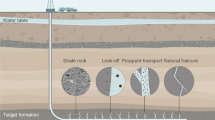
Abstract
The significance of the tracer testing technique is widely accepted in reservoir performance analysis in hydrology as well as in hydrocarbon exploration and production. The subsurface reservoir delineation for hydrocarbon exploration and optimum production is one of the most critical aspects of petroleum system analysis. The quality of the reservoir and its performance prediction require extensive knowledge of qualitative reservoir geology, its depositional environment, facies heterogeneity and engineering properties of subsurface formations. Tracer testing is amongst the few techniques available in the oil and gas (O&G) industry, which stands up to these expectations and is successfully used for quantitative determination and analysis of sub-seismic scale structural and stratigraphic heterogeneities. Tracer testing is also being utilized in determining residual oil saturation (Sor) and lateral correlation of reservoir properties in the subsurface. Apart from the O&G industry, the concentration-based applications of tracer testing have been proved in hydrology, geothermal and medical science. A comprehensive review is presented to explain the application of tracer testing technique to investigate porous media, mainly in O&G industry. The type of tracers used, their selection criteria, concentration, and natural versus gradient and qualitative to a quantitative application are discussed in the current review. Generally, two types of tracers (chemical and radioactive) are preferred in the petroleum industry for gas/fluid flow assessment, waterflood optimization and establishing connectivity between multiple wells. The current paper reviews both types of tracer tests, namely single well and inter well, in detail discussing the objectives, calculations, designing, injection, sampling, laboratory analysis and knowledge integration. The preliminary aim was to provide a review of the tracer testing technique used in reservoir evaluation and well-to-well connectivity analysis.
Similar content being viewed by others
Introduction
The recent developments of nano-technology and eco-friendly chemicals have boasted the applications of smart tracers to determine and characterize the flow behavior of gases and fluids in porous media. Tracers may be of radioactive or chemical compounds used to monitor the migration pattern of gases and fluids through the movement of tracers in subsurface lithologies mainly in reservoir formations. This may sound very simple, however, to gain the right kind of information; one needs sophisticated techniques, specific tools and data analysis skills. Tracers are being used in the O&G industry for a long time in five spot flood patterns (Baldwin M Aem 1966; Brigham et al. 1965) and in reservoir zones (Abbaszadeh-Dehghani and Brigham 1984). Application of tracers testing is proven in sanitary systems (Straub and Hagee 1957), water carven, hydro-geological, geothermal studies (Chrysikopoulos 1993), and O&G industries for reservoir studies and inter-well connectivity analysis (Davis et al. 1980). The application of tracers in the oil field was started in the mid-twentieth century (Hutchins et al. 1991; Serres-Piole et al. 2011, 2012). Nowadays, tracers and nanoparticles are being commonly used in various O&G drilling and testing operations. Tracers are the chemical or other materials that are placed in the borehole fluid to detect and infer drilled fluid pathways, migration mechanism and well-to-well connectivity. It is extensively used in well-drilling operations, water flooding studies, treatment, and various other operations at the exploration and production (E&P) stage to scale how the hydrocarbons migrate through reservoir both qualitatively and quantitatively and are also used as a significant tool for estimating Sor and enhanced oil recovery (EOR) analysis for flood optimization (Bjornstad et al. 1990; Clayton 1967; Michael Shook et al. 2004; Michael Shook et al. 2017; Sun et al. 2021; Sun and Ertekin 2020). They are applied for diagnostics where quantification of production and communication is the goal and must be foreign to the reservoir environment. Apart from the E&P industry, this method is used in contaminant mapping of hazardous chemicals, radioactive disposal sites, and many other environmental studies. Strontium isotopes have been proven as a good tool for tracing (åberg 1995; Hirsch et al. 2005; R. Tang et al. 2011; Zemel 1995h).
