rated for maximum pressure and temperature of 600 bar and 150 °C. These sight glasses capable the operator to monitor the internal contents of the measuring chamber and dispatch the images of the formed drop at the tip of the injection needle to the drop shape analysis software. The required drop was formed using two manual hydraulic pumps that can inject the drop and bulk fluids into the main chamber at the desired pressure. After forming the drop, a CCD camera equipped with a macro-lens (Computar, Japan) records the required images every second and delivers them to the online software where the IFT values can be calculated using the drop shape analysis approach (Lashkarbolooki et al. 2019b). It should be noted that the used software is rapid enough to capture the required images as the drop was formed. On the other hand, the IFT variation in the current investigation was slow enough to be measured with enough reliability. In other words, since no chemical surfactants existed in the current work and the possible effect of swelling on IFT variation is a time-consuming phenomenon, there was no limitation to utilize the software for IFT and swelling calculations.
Besides, the required pressure in the main measuring chamber and drop and bulk accumulators was provided using two separate manual hydraulic pumps. On the other side, the temperature of the main measuring chamber was controlled using a PID controller coupled with PT-100 with an accuracy of 0.1 K using heating elements implanted in the main chamber body.
For the preparation of carbonated water solutions, about 80% of the visual cell was filled with water, and then, the pressure of the visual cell and the CO2 tank was elevated to the desired value using the manual pump. As the pressure reached a plateau (which is an indication of equilibration of water and CO2), the oil drop was injected into the bulk phase to form a drop at the tip of the nozzle. Since the excess amount of CO2 was injected at the top of the bulk phase, it is completely obvious that the solution was always in equilibrium and the aqueous phase was completely saturated with CO2. The noteworthy point is that the IFT values were measured three times for at least 1 h to ensure reaching equilibrium.
Decay model
To correlate DIFT values of crude oil and studied aqueous solutions, an exponential decay model was used:
where \({{\varvec{\gamma}}}_{0},{{\varvec{\gamma}}}_{{\varvec{t}}}\boldsymbol{ },{{\varvec{\gamma}}}_{{\varvec{e}}}\) are the initial IFT, IFT at time t and EIFT, respectively, and \({\varvec{\tau}}\) is an adjustable parameter called adsorption or relaxation time. Despite the simplicity of this model (a model with one adjustable parameter), it has an acceptable level of accuracy to predict the DIFT behavior of crude oil/ water and crude oil/carbonated water systems (Hamidian et al. 2019; Lashkarbolooki and Ayatollahi 2018b; Lashkarbolooki et al. 2017).
Results and discussion
Evaluation of EIFT of crude oil/CO2
The EIFT values of crude oil/CO2 were measured as a function of pressure at different operating temperatures (see Fig. 2a). According to the measured IFT values, no dynamic behavior was obtained for crude oil/CO2 since the EIFT was obtained at the first contact of crude oil and CO2. From a closer look at Fig. 2a, one can conclude that both temperature and pressure influenced the EIFT of the crude oil/CO2 system. At each isotherm of 25 °C, 50 °C, and 75 °C, EIFT values were reduced as the pressure was enhanced due to higher CO2 solubility in crude oil as the pressure was increased. On the other hand, the CO2 solubility reduces as the temperatures increases which directly leads to higher IFT values.
a EIFT between crude oil and CO2 at three constant temperature versus pressure, b density of CO2 obtained from NIST (Lemmon et al. 2010) and its relation with intersection point and c relation between intersection point, inflection and supercritical point
Commonly, the EIFT of crude oil experiences a reducing pattern as a function of pressure (Cao and Gu 2013; Wang and Gu 2011; Wang et al. 2010; Zolghadr et al. 2013). Respecting this fact, two pressures of 500 and 4500 psi were examined in a wide range of temperatures. The results illustrated that the EIFT values decrease sharply at the first examined pressure, while it faced a gradual change for the second examined pressure. The lines of the first interval correlated with the contiguity of CO2 and light components, whereas the second interval lines demonstrate the adjacency of CO2 with heavier components (Nobakht et al. 2008; Zolghadr et al. 2013). According to the results depicted in Fig. 2a, it is obvious that the lower oil extraction at lower temperatures can lead to CO2 contiguity with lighter components leading to easier dissolution. In contrast, an increase in the temperature leading to higher oil extraction which exposes CO2 to heavy components causes IFT reduction due to a reduction in CO2 dissolution in oil (Zolghadr et al. 2013).
