Abstract
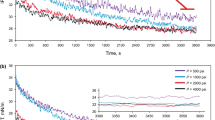
The current investigation was performed to examine the effect of an ionic liquid (IL)-based surfactant, namely 1-dodecyl-3-methylimidazolium chloride ([C12mim][Cl]) dissolved in distilled-carbonated water and brine-carbonated water, on the swelling factor and interfacial tension (IFT). In this way, the concentration of IL, temperature, and pressure were varied between 0–500 ppm, 298–338 K, and 34–374 bar, respectively. The results reveal that the presence of salinity reduces the swelling factor due to the lower solubility of carbon dioxide (CO2) in water. Besides, the swelling factors measured under different pressures and temperatures reveal a direct relationship between the swelling factor and these two thermodynamic parameters. Finally, the swelling factor measurements reveal a significant effect of [C12mim][Cl], even at low concentrations of 500 ppm, on the swelling factor reduction, which means lower liberation of CO2 from CW. On the other hand, the IFT measurements reveal that, although both temperature and pressure enhancement can reduce the IFT values of the CW/crude oil, the effect of IL (500 ppm) can reduce the IFT value from 28.70 mN m−1 to a value of 0.98 mN m−1, which is about a 30 folds reduction in its original value. This makes it possible to consider IFT reduction as one of the main mechanisms for the proposed innovative EOR method. To sum up, it seems that the proposed method can activate the swelling factor and IFT reduction as the effective mechanisms and even enhance the potential of the CW approach for better CO2 sequestration and storage.






Similar content being viewed by others
References
Abedini A, Mosavat N, Torabi F (2014) Determination of minimum miscibility pressure of crude oil–CO2 system by oil swelling/extraction test. Energ Technol 2:431–439
Ahmadi MA, Zeinali Hasanvand M, Behbahani SS, Nourmohammad A, Vahidi A, Amiri M, Ahmadi G (2016) Effect of operational parameters on the performance of carbonated water injection: experimental and numerical modeling study. J Supercrit Fluids 107:542–548
Aki SN, Mellein BR, Saurer EM, Brennecke JF (2004) High-pressure phase behavior of carbon dioxide with imidazolium-based ionic liquids. J Phys Chem B 108:20355–20365
Austad T, RezaeiDoust A, Puntervold T (2010) Chemical mechanism of low salinity water flooding in sandstone reservoirs. In SPE improved oil recovery symposium; Society of Petroleum Engineers
Aveyard R, Binks B, Chen J, Esquena J, Fletcher P, Buscall R, Davies S (1998) Surface and colloid chemistry of systems containing pure sugar surfactant. Langmuir 14:4699–4709
Barclay TH, Mishra S (2016) New correlations for CO2-Oil solubility and viscosity reduction for light oils. J Pet Explor Prod Technol 6:815–823
Coninck H, Loos M, Metz B, Davidson O, Meyer L (2005) IPCC special report on carbon dioxide capture and storage. Intergovernmental Panel on Climate Change
Corvo MC, Sardinha J, Casimiro T, Marin G, Seferin M, Einloft S, Menezes SC, Dupont J, Cabrita EJ (2015) A rational approach to CO2 capture by imidazolium ionic liquids: Tuning CO2 solubility by cation alkyl branching. Chemsuschem 8:1935–1946
Dharaskar Swapnil A (2012) Ionic liquids (a review): the green solvents for petroleum and hydrocarbon industries. Res J Chem Sci ISSN 2231:606X
Domańska U (2005) Solubilities and thermophysical properties of ionic liquids. Pure Appl Chem 77:543–557
Duan Z, Sun R, Zhu C, Chou I-M (2006) An improved model for the calculation of CO2 solubility in aqueous solutions containing Na+, K+, Ca2+, Mg2+, Cl−, and SO42−. Mar Chem 98:131–139
Fan H-F, Li Z-B, Liang T (2007) Experimental study on using ionic liquids to upgrade heavy oil. J Fuel Chem Technol 35:32–35