Practically, the tracers can be used as a tool to improve the predictability of internal properties of the reservoirs like porosity, permeability, and tortuosity. It is generally used to quantify the sweep efficiency (volumetric) between injector and producer wells (Cobb and Marek 1997; Norman et al. 2006), identification of offending injectors or other uncertainties of injector wells (Aanonsen et al. 1995; Güyagüler and Horne 2004), directional flow trend analysis to analyze injection and withdrawal rates at paired wells, to delineate the subsurface geological discontinuities (faults, fractures, deformation bends, and other structural and stratigraphic uncertainties) and flow behavior analysis (Furuse and Toda 1999; Klett et al. 1981; Wang et al. 2018), and inter-well volumetric sweep efficiency (Wagner 1977) analysis (tracing) using sweep-improvement treatments. Based on the aims and objectives of the project, the tracers can be widely categorized as active and passive tracers. The key objectives and economics of a tracer testing operation must be defined before its application or analysis (Abbaszadeh 1995; Du and Guan 2005). Nowadays, forward mathematical modeling, artificial intelligence and machine learning (AI/ML) techniques are being popular for the analysis of recorded data. AI/ML techniques are also used to develop a comprehensive tracer testing plan and amalgamate it along with the petrophysical interpretations done using the above-mentioned techniques (Joshi et al. 2021; Khilrani et al. 2021; Knackstedt et al. 2009; Sprunger et al. 2021). The general characteristics of the tracer are shown in Table 1.
The current research work attempts to review the application of the tracer technique in inter-well and single-well testing in the petroleum industry. The systematic flow of the contents discussed in the subsequent sections of this paper is shown in Fig. 1.
Types of tracers
The three important types of tracers are: (i) radioactive tracers and (ii) chemical tracers and (iii) trace substance tracers (Fig. 2). For decades, radioactive tracers were well known for an assortment of investigational purposes. However, with time, advancements in chemical tracers have benefited to E&P industry to diagnose subsurface heterogeneities and recognize reservoir quality, recovery factors and measure EOR efficiency. Radioactive isotopes are utilized to label chemical tracers to give insightful tools of high sensitivity and selectivity. The tracer properties are characterized exclusively by their chemical composition (Zemel 1995c). The application of the different types of tracers is discussed in the subsequent sections.
Radioactive tracers
The radioactive tracers were popular for a variety of investigational purposes and have been broadly accepted to study the aspects of subsurface drilling, production/ injection and development (Abernathy et al. 1994). The radioactive tracers are been injected directly into the well-bore, using a funnel. The use of radioactive materials near the well-bore diagnostics is limited to a few feet of depth of signal; the gamma rays are absorbed by dense materials such as steel and cement reservoir, rocks and fluids (Zemel 1995a). This causes the data interpretation to be limited, close to the well-bore when looking for horizontal diagnostic applications. Also, the radioactive tracers are limited to a few unique identifiable radioactive isotopes, half-life, and decay (Zemel 1995b). Health and safety management during the use and disposal of radioactive tracers can be risky, and users must adhere to the environmental hazard rules and policies (Zemel 1995h). Abernathy et al. 1994 have explained the basic methods of working and safe handling of radioactive materials depicted in Fig. 3.
Radioactive water tracers are employed in well-to-well studies to determine the movement and saturation of subterranean fluids. These tracers including radioactive gas tracers are widely used to solve the large number of environmental problems, mostly having an insignificant impact on oilfield studies. Increasingly, these minimal effects are moving into the mainstream of oilfield operations. Because of the minute quantities of either radium-226 or its daughter radon-222, used in routine oilfield activities, naturally occurring radioactive material have a minimal impact on the economic side of the oil business. In the areas, where the scale is brought to the surface for removal, the concentration of radioactivity is usually high enough. Thus, special handling is required for the same (Smith 1987). Other environmental concerns include oily water and oil spills. The oil tracers must meet the following criteria: (a) must be stable to the environment and (b) must have high sensitivity for detection and velocity profile (McLeod et al. 1971).
Chemical tracers
The effectivity of chemical tracers has been proved in petroleum exploration due to their rapid and cost-effective nature. This technique has been successfully used in fluid flow pathways and gradient analysis and monitoring in complex subsurface conditions (Zemel 1995c). Chemical tracers are used to measure the concentration of a chemical substance in a fluid, while physical tracers are used to measure temperature. Active tracers change the flow of the fluid dynamically by changing fluid parameters that appear in the equation of motion, such as density or viscosity (Ellis et al. 2016), whereas passive tracers do not influence flow (Y.-C. Chen et al. 2021). Environmentalists have traditionally been hostile to the O&G industry (Matthiessen 2000; Vora et al. 2021). Some of the environmental problems where chemical tracers can be effectively utilized are oil spills, oily water, quantitative oil field measurements, and water flooding analysis (Zemel 1995h). Chemical tracers are expected to perform at high temperature and pressure conditions. Although they can sustain extremely high pressure (as encountered in the subsurface), chemical tracers tend to degrade at temperatures above 95 degrees celcius (Gombert et al. 2017). Chemical tracers can be further divided into three subtypes: (i) chemical gas tracers, (ii) chemical liquid tracers and (iii) solid particulate tracers (Fig. 2).