As aforementioned, the VIT method is one of the reliable and fast methods for MMP calculation in which the EIFT values of first and second intervals at each temperature extrapolate to zero and the MMP and Pmax (first contact miscibility pressure) values are, respectively, determined (Gu et al. 2013). According to Fig. 2a and Table 1, the MMP values of 960 psi, 1506 psi, and 2200 psi were obtained for temperatures of 25 °C, 50 °C, and 75 °C, respectively. A glance into the MMP values calculated in this stage revealed that temperature enhancement leads to higher required pressures for complete miscibility of CO2 into the crude oil probably due to the lower CO2 solubility at the higher temperatures (Enick and Klara 1990; Hutin et al. 2014).
The Pmax values also increased from 1038 to 2555 psi and 3677 psi as temperature increased from 25 to 50 and 75 °C. To identify the intersection point where the slope of the linear curve changes, the equations of lines for two intervals in each temperature were calculated. The tabulated equations along with their correlation coefficient (R2) revealed the successfulness of the utilized equations to correlate the EITF value with an acceptable level of accuracy (see Table 1). Furthermore, CO2 density at each temperature and pressure and its relationship with intersection points at different temperatures is depicted in Fig. 2b. This figure revealed that the density of crude oil has an inflection point as temperature changes. Moreover, the density value which is pressure-dependent at each isotherm was depicted and the differences between intersection, inflection and supercritical points are shown in Fig. 2c. For temperature of 25 °C which inflection point and CO2 phase change from the gas phase to the liquid phase are the same, and the intersection and inflection points are similar to each other. (Their difference is 0 in Fig. 2c) However, for temperatures of 50 and 75 °C which inflection points are not coincided to change of CO2 phase from the gas phase to supercritical phase, intersection points were lower compared with the inflection point (200 psi and 150 psi lower than inflection points, respectively) and higher than the point in which the CO2 phase changes (220 psi and 710 higher than a supercritical point). In other words, an increase in temperature from 50 to 75 °C increases the difference between supercritical and intersection points which moves the system toward higher pressures to reach MMP pressure. Also, the results revealed a linear correlation between the intersection point and CO2 density.
The depicted slopes and intercepts of VIT lines of the first and second intervals (Figs. 3a and b) show that the slopes and intercepts of EIFT versus temperature curves almost decrease with increasing temperature in the same pattern.
Figure 4 shows that although the slopes of the first interval lines are more than the second interval at all the studied temperatures, the same variation for the slope was observed which depends on temperature.
A closer examination of the results depicted in Fig. 5 demonstrated that increasing the temperature reduces the slope of EIFT lines consequently enhancing MMP, Pmax, and intersection point values although there is almost a linear relationship between intersection point, MMP, and Pmax values and temperature. Among the examined parameters, the enhancement rate of Pmax versus T is the highest among these three parameters which are attributed to the higher slope of the second interval. So, higher pressure is required to achieve the first contact miscibility at elevated temperatures. Since intersection points are calculated by equality of first and second intervals lines, it will also increase as a function of temperature. For this type of crude oil, it can be concluded that the performance of CO2 injection can be higher for a reservoir with a lower temperature since a lower pressure is required to reach miscibility conditions and extraction of trapped oil. According to these findings, the CO2 injection for this type of crude oil is more practical if the reservoir temperature is low.