Fan Z-X, Wang T-F, He Y-H (2009) Upgrading and viscosity reducing of heavy oils by [BMIM][AlCl4] ionic liquid. J Fuel Chem Technol 37:690–693
Foroozesh J, Jamiolahmady M (2016) Simulation of carbonated water injection coreflood experiments: an insight into the wettability effect. Fuel 184:581–589
Foroozesh J, Jamiolahmady M, Sohrabi M (2016) Mathematical modeling of carbonated water injection for EOR and CO2 storage with a focus on mass transfer kinetics. Fuel 174:325–332
Fu S, Zhou W, Wang Z, Yin B, Liu J, Sun D, Wei X (2008) Study on dynamic interfacial tension of 3-dodecyloxy-2-hydroxypropyl trimethyl ammonium bromide at liquid/liquid interface. Fluid Phase Equilib 269:93–97
Gao P, Towler B (2012) Integrated investigation of enhanced oil recovery in South Slattery Minnelusa Reservoir, part 2: CO2 miscible injection. Pet Sci Technol 30:2543–2551
Gao P, Towler BF, Pan G (2010) Strategies for evaluation of the CO2 miscible flooding process. In: Abu Dhabi International petroleum exhibition and conference; Society of Petroleum Engineers
Ghosh KK, Sharma P (2003) Reactivities of hydroxamic acid in surfactant-poly (ethylene glycol) couples. Colloids Surf A 231:113–121
Guzman-Lucero D, Flores P, Rojo T, Martínez-Palou R (2010) Ionic liquids as demulsifiers of water-in-crude oil emulsions: study of the microwave effect. Energy Fuels 24:3610–3615
Healy R, Holstein E, Batycky J (1994) 3 Status of miscible flooding technology. In: 14th World Petroleum Congress
Hezave AZ, Dorostkar S, Ayatollahi S, Nabipour M, Hemmateenejad B (2013) Dynamic interfacial tension behavior between heavy crude oil and ionic liquid solution (1-dodecyl-3-methylimidazolium chloride ([C12mim][Cl]+ distilled or saline water/heavy crude oil)) as a new surfactant. J Mol Liq 187:83–89
Honarvar B, Azdarpour A, Karimi M, Rahimi A, Afkhami Karaei M, Hamidi H, Ing J, Mohammadian E (2017) Experimental investigation of interfacial tension measurement and oil recovery by carbonated water injection: a case study using core samples from an Iranian carbonate oil reservoir. Energy Fuels 31:2740–2748
Hu Y-F, Li S, Chu Y-P, Guo T-M (2004) Wax precipitation in three Chinese reservoir oils under carbon dioxide (CO2) injection. Energy Fuels 18:1183–1186
Jarrell PM, Fox CE, Stein MH, Webb SL (2002) Practical aspects of CO2 flooding. Soc Pet Eng Richardson, TX
José-Alberto M-H, Jorge A (2011) Current knowledge and potential applications of ionic liquids in the petroleum industry. Ionic Liq Appl Perspect
Kumełan J, Kamps AP-S, Tuma D, Maurer G (2006a) Solubility of CO2 in the ionic liquid [hmim][Tf2N]. J Chem Thermodyn 38:1396–1401
Kumełan J, Pérez-Salado Kamps Á, Tuma D, Maurer G (2006b) Solubility of CO2 in the ionic liquids [bmim][CH3SO4] and [bmim][PF6]. J Chem Eng Data 51:1802–1807
Lashkarbolooki M, Riazi M, Ayatollahi S (2017) Effect of CO2 and natural surfactant of crude oil on the dynamic interfacial tensions during carbonated water flooding: experimental and modeling investigation. J Petrol Sci Eng 159:58–67
Lashkarbolooki M, Riazi M, Ayatollahi S (2018) Experimental investigation of dynamic swelling and Bond number of crude oil during carbonated water flooding. Effect Temp Press Fuel 214:135–143
Lashkarbolooki M, Hezave AZ, Ayatollahi S (2019) Swelling behavior of heavy crude oil during injection of carbonated brine containing chloride anion. J Mol Liq 276:7–14
Lemos RC, da Silva EB, dos Santos A, Guimaraes RC, Ferreira BM, Guarnieri RA, Dariva C, Franceschi E, Santos AF, Fortuny M (2010) Demulsification of water-in-crude oil emulsions using ionic liquids and microwave irradiation. Energy Fuels 24:4439–4444
Ligthelm DJ, Gronsveld J, Hofman J, Brussee N, Marcelis F, van der Linde H (2009) Novel waterflooding strategy by manipulation of injection brine composition. In: EUROPEC/EAGE conference and exhibition; Society of Petroleum Engineers