Chemical gas tracer
In 1946, helium was being used as a tracer, under gas injection (Tayyib et al. 2019). As of today, sulfur hexafluoride (SF6) is the most prevalent chemical gas tracer (Fig. 3c). Per fluro carbon (PFC) group of tracer has found the most widely used chemical gas tracers and found to have wide applications. Chemical inertness, high stability, high detectability make PFCs an excellent tracer to investigate O&G and other wide-ranging subsurface/surface problems. The most frequent compounds are per fluro di-methyl cyclo butane (PDMCB), per fluro methyl cyclo pentane (PMCP), per fluro methyl cyclo hexane (PMCH), and 1, 2- and 1, 3-per fluro di-methyl cyclo hexane (1, 2-/1, 3-PDMCH). Quang et al. 2007 have researched the process and preparation of very common tracers such as Au-198, Ar-41, and CH (sub 3) Br-82. These tracers are injected through a line connected to the Christmas tree, for gaining the cooling effect. Sulfur hexafluoride too is employed and segregated on a GC column, with an electron capture detector used to monitor it (Dugstad 1992; Tayyib et al. 2019; Zemel 1995f).
Chemical liquid tracer
In the early days of tracing, dyes were commonly used in the oil industry to track fluid flow. Yet, downhole interactions with the reservoir rock causes losses, which leads to limiting the use of dyes. Recently, dye tracer experiments were directed to survey the impact of changing water hydraulic properties after oil mud wastewater (OMW) application on the generation of solute transport pathways (Mahmoud et al. 2010; Tayyib et al. 2019). Tracer studies have also been used to add the data provided by other reservoir characterization methods such as pulse and interference testing (Hutchins et al. 1991; Serres-Piole et al. 2011), thereby helping develop a new type of water tracer, which can be used in oil-filled reservoir investigations. Ethanol can correlate the population density and originate from multiple volatile chemical product (VCP) sources. Ethanol and fragrances are among the amplest and most reactive volatile organic compounds (VOCs) associated with VCP emissions (Gkatzelis et al. 2020). During the data acquisition phase, the chemical liquid tracers are injected into the formation through the annulus or the well-bore (through a line connected to the Christmas tree). Some common examples are ammonium thiocyanate (NH4SCN), N-propyl alcohol (C3H8O), isopropyl alcohol (C3H8O), N butanol (C4H10O), iso-butanol (C4H10O), and tertiary butanol (C4H10O).
Solid particulate tracers
There is a difference between the types of solids, as they are not alike in what they can measure. Particulate tracers are having some issues that are not resolved, which makes them only quantitative and limited to phase flow analysis to determining the fluid mobility and migration pathways. Naturally occurring markers, DNA, are some examples that fall in the category (Tayyib et al. 2019). Solid particulate tracing (SPT) is a quick and easy approach to characterize oil fields, determine solute transfer limitations in subterranean reservoirs, and tracing impurities. Key limits, such as a reservoir's effective porosity and permeability, can be attained by executing multi-tracer experiments and interpreting the tracer advancement timings into topographic maps. The modular nature of DNA enables the creation of an almost infinite number of distinct tracers. Because an infinite number of tracers, each with a unique DNA identification, may be used concurrently and discriminated at extremely low concentrations, the use of non-toxic synthetic DNA tracers appears promising (Kong et al. 2018; Mikutis et al. 2018; Pang et al. 2020).
Trace substance tracers
Trace substances or minor elements are chemical substances whose concentration (or any other measure of amount) is very less. They are generally classified into two categories: essential and nonessential trace substance tracers. Other than these two subcategories, the rare earth elements are also counted into the substance tracers. The humic substances play an important role in controlling metal speciation, as well as the trace element mobility in water and soil (Pédrot et al. 2010). For colloidal organic matter composition, they have resulted to be very efficient on the functional sites and quite complexes formed on the humic molecules (Catrouillet et al. 2019).
Types of tracer test
Traditional hydrocarbon exploration studies include subsurface geological and geophysical data acquisition, processing, petroleum system analysis (interpretation), reservoir facies and heterogeneity modeling, drilling, well-logging, coring and well testing. It is dominant that these studies be synchronized to have a vivid picture of porous reservoir conditions in the subsurface and to have a detailed reservoir model with associated risk and uncertainties (Abbaszadeh 1995). Generally, two types of tracer tests are preferred (i) inter-well tracer test (IWTT) and (ii) single-well tracer test (SWTT).