pH effect on the IFT
Another important parameter is the pH of the solution since it affects the ionization of natural surface-active agents (Hutin et al. 2014). The evaluation of the pH effect on the DIFT and EIFT (Fig. 6 and Fig. 7a) shows that in the presence of acidic solutions (pH = 2 and 4) and weak alkaline solutions (pH = 8 and 10), the differences between the initial and EIFT values were insignificant which means that surface-active materials are not capable to correctly pack at the interface as a function of time (see Fig. 7). But, the results illustrated that a lower initial IFT value and a reduction trend for DIFT were observed as the solutions become more basic (pH = 11 to 14). Generally, when crude oil comes into contact with the aqueous phase, the active materials that exist in crude oil can transfer from the oil phase to the aqueous phase leading to an IFT reduction time dependently. Ionization of both basic and acidic constituents accelerates this partition leading to more IFT reduction. The point that must be considered is that the effectiveness of pH variation on IFT highly depends on basic and acidic constituents of oil and their ionization (Hutin et al. 2014). It shows that in the presence of active alkaline, the acidic components of crude oil have more influences which can cause a reduction in IFT values. Since the ionization of acidic components is more influential at high pH values, the lowest IFT value of 0.3 mN/m was observed at a pH of 14. In detail, IFT values sharply decreased at higher pH values since the acidic species that existed on the surface of crude oil are saponified (Hutin et al. 2016, 2014). Moreover, the slight change of EIFT at pH between 2 and 10 shows that the basic constituents could not be ionized properly at this range of pH. So, basic species are not active enough at the interface to decrease the IFT values in this pH interval. Furthermore, due to the low ionization of basic components that existed at the interface at low pH values, no significant effect on the IFT values was observed as the pH of solutions was decreased.
Furthermore, the adsorption time values calculated by the MED model (Fig. 7b) demonstrated that the basic solutions have a more effective role than the acidic ones in the ionization and packing of acidic species present in the oil phase. This phenomenon causes a lower relaxation time of surface-active materials in contact with alkaline solutions compared to those obtained for acidic ones.
Evaluation of IFTs of crude oil/carbonated water and crude oil/ water
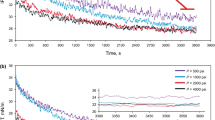
DIFT values of crude oil/water system were measured at two different pressures of 500 psi and 4500 psi and different temperatures of 25 °C, 50 °C, and 75 °C (see Figs. 8a and b). In all the studied cases, since natural surfactants of crude oil such as asphaltene and resin fractions move into the interface as time passes, IFT decreases as a function of time till reaching an equilibrium state and this is the equality of adsorption/ desorption rate of natural surfactants indicates the achievement of EIFT. As shown in Fig. 8, IFT decreases when the temperature increases from 25 to 50 °C for both examined pressures, while the IFT value experiences an increase if the temperature increases from 50 to 70 °C. This temperature in which the IFT variation behavior shows different patterns is known as phase inversion temperature (PIT).
In the next stage, the results of the empirical decay model against log ((γ0−γt)/(γt−γe)) were also evaluated to ensure reaching to an equilibrium state (see Fig. 9). As it is clear, the DIFT values can be divided into four parts: (1) induction time, (2) rapid fall time, (3) meso-equilibrium time, and (4) equilibrium time (Hua and Rosen 1988; Lashkarbolooki and Ayatollahi 2017; Lashkarbolooki et al. 2016a). The required time to reach the meso- equilibrium can be estimated by calculating of adsorption time from Eq. 1.
The effect of temperature on the DIFT between crude oil and carbonated water at two different pressures of 500 and 4500 psi as shown in Figs. 10a and b, respectively. As shown in Fig. 10, increasing the temperature from 25 °C to 50 °C for both examined pressures led to an increase in the EIFT, while EIFTs were decreased as the temperature was enhanced from 50 to 75 °C.
For more clarification, the EIFT values of water/crude oil and carbonated water/crude oil systems at all the studied operating conditions were compared (see Fig. 11a). Under the constant temperatures, pressure almost has no considerable effect on the EIFT of both solutions including water and CW. It should be noted that as CO2 was dissolved in the water phase, the aqueous pH solution was decreased due to the carbonic acid formation. As aforementioned, pH reduction had no considerable effect on the IFT of the studied crude oil. Despite this, the adsorption time of the crude oil/aqueous phase was considerably affected by CO2 content and lower times were required to achieve the equilibrium at high temperatures and pressures. This observation confirmed that the interfacial behavior of crude oil is completely different if the CO2 existed in the solution or not (both gas and supercritical phases). In detail, the IFT of crude oil regardless of the CO2 status (being gaseous or supercritical) experienced a sharp reduction as the pressure was changed. However, the crude oil/CW IFT almost remained unaffected by the CO2 content of the aqueous phase similar to the trend observed for the water phase.