McGuire P, Chatham J, Paskvan F, Sommer D, Carini F (2005) Low salinity oil recovery: an exciting new EOR opportunity for Alaska's North Slope. In: SPE western regional meeting; Society of Petroleum Engineers
Merchant DH (2010) Life Beyond 80: A look at conventional WAG recovery beyond 80% HCPV injected in CO2 tertiary floods. In: SPE international conference on CO2 capture, storage, and utilization; Society of petroleum engineers
Morrow NR, Tang G-Q, Valat M, Xie X (1998) Prospects of improved oil recovery related to wettability and brine composition. J Petrol Sci Eng 20:267–276
Mosavat N, Torabi F (2014) Application of CO2-saturated water flooding as a prospective safe CO2 storage strategy. Energy Procedia 63:5408–5419
Mosavat N, Abedini A, Torabi F (2014) Phase behaviour of CO2–brine and CO2–oil systems for CO2 storage and enhanced oil recovery: experimental studies. Energy Procedia 63:5631–5645
Murillo-Hernández JA, García-Cruz I, López-Ramírez SN, Duran-Valencia C, Domínguez JM, Aburto J (2009) Aggregation behavior of heavy crude oil–ionic liquids solutions by fluorescence spectroscopy. Energy Fuels 23:4584–4592
Painter P, Williams P, Mannebach E (2010a) Recovery of bitumen from oil or tar sands using ionic liquids. Energy Fuels 24:1094–1098
Painter P, Williams P, Lupinsky A (2010b) Recovery of bitumen from Utah tar sands using ionic liquids. Energy Fuels 24:5081–5088
Riazi M, Golkari A (2016) The influence of spreading coefficient on carbonated water alternating gas injection in a heavy crude oil. Fuel 178:1–9
Riazi M, Sohrabi M, Jamiolahmady M, Ireland S (2009) Oil recovery improvement using CO2-enriched water injection. In: EUROPEC/EAGE conference and exhibition; Society of petroleum engineers
Riazi M, Sohrabi M, Jamiolahmady M (2011) Experimental study of pore-scale mechanisms of carbonated water injection. Transp Porous Media 86:73–86
Schilderman AM, Raeissi S, Peters CJ (2007) Solubility of carbon dioxide in the ionic liquid 1-ethyl-3-methylimidazolium bis (trifluoromethylsulfonyl) imide. Fluid Phase Equilib 260:19–22
Shaker Shiran B, Skauge A (2013) Enhanced oil recovery (EOR) by combined low salinity water/polymer flooding. Energy Fuels 27:1223–1235
Shakiba M, Ayatollahi S, Riazi M (2016) Investigation of oil recovery and CO2 storage during secondary and tertiary injection of carbonated water in an Iranian carbonate oil reservoir. J Petrol Sci Eng 137:134–143
Shariati A, Peters C (2004) High-pressure phase behavior of systems with ionic liquids: II. The binary system carbon dioxide+ 1-ethyl-3-methylimidazolium hexafluorophosphate. J Supercrit Fluids 29:43–48
Siedlecka EM, Czerwicka M, Neumann J, Stepnowski P, Fernández J, Thöming J (2011) Ionic liquids: methods of degradation and recovery. In: Ionic liquids: theory, properties, new approaches; IntechOpen
Simon R, Graue D (1964) Generalized correlation for predicting solubility swelling+ viscosity behavior of CO2-crude oil systems. J Pet Technol Soc Pet Eng 222 Palisades Creek Dr, Richardson, TX 75080, 1028
Sohrabi M, Riazi M, Jamiolahmady M, Ireland S, Brown C (2008) Carbonated water injection for oil recovery and CO2 storage. In: Sustainable energy UK conference: meeting the science and engineering challenge, Oxford, UK
Sohrabi M, Kechut NI, Riazi M, Jamiolahmady M, Ireland S, Robertson G (2011) Safe storage of CO2 together with improved oil recovery by CO2-enriched water injection. Chem Eng Res Des 89:1865–1872
Sohrabi M, Kechut NI, Riazi M, Jamiolahmady M, Ireland S, Robertson G (2012) Coreflooding studies to investigate the potential of carbonated water injection as an injection strategy for improved oil recovery and CO 2 storage. Transp Porous Media 91:101–121