Inter-well tracer test
Improving reservoir descriptions by defining inter-well communication and flow paths are shown in Fig. 4. When fluids are being administered in a set pattern (5-spot, 9-spot, etc.) and each well is tagged with a distinct tracer, directional flow trends can be seen from the early tracer breakthrough at producers in a preference direction from the injectors. While directional flow trends are common, changing the injection pattern and/or injection and withdrawal rates at chosen wells can typically increase inter-well sweep efficiency (Fig. 5). The oil saturation that remained in the reservoir after the production is calculated and termed as residual oil saturation (Du and Guan 2005; Tang and Harker 1991), which can be determined using an inter-well tracer test.
Tracers are also used to investigate communication across faults and deformation bends and to determine facies heterogeneity, compartmentalization, and fluid loss. Faults having larger stratigraphic displacement, sub-seismic-scale deformation bands, and permeability pinch outs (stratigraphic wedge out) can serve as obstacles to the movement of fluids and gases hydrocarbons through porous reservoir rocks (Hutchins et al. 1991; Knackstedt et al. 2009). Bottom hole pressure build-up studies in surrounding wells are usually used to find such impediments. However, the path of these barriers can be further defined using IWTT. Generally, the coarser clastic reservoirs deposited or matured in a compressive stress regime show the development of deformation bends that can drastically reduce the lateral permeability of the reservoir (Zhang and Ertekin 2019). The tracer technique can help in delineating these zones and associated deformation zones (Agency 2004; Peralta Gomes et al. 2018; Pérez-Romero et al. 2020; Schümann and Fossen 2018; Wrona et al. 2019). Objectives (qualitative and quantitative) of IWTT are described by (Sanni et al. 2015) and are depicted in Fig. 4.
The estimation of tracer quantity needed the following data related to reservoir zonation and its facies continuity: (i) reservoir thickness map (isopach/isochron) and pay zones details along with hydrocarbon contacts. (ii) Porosity, which is the percentage of void spaces present in the reservoir. For IWTT total porosity (φ) is preferred. (iii) Water saturation (Sw), which is defined as the fraction of pore spaces occupied in the reservoir by water. (iv) The tracer detection limit—It is the lowest quantity of tracer that can be distinguished at the detection point (producing well) in a given time. (v) The surface distance between the injection well and producing wells (If there are multiple injectors, the distance factor should be carefully calculated). (vi) The safety factor depends greatly on the water concentration of the reservoir and nearby aquifers, and the values vary between 10 and 100. It accounts for the dilution of tracer concentration when tracer-laden fluid comes in contact with water present in the reservoir or aquifer (Aydin and Akin 2001; Field 2003; Mohammadi et al. 2021).
For radioactive tracers
For chemical tracers
where
RDF = Radiation dilution factor
DL = Detection limit
h = Reservoir pay thickness (m)
φ = Porosity
Sw = Water saturation
L = Distance between injector and producer
t1/2 = half-life of a radioactive isotope (for chemical tracers it is infinite)
MDL = Minimum detection limit
t = Total time considered for the project
α = Dispersion constant, i.e., how the chemical has been dispersed in the reservoir, basically the adsorption level.
Single-well tracer testing
The SWTT is used to determine the remaining oil saturation in a near wellbore region (AlAbbad et al. 2016; Ferreira et al. 1992). This method exploits the time lag of back-produced ester vs. hydrolyzed alcohol (Fig. 6). The time delay is due to chromatographic separation between passive (alcohol) and partitioning tracer (acetate) and is related to saturation of a stagnant phase (oil) and the tracer’s partition coefficients between the flowing (water) and stagnant phase for the tracer. The tracer testing is a reliable in situ measurement of remaining oil saturation, which defines the target for enhancing oil recovery and at the same time allows us to estimate how much bypassed oil could be in the field (Berkeley and Oak Ridge 2009; Braconnier et al. 2019; Deans and Mut 1997). Other than that, it does not alter the wettability of the formation. It helps in covering larger pore volume for residual oil saturation compared to other residual oil saturation calculation tools. Adding to the above, the SWTT is not suitable for the heterogeneous reservoir. If the reservoir is heterogeneous, the flow back of chemicals to the wellbore is not sure and recovery could be uncertain or very low (C. Chen et al. 2014; Ding et al. 2017).