It is also observed that temperature has a considerable influence on the IFT values compared with pressure since it can influence both the entropy and packing of natural surfactants at the interface (Duan et al. 2006; Lashkarbolooki and Ayatollahi 2018a; Lashkarbolooki et al. 2016a). In detail, an increase in the temperature leads to an increase in the molecular movement and even reduces the IFT. For the water/crude oil system, as the temperature increases from 25 to 50 °C, natural surfactants can easily move to the interface due to the molecules movement enhancement, while as the temperature rises to 75 °C, the higher motion of molecules impedes their proper packing. According to these facts, it seems that the best packing of natural surfactants can occur at a temperature of 50 °C so that increasing entropy is the dominant parameter in the first interval, while the reduction of packing due to reduction of surface excess concentration of active agents can be considered as a dominant factor in the second interval (Lashkarbolooki et al. 2016b). Besides, the measurements revealed that EIFT increases if temperature enhances from 25 to 50 °C, but it again decreases as temperature further increases to a value of 75 °C which indicates the temperature of 50 °C as the phase inversion temperature (PIT). Totally, according to Fig. 11a, no considerable positive effect was observed as CO2 dissolved in the water phase except for a little more IFT reduction in the temperature of 25 °C, while at the elevated temperatures of 50 and 75 C, IFTs of crude oil/carbonated water were higher than IFT of crude oil/deionized water due to a reduction in the surface activity of natural materials and their orientation at the interface comes from the higher entropy and CO2 partitioning.
Measuring the oil swelling at the presence of carbonated water for two different pressures of 500 and 4500 psi during 400 s measurements revealed no considerable change in this parameter for the pressure of 500 psi as a function of temperature, while this factor experienced an enhancement as the pressure was raised to 4500 psi (see Fig. 12). It was expected to observe a reduction in the oil swelling rate as the temperature was increased (at constant pressure) since the amount of dissolved CO2 in the aqueous phase decreases with temperature, while a contradicting trend was observed for high pressure. These observed trends can be correlated with the higher CO2 content as pressure increases, while enhancement of swelling factor at elevated temperature can be attributed to higher partitioning of CO2 from the aqueous phase toward the crude oil phase because of hydrogen bond reduction. Figure 12b also shows that temperature introduces a higher impact on the partitioning of CO2 and oil swelling factor than pressure.
Conclusion
The current study is concentrated on the influence of CO2 on the surface properties of crude oil/carbonated water solutions as functions of pressure and temperature. The obtained results can be classified as below:
-
1.
Considering the crude oil/CO2 IFT values, two distinguished pressure intervals were observed for all the studied temperatures. That is, the EIFT values decreased sharply and gradually in the first and second intervals, respectively, with no discontinuities in the supercritical CO2 conditions.
-
2.
Similar trends were obtained for the intercepts and slopes of two-interval lines as a function of temperature.
-
3.
There was rather a linear relationship between the intersection point, MMP, and Pmax values with temperature.
-
4.
Although the same intersection and inflection points (equal to CO2 phase changes from the gas phase to liquid phase) were observed at 25 °C, the inflection points (unequal to change of CO2 phase point from the gas phase to supercritical phase) introduce no coincide with intersection points at 50 and 75 °C. The difference between supercritical and intersection points increases as temperature increases from 50 to 75 °C leading to the higher required pressure for MMP.
-
5.
Since changing pressure is ineffective on the IFT of crude oil–water and crude oil/CW in contrast to the crude oil/CO2 IFT (significant reduction as pressure enhances), it is possible to conclude that CO2 content has no considerable influence on the IFT variation of crude oil/aqueous solution.
-
6.
Since IFT remained rather intact as the aqueous phase pH was decreased, carbonic acid formation by the dissolution of CO2 in the solution is not a proper way for IFT reduction.
-
7.
Two different trends were observed for phase inversion temperature of crude oil/water and crude oil/carbonated water systems. In the case of crude oil/water, increasing temperature from 25 to 50 °C led to a reduction in IFT values while further increase in temperature to 75 °C led to an increase in IFT. However, a reverse trend was obtained for the crude oil/carbonated water system which was probably related to different effects such as entropy, micro-emulsion formation, swelling of crude oil, and packing and orientation of natural surfactants and CO2 at the fluid/fluid interface.
-
8.
Higher oil swelling factor at higher pressure (4500 psi) can be attributed to the upper CO2 content, while higher swelling factor at elevated temperature (75 °C) can be attributed to the greater partitioning of CO2 from the aqueous phase toward the crude oil phase.