Sohrabi M, Emadi A, Farzaneh SA, Ireland SA (2015) thorough investigation of mechanisms of enhanced oil recovery by carbonated water injection. In: SPE annual technical conference and exhibition; society of petroleum engineers
Somasundaran P, Zhang L (2006) Adsorption of surfactants on minerals for wettability control in improved oil recovery processes. J Petrol Sci Eng 52:198–212
Soriano AN, Doma BT Jr, Li M-H (2008a) Solubility of carbon dioxide in 1-ethyl-3-methylimidazolium 2-(2-methoxyethoxy) ethylsulfate. J Chem Thermodyn 40:1654–1660
Soriano AN, Doma BT Jr, Li M-H (2008b) Solubility of carbon dioxide in 1-ethyl-3-methylimidazolium tetrafluoroborate. J Chem Eng Data 53:2550–2555
Stiles L, Magruder J (1992) Reservoir management in the means San Andres Unit. J Petrol Technol 44:469–475
Tang G, Morrow NR (1997) Salinity, temperature, oil composition, and oil recovery by waterflooding. SPE Reserv Eng 12:269–276
Verma MK (2015) Fundamentals of carbon dioxide-enhanced oil recovery (CO2-EOR): A supporting document of the assessment methodology for hydrocarbon recovery using CO2-EOR associated with carbon sequestration; US Department of the Interior, US Geological Survey Washington, DC
Welker J (1963) Physical properties of carbonated oils. J Petrol Technol 15:873–876
Wideroee HC, Rueslaatten H, Boassen T, Crescente CM, Raphaug M, Soerland GH, Urkedal H (2010) Investigation of low salinity water flooding by NMR and CryoESEM. In: Proceedings of the 2010 International symposium of the society of core analysts, Halifax, Nova Scotia, 4–7
Yang D, Tontiwachwuthikul P, Gu Y (2005) Interfacial tensions of the crude oil+ reservoir brine+ CO2 systems at pressures up to 31 MPa and temperatures of 27 C and 58 C. J Chem Eng Data 50:1242–1249
Ye Z, Zhang F, Han L, Luo P, Yang J, Chen H (2008) The effect of temperature on the interfacial tension between crude oil and gemini surfactant solution. Colloids Surf A 322:138–141
Yim J-H, Ha S-J, Lim JS (2018) Measurement and correlation of CO2 solubility in 1-butyl-3-methylimidazolium ([BMIM]) cation-based ionic liquids:[BMIM][Ac],[BMIM][Cl],[BMIM][MeSO4]. J Supercrit Fluids 138:73–81
Zabihi S, Rahnama Y, Sharafi A, Borousan F, Zeinolabedini Hezave A, Shirazian S (2020) Experimental solubility measurements of fenoprofen in supercritical carbon dioxide. J Chem Eng Data 65:1425–1434
Zaker S, Parvizi R, Hosseini S, Ghaseminejad E (2020) Crude oil behavior during injection of solutions containing MgSO4 in the presence and absence of CO2. Energy Sources Part A Recov Util Environ Effects 1–18
Zaker S, Sharafi A, Parvizi R, Esmaeili-Faraj SH, Ghaseminejad E (2020b) Swelling behavior of heavy crude oil in carbonated water at the presence of Na2 SO4 and Mg2SO4. J Pet Explor Prod Technol 10:2759–2769
Zhao G-X, Zhu B-Y (2003) Principles of surfactant action. China Light Industry Press, Beijing, p 66
Zhao Z, Bi C, Li Z, Qiao W, Cheng L (2006) Interfacial tension between crude oil and decylmethylnaphthalene sulfonate surfactant alkali-free flooding systems. Colloids Surf, A 276:186–191
Zhao Z, Bi C, Qiao W, Li Z, Cheng L (2007) Dynamic interfacial tension behavior of the novel surfactant solutions and Daqing crude oil. Colloids Surf A 294:191–202
Zolghadr A, Escrochi M, Ayatollahi S (2013) Temperature and composition effect on CO2 miscibility by interfacial tension measurement. J Chem Eng Data 58:1168–1175
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Alabdulbari, O.A.A., Alabid, F.S.R. & Hosseini, S. Effects of formation brine, [C12mim] [Cl] concentration, temperature and pressure on the swelling factor and IFT of the carbonated water/heavy crude oil system. Braz. J. Chem. Eng. 39, 289–300 (2022). https://doi.org/10.1007/s43153-021-00210-6
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s43153-021-00210-6