The injected slug volume depends on the following factors: (a) the radius of investigation, which is the area around the well bore in which the tracer test needs to be conducted and readings are to be taken. (b) The injection and production rates, basically defined as the rate of injection and production, affect the volume of a slug to be injected. (c) The hydrolysis reaction rate, which is defined as the partitioning tracer reacts with water and produces a passive tracer (Field 2003). The rate of this reaction affects the volume of a slug to be injected. The slug volume can be estimated using the following equation,
where
R = radius of investigation
H = thickness of the formation
Φ = porosity
Sw = average water saturation
β = Retardation factor (there is a predetermined range of retardation factor for calculating the slug volume, we assume one and use it for calculation)
Slug size = (1/3) * (slug volume) containing partitioning tracer and material balance tracer.
Push volume = (2/3) * (slug volume) containing only material balance tracer.
Design phase
Proper planning and execution are imperative for the success of a tracer test. There are many phases required in a tracer test (Fig. 7) of which the design phase is the most important. Once the tracer is designed and injected into the borehole, it will be difficult to recover/ modify the tracer injection quantities or types. There are two sections of designing phase,
-
(i)
(i) Instrument Checking- It helps in determining the minimum detection limit of a tracer and efficient working of the instrument (done approximately once in a decade).
-
Step 1—Prepare a high concentration solution of the tracer, for example. 106 ppm, i.e., the stock solution.
-
Step 2—Further prepare more diluted samples by taking parts of the stock solution and then diluting it. For example, in order to produce 105 ppm solution, take 10 ml stock solution and dilute it to make 100ml of the solution. Using equation N1V1 = N2V2 where N1 = 106 ppm, V1 = 10 ml, V2 = 100 ml, we get N2 = 105 ppm. Similarly to make 104 ppm solution, take 1 ml stock solution and dilute it to 100ml. Prepare a range of different concentration solutions (1ppm to 106 ppm)
-
Step 3—Add the same amount of reagent to each sample and allow it to react. Then, one can analyze the variation and change in color.
-
Step 4—After analyzing the color, one can get an optimum range. The steps will be further repeated within that optimum range to get the minimum detectable concentration of the tracer.
-
-
(ii)
Field-Wise Designing Programme
For static conditions
-
Step 1—Make a specific concentration solution (for example. 0.5% formation water solution)
-
Step 2—Take 2 vials from the solution at t=0 days to study its properties. Take one vial with formation rocks and one vial without formation rocks.
-
Step 3—Keep the solution at the desired temperature and pressure conditions, so that the reservoir environment could be simulated. Again take 2 vials after t=1 day, one vial with formation rocks and one without.
-
Step 4—Repeat step 3 for t=2, t=3 and t=4 days.
-
In total, one generates 10 samples for a specific concentration solution. Change a parameter, i.e., either the percentage of the fluid (formation water or injected water) and obtain 10 samples for each unique combination of parameters. Ultimately, 5–6 times more samples are generated compared to the initial conditions.
For dynamic conditions
The core is put up in a core plug in a core flooding apparatus. The core is dipped into the formation water and left there for a few days depending on the objectives of the project. This helps in saturating the dry core with the formation water. The plug is then inserted into a still jacket, which has a rubber slip. The rubber slip has a diameter that can accommodate the core plug (Fig. 8). Then, the slip is flushed with silicon oil or water. Continuous injection of water or silicon oil creates pressure over the slip. The slip contracts due to the build-up pressure and ultimately the core plug starts feeling the effects of pressure. Eventually, the plug is brought to the reservoir pressure and pseudo-reservoir conditions are created. Then, oil is passed through the still jacket. It displaces some water and thus gives us the oil saturation (Alrumah and Ertekin 2019). Before the tracer test is run, some oil has already been produced from the reservoir and some water injection has also been done. To account for these factors, water is again passed through the jacket. Now the tracer-laden fluid is injected into the core plug, and results are analyzed.
Schematics of a core flooding apparatus (Hematpour et al. 2016)
For SWTT partitioning tracers are used. Partitioning tracers are the tracers that have an affinity for multiple phases and therefore a partition between two or more phases. Generally, ethyl acetate (CH3COOC2H5), also called Ester, is used as a primary tracer for SWTT. It is a less polar or more nonpolar compound; therefore, it has more affinity toward oil. Ester reacts with water; it is injected through at the reservoir pressure and temperature and undergoes a hydrolysis reaction.