Abbreviations
- CW:
-
Carbonated water
- DIFT:
-
Dynamic interfacial tension (mN/m)
- DW:
-
Deionized water
- EIFT:
-
Equilibrium interfacial tension (mN/m)
- EOR:
-
Enhanced oil recovery
- IFT:
-
Interfacial tension (mN/m)
- Li :
-
Line number
- MMP:
-
Minimum miscibility pressure (psi)
- MED model:
-
Mono-exponential decay
- PID:
-
Proportional integral derivative
- PIT:
-
Phase inversion temperature (°C)
- PT:
-
Platinum resistance thermometer
- Pmax :
-
First contact miscibility pressure (psi)
- R2 :
-
Correlation coefficient
- VIT:
-
Vanishing Interfacial Tension
- \({{\varvec{\gamma}}}_{{\varvec{e}}}\) :
-
Equilibrium IFT (mN/m)
- \({{\varvec{\gamma}}}_{0}\) :
-
Initial IFT (mN/m)
- \({{\varvec{\gamma}}}_{{\varvec{t}}}\) :
-
IFT at time t (mN/m)
- \({\varvec{\tau}}\) :
-
Relaxation time (sec)
References
Ahmad W, Vakili-Nezhaad G, Al-Bemani AS, Al-Wahaibi Y (2016) Experimental determination of minimum miscibility pressure. Procedia Eng 148:1191–1198
Ahmadi MA et al (2016) Effect of operational parameters on the performance of carbonated water injection: experimental and numerical modeling study. 107: 542-548
Alizadeh A., Khishvand M., Ioannidis M, Piri MJF (2014) Multi-scale experimental study of carbonated water injection: an effective process for mobilization and recovery of trapped oil. 132: 219-235
Al-Jarba M, Al-Anazi B (2009) A comparison study of the of the CO2-Oil physical properties literature correlations accuracy using visual basic modelling. Nafta 60(5):287–291
Alomair OA, Garrouch AA (2016) A general regression neural network model offers reliable prediction of CO2 minimum miscibility pressure. J Petr Explor Prod Technol 6(3):351–365
Bai J-M, Fan W-Y, Nan G-Z, Li S-P, Yu B-S (2010) Influence of interaction between heavy oil components and petroleum sulfonate on the oil–water interfacial tension. J Dispersion Sci Technol 31(4):551–556
Bisweswar G, Al-Hamairi A, Jin S (2020) Carbonated water injection: an efficient EOR approach. A review of fundamentals and prospects. J Petr Explor Prod Technol, 10(2): 673–685
Bougre E, Gamadi T (2021) Enhanced oil recovery application in low permeability formations by the injections of CO2, N2 and CO2/N2 mixture gases. J Petr Explor Prod 11(4):1963–1971
Buckley JS (1996) Mechanisms and consequences of wettability alteration by crude oils, Heriot-Watt University
Cao M, Gu Y (2013) Oil recovery mechanisms and asphaltene precipitation phenomenon in immiscible and miscible CO2 flooding processes. Fuel 109:157–166
Choubineh A, Helalizadeh A, Wood DA (2019) The impacts of gas impurities on the minimum miscibility pressure of injected CO2-rich gas–crude oil systems and enhanced oil recovery potential. Pet Sci 16(1):117–126
Cratin PD (1993) Mathematical modeling of some pH-dependent surface and interfacial properties of stearic acid. J Dispersion Sci Technol 14(5):559–602
Crawford H, Neill G, Bucy B, Crawford P (1963) Carbon dioxide-a multipurpose additive for effective well stimulation. J Petrol Technol 15(03):237–242
De Nevers N (1966) Carbonated waterflooding. World Oil;(United States), 163(4)
Duan Z, Sun R, Zhu C, Chou I-M (2006) An improved model for the calculation of CO2 solubility in aqueous solutions containing Na+, K+, Ca2+, Mg2+, Cl−, and SO42−. Mar Chem 98(2–4):131–139
Duguid A, Radonjic M, Scherer GW (2011) Degradation of cement at the reservoir/cement interface from exposure to carbonated brine. Int J Greenhouse Gas Control 5(6):1413–1428
Enick RM, Klara SM (1990) CO2 solubility in water and brine under reservoir conditions. Chem Eng Commun 90(1):23–33
Escrochi M, Mehranbod N, Ayatollahi S (2013) The gas–oil interfacial behavior during gas injection into an asphaltenic oil reservoir. J Chem Eng Data 58(9):2513–2526