Ethanol thus formed in this in situ reaction works as a secondary or passive tracer, i.e., the tracer knowingly formed after the reaction of the primary tracer with water. Ethanol thus formed is a more polar compound and thus has an affinity toward water. Ethanol is more soluble in water and less soluble in oil, whereas the ester is more soluble in oil and less soluble in water.
A tracer's partitioning coefficient (k) is calculated. It is the ratio of the tracer concentration in oil to the tracer concentration in water. Different laboratory tests are done to determine the partitioning coefficient.
where
K = partitioning coefficient.
Co = concentration of ester in oil.
Cw = concentration of ester in water.
Three factors affect the partitioning coefficient. They are-
-
Oil composition If the components are more polar, they will avoid ester from dissolving in them. Therefore, oil composition should be known.
-
Salinity—Higher the salinity, lower the interstitial spaces between the molecules of water and thus lower the stability.
-
Temperature—Higher the temperature, the higher the solubility. It should be kept in mind that the temperature should not be high enough to vaporize the tracer.
Operation execution phase
This phase involves the field execution of the tracer test. It is further subdivided into two stages:
Injection sampling
This stage demands the involvement of a reservoir engineer, chemist, and field specialist. Specific tracer injection equipment is needed to inject the tracer into the wellbore. The first piece of equipment needed is a tank, made up of steel, with an inbuilt stirrer. The stirrer rotates, creating turbulence and thus helping the tracer to get dissolved in the injection water. The quantity of the sampling is highly dependent on the project objectives and requirements, but the maximum capacity of the tank can be up to 50,000 L. The tank may have considerable height. Therefore, to check the contents and supervise the mixing procedure, one needs to climb the tank and see through a steel net or sieve. Once the injection fluid is prepared, it needs to be transferred/ injected from the tank to the borehole through concealed pipes (Cubillos et al. 2007; Hadi et al. 2016). While injecting the fluid into the borehole, injector and producer wellbore volumes are also considered (Davarpanah et al. 2019; Sallam et al. 2015). For example, to inject 50m3 of injection fluid into a well whose wellbore volume is 10m3, an additional 20m3 (10m3 for injector well and 10m3 for producer well) of push volume needs to be injected after injection of tracer fluid. Push volume compensates for the injector and producer wellbore volume. The push volume occasionally contains some chemicals that wash off the tracer stuck on the wellbore wall and further mix them up with the initial injected fluid. Once the fluid has been injected, a proper sampling frequency plan needs to be followed. Improper sampling might give false results, and excessive sampling could increase the monetary cost of the test and scant sampling could lead to missing important details like breakthrough time, etc. The sampling interval is directly proportional to the distance between the injector and producer well. Lesser the distance, the lesser the interval (Stockinger et al. 2016). Water samples (for an individual well) may be stored in bottles that normally are collected from the separator. Water sampling is cheap, and frequent sampling is advised (Stockinger et al. 2016). Only a portion of the gathered samples must be tested at first; if no tracer is identified, intermediate samples can be discarded. Once a tracer has been identified, samples are studied backward until a tracer breakthrough is discovered. Some tracers may biodegrade after sampling in certain circumstances. To circumvent this, a biocide might be given to the sample just after it is collected. Bacterial growth can be inhibited by adding 0.1 ppm NaN3 to the stock solution. Samples need to be sent for analysis as soon as they are produced to optimize the results. Once the breakthrough is achieved, the sampling frequency interval is kept static and then gradually decreased.
The plot of tracer concentration detected vs. time when the sample was procured has a bell-shaped curve with the peak denoting the breakthrough (Chrysikopoulos 1993; Field 2003; Hutchins et al. 1991; Mohammadi et al. 2021; Sanni et al. 2015; J. S. Tang and Harker 1991). Improper injection and sampling phase could lead to the failure of the tracer test. There could be a loss of the fluid injected, and thus, improper or no results would be obtained. The reasons for fluid loss could be-
-
a)
There could arise a case where none of the tracers reaches the surface through the production well. This could be attributed to the fact that there might be fractures or high permeability formations or regions that could have swayed the tracer-induced fluid into an altogether different area that is not covered in the sampling program (R. Zhang et al. 2021).
-
b)
Fractures or high permeability channels could also lead to insignificant pressure buildup at the injector even after the entire calculated fluid volume has been injected. The insignificant pressure could denote a short-cut between the injector and producer, into which the fluid has traveled, thus giving false or negative results (Na et al. 2020).
-
c)
Improper pretest simulation study of the ‘to be done’ tracer test could pose as a hindrance to flow and might result in flow loss (Silin and Tsang 2003).
-
d)
Even if the tracer test is perfectly planned and executed, a poor sampling program could lead to the failure of the objectives of the test. Low or insufficient sampling could lead to false results, which could ultimately lead to declaring that the injected fluid has been lost to the formation (Field 2002, 2003).
-
e)
Improper study of the subsurface could also be a factor. If the reservoir is naturally fractured and it is not taken into account, it could lead to the injected fluid bypassing the monitoring well and could go to places very far away using the natural fractures of the reservoir (Harvey et al. 1996; King et al. 1997).
-
f)
Another reason could be the presence of an aquifer. The tracer has a specific concentration in the injected water. If this injected fluid comes in contact with the aquifer, it could further dilute the concentration of the tracer. The dilution could be so severe that it could drop the concentration of the tracer below the minimum detection limit and the tracer could go unnoticed by the producer (Du and Guan 2005).
For SWTT (Fig. 6), the primary tracer is injected via water into the wellbore followed by rinsing the well-bore with an adequate volume of water or chemical so that there is no retention of its mass (Löfgren et al. 2007). Once the fluid is injected, the well is shut off for a calculated period. Shutting off the well provides a calm subsurface environment for the tracer to react with the fluids present in the injection zone. The primary tracer used in SWTT further breaks down into active and passive tracers. Another compound, i.e., IPA, a material balance tracer, is also injected along with the ester to determine the total recovery. This IPA is inert to oil, water and other tracers. As the reaction takes place, an ester is partitioned into ethanol. Some amount of ester dissolves into oil and some remains in water depending upon its partitioning coefficient (AlAbbad et al. 2016; Serres-Piole et al. 2012; Tayyib et al. 2019). Of the amount that remains in the water, ethanol generates that further dissolves in water and disturbs the partitioning equilibrium between ester in oil and ester in water. To account for this disturbance, some ester moves from oil to water to maintain the partitioning coefficient (Michael Shook et al. 2004; Löfgren et al. 2007). Once the shut-in period is complete, production begins and sampling starts. Initially, large amounts of ethanol are received along with minute quantities of ester. This is followed by a surge in the amount of ester detected. It is critical to avoid cross-contamination due to the highly sensitive analytical procedures required. Tracer activities must be meticulously planned to avoid any close contact between injection and sampling equipment (Michael Shook et al. 2004; Löfgren et al. 2007; Mahmoud et al. 2010). If injection pumps or tracer containers are transported after injection in the same vehicle or housed in the same building as sample bottles or sample equipment, it could be a source of contamination.
Laboratory analysis
This stage primarily involves a specialist chemical engineer for methodical detection and interpretation of the collected tracer samplings. The sample collected needs to be processed/ filtered prior to analysis (AlAbbad et al. 2016; Bjornstad et al. 1990; Michael Shook et al. 2017; Serres-Piole et al. 2011; Stockinger et al. 2016). The concentrations of the tracers are measured using several procedures in the laboratory. Various perspectives will have varying degrees of uncertainty; therefore, it is important to distinguish between the detection limit and the quantification limit (Serres-Piole et al. 2012). It can be difficult to acquire precise quantification of the tracer at concentrations near the detection limit; as a result, some laboratories just declare "detected" without quantification when the concentration is low. The samples can be analyzed in a variety of ways. The approaches that are most regularly utilized are listed here.
(i) Gas chromatography
Chromatography is defined as the process that is used to separate components present in a mixture (Ermagambet et al. 2016). There are two basic phases present in a chromatography experiment: the mobile phase and the stationary phase. The mobile phase dissolves the mixture to be separated in itself and then transports it through the stationary phase (Eiceman et al. 2002). The main phenomenon behind the separation is the different travel velocities of different components while traveling through the stationary phase. The nature and properties of the mobile phase and the stationary phase affect the velocity of the components. The difference in velocity leads to a difference in the travel time between components going through the stationary phase (Santos and Galceran 2002). This difference in travel time is defined as the retention time.