Foroozesh J, Jamiolahmady M, Sohrabi MJF (2016) Mathematical modeling of carbonated water injection for EOR and CO2 storage with a focus on mass transfer kinetics. 174: 325–332
Gogoi S, Gogoi SB (2019) Review on microfluidic studies for EOR application. J Pet Explor Prod Technol 9(3):2263–2277
Green D, Willhite G (1998) Enhanced Oil Recovery, Vol. 6, 18–27. Richardson, Texas: Textbook Series, SPE
Gu Y, Hou P, Luo W (2013) Effects of four important factors on the measured minimum miscibility pressure and first-contact miscibility pressure. J Chem Eng Data 58(5):1361–1370
Hamidian R, Lashkarbolooki M, Amani H (2019) Ion type adjustment with emphasize on the presence of NaCl existence; measuring interfacial tension, wettability and spreading of crude oil in the carbonate reservoir. J Petrol Sci Eng 182:106266
Holm L, Josendal V (1974) Mechanisms of oil displacement by carbon dioxide. J Petrol Technol 26(12):1427–1438
Honarvar B et al (2017) Experimental investigation of interfacial tension measurement and oil recovery by carbonated water injection: a case study using core samples from an iranian carbonate oil reservoir. Energy Fuels 31(3):2740–2748
Hua XY, Rosen MJ (1988) Dynamic surface tension of aqueous surfactant solutions: I. Basic paremeters. J Colloid Interface Sci 124(2):652–659
Hutin A, Argillier J-FO, Langevin D (2014) Mass transfer between crude oil and water. Part 1: Effect of oil components. Energy Fuels 28(12):7331–7336
Hutin A, Argillier J-F, LangevinD (2016) Influence of pH on oil-water interfacial tension and mass transfer for asphaltenes model oils. Comparison with crude oil behavior. Oil Gas Sci Technol–Revue dIFP Energies nouvelles, 71(4): 58
Jaeger PT, Eggers R (2012) Interfacial properties at elevated pressures in reservoir systems containing compressed or supercritical carbon dioxide. J Supercritical Fluids 66:80–85
Jaeger P, Pietsch A (2009) Characterization of reservoir systems at elevated pressure. J Petrol Sci Eng 64(1–4):20–24
Keleşoğlu S, Meakin P, Sjøblom J (2011) Effect of aqueous phase pH on the dynamic interfacial tension of acidic crude oils and myristic acid in dodecane. J Dispersion Sci Technol 32(11):1682–1691
Lashkarbolooki M, Ayatollahi S (2017) Experimental and modeling investigation of dynamic interfacial tension of asphaltenic–acidic crude oil/aqueous phase containing different ions. Chin J Chem Eng 25(12):1820–1830
Lashkarbolooki M, Ayatollahi S (2018a) Effects of asphaltene, resin and crude oil type on the interfacial tension of crude oil/brine solution. Fuel 223:261–267
Lashkarbolooki M, Ayatollahi S (2018b) Evaluation of effect of temperature and pressure on the dynamic interfacial tension of crude oil/aqueous solutions containing chloride anion through experimental and modelling approaches. Canadian J Chem Eng 96(6):1396–1402
Lashkarbolooki M, Ayatollahi S (2018c) Experimental investigation on CO2-light crude oil interfacial and swelling behavior. Chin J Chem Eng 26(2):373–379
Lashkarbolooki M, Ayatollahi S, Riazi M (2016a) Mechanistic study on the dynamic interfacial tension of crude oil+ water systems: experimental and modeling approaches. J Ind Eng Chem 35:408–416
Lashkarbolooki M, Vaezian A, Hezave AZ, Ayatollahi S, Riazi M (2016b) Experimental investigation of the influence of supercritical carbon dioxide and supercritical nitrogen injection on tertiary live-oil recovery. J Supercritical Fluids 117:260–269
Lashkarbolooki M, Riazi M, Ayatollahi S (2017) Effect of CO2 and natural surfactant of crude oil on the dynamic interfacial tensions during carbonated water flooding: experimental and modeling investigation. J Petrol Sci Eng 159:58–67