To explain the concept of chromatography, refer to Fig. 9. The pipette-A consists of a stationary phase (light blue color), which is nonpolar in this case (based on assumption). A sample is loaded onto the stationary phase, which always is solid in nature. The loaded sample is composed of two different components, the more polar or less nonpolar component (violet color) and the less polar or more nonpolar component (orange color). A polar type of solvent is used as the mobile phase is added (Pipette-B, Fig. 9). Now once the solvent gets past the loaded sample, it attracts a similar natured more polar component (Pipette-C, Fig. 9). This results in the segregation of the components and different speeds of the components. The one more attracted to the stationary phase moves slowly. The one attracted to the solvent moves fast and reaches the nozzle (Pipette-D, Fig. 9). This process helps in the segregation of the components (Gin and Imwinkelried 2018; Zuo et al. 2013).
This method is applicable when the different components display various colors. When there is no segregation based on color; then, gas chromatography is preferred. It is applicable for volatile tracers (example hydrocarbon tracers) only. The tracer sample is obtained in the liquid state in a vial with a rubber cork topped with an aluminum sheet with a small hole in it. The vial is heated to vaporize the molecules present in the fluid (Zuo et al. 2013). The vapor gets trapped between the rubber cork and the liquid interface. Once the vapors are trapped, an empty syringe is inserted into the vial through the small hole in the aluminum covering. The needle of the syringe punctures right through the rubber cork into the vial. The vapors are sucked into the syringe and are ready for analysis. The contents of the syringe are emptied into the gas sampling valve (GSV). A beaker of water is placed at the vent point of GSV (Cuddeback et al. 2002). Bubbles occurring on its surface confirm the presence and passage of gas. A gas chromatograph consists of different sections that perform various specialized functions. One of its sections prepares the sample that is injected into the already loaded stationary phase, which is prepared in another section, through insulated pipes. A mobile phase is then added to this section. The mobile phase is always a gas such as nitrogen and helium. For detecting various components present in the sample, different detectors are placed at the nozzle of the tube (Ashworth 1964). The commonly used detectors are-
-
a)
Flame ionization detector (FID)
A flame is placed at the nozzle of the pipette. It breaks the leaving molecule into its components. This ignition of the leaving molecule changes its structure leading to breaking of the bonds and development of free electrons, which can move freely. This FID detects the free electron and gives a voltage corresponding to the concentration of the electrons detected (Hage 2018; Hinshaw 2006). This voltage reading is recorded and is plotted on a graph for comparison and further analysis. It gives a bell-shaped curve, and the area under the curve gives the relative concentration of molecules. This area is used to calculate the tracer quantity recovered. Gases used for ignition in FID are hydrogen and air.
(b) Electron capture detector (ECD)
It works for electronegative elements only. A flame is provided at the nozzle, and the molecule is broken into components. Once the components are segregated, they are bombarded with electrons. The electronegative components of the molecule attract the electron to complete their octet. The electrons that are not attracted by the electro-negative elements reach the detector (Bunert et al. 2017). A voltage fluctuation is developed with respect to the received electrons, and a graph is plotted (Lasa et al. 1994). Even if two components show a similar voltage fluctuation, they can be differentiated based on the time at which they are received. If two components come out at the same time, a temperature difference is provided to further segregate them out (Pasco and Schwarz 1983). This process is called temperature ramping.
(ii) Spectrophotometer
The basic principle behind the working of the spectrophotometer is the Beer–Lembert law. The law states that there is a linear relationship between the concentration and absorbance of a solution. It associates the attenuation of light with the characteristics or properties of the material it is forced to pass through (Mayerhöfer et al. 2019; Swinehart and Schmidt 1967). This method of tracer detection and calculation is used for nonvolatile chemical tracer detection. The sample solution is diluted with a coloring agent, which imparts color when reacted with tracer molecule. After a specific standard time has been given to the colored solution, it is put up in a test tube for further investigation. Spectrophotometer passes light through this test tube and the colored molecules absorb light. The light absorbed (decrease in the amplitude of the incident light) (Gin and Imwinkelried 2018) is proportional to the concentration of molecules present.
(iii) Liquid scintillation counter
It is used for radioactive tracer detection. The tracer always keeps on emitting beta particles (β particles). The solution is bombarded with photons, which react with β particles and emit a different compound and detected at the detectors (Hou 1989; Hou and Dai 2020). For SWTT, the concentration of ethanol and ester received vs. the production done is plotted on a graph. This graph gives two bell-shaped curves each having a distinguished peak. The horizontal distance between the two peaks gives the retardation factor (Fig. 10).
Plotting of the passive and partitioning tracer concentration received vs. production done for an SWTT (Bu et al. 2014)
β = Retardation factor.
Qa = Total liquid production value when peak of