Lashkarbolooki M, Hezave AZ, Ayatollahi S (2019a) The role of CO2 and ion type in the dynamic interfacial tension of acidic crude oil/carbonated brine. Pet Sci 16(4):850–858
Lashkarbolooki M, Hezave AZ, Ayatollahi S (2019b) Swelling behavior of heavy crude oil during injection of carbonated brine containing chloride anion. J Mol Liq 276:7–14
Lemmon E, Huber M, McLinden M (2010) NIST Standard Reference Database 23, Reference Fluid Thermodynamic and Transport Properties (REFPROP). R1234yf. fld file dated December, 22
Mahinpey N, Ambalae A, Asghari K (2007) In situ combustion in enhanced oil recovery (EOR): a review. Chem Eng Commun 194(8):995–1021
Mosavat N, Torabi F (2013) Performance of secondary carbonated water injection in light oil systems. Ind Eng Chem Res 53(3):1262–1273
Nobakht M, Moghadam S, Gu Y (2008) Mutual interactions between crude oil and CO2 under different pressures. Fluid Phase Equilib 265(1–2):94–103
Peters RA (1931) Interfacial tension and hydrogen-ion concentration. Proceedings of the Royal Society of London. Series A, Containing Papers of a Mathematical and Physical Character, 133(821): 140–154
Riazi M (2011) Pore scale mechanisms of carbonated water injection in oil reservoirs, Heriot-Watt University
Rostami A, Arabloo M, Kamari A, Mohammadi AH (2017) Modeling of CO2 solubility in crude oil during carbon dioxide enhanced oil recovery using gene expression programming. Fuel 210:768–782
Sasaki K, Sugai Y, Chanmoly O, Kono H (2013) CO2 solubility characteristics of crude oils related to carbon capture and utilization (CCU), Proceeding Heavy Oil Congress, pp. 1–4
Walsh SD, Mason HE, Du Frane WL, Carroll SA (2014) Mechanical and hydraulic coupling in cement–caprock interfaces exposed to carbonated brine. Int J Greenhouse Gas Control 25:109–120
Wang X, Gu Y (2011) Oil recovery and permeability reduction of a tight sandstone reservoir in immiscible and miscible CO2 flooding processes. Ind Eng Chem Res 50(4):2388–2399
Wang X, Zhang S, Gu Y (2010) Four important onset pressures for mutual interactions between each of three crude oils and CO2. J Chem Eng Data 55(10):4390–4398
Wang J, Dong M, Li Y, Gong H (2015) Prediction of nitrogen diluted CO2 minimum miscibility pressure for EOR and storage in depleted oil reservoirs. Fuel 162:55–64
Zhao Y, Song Y, Liu Y, Liang H, Dou B (2011) Visualization and measurement of CO2 flooding in porous media using MRI. Ind Eng Chem Res 50(8):4707–4715
Zhu C-F et al (2021) Experimental study of enhanced oil recovery by CO2 huff-n-puff in shales and tight sandstones with fractures. Pet Sci 18(3):852–869
Zihao Y, Min J, Mingyuan L, Zhaoxia D, Peng Y (2011) Implication of geochemical simulation for CO2 storage using data of York reservoir. Chin J Chem Eng 19(6):1052–1059
Zolghadr A, Escrochi M, Ayatollahi S (2013) Temperature and composition effect on CO2 miscibility by interfacial tension measurement. J Chem Eng Data 58(5):1168–1175
Acknowledgements
The authors are grateful to thank Mr. Ali Zeinolabedini Hezave for their kind help to provide required equipment including HP-HT pendant drop interfacial tension.
Funding
The authors acknowledge the funding support of Babol Noshirvani University of Technology through Grant program No. BNUT/393055/2020.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
Authors have declared that no competing interests exist.
Ethical approval
The research does not require any ethical clearance issue.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Farahabadi, Z.T., Lashkarbolooki, M. Effect of CO2 on the interfacial tension and swelling of crude oil during carbonated water flooding. J Petrol Explor Prod Technol 13, 353–364 (2023). https://doi.org/10.1007/s13202-022-01554-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13202-022-01554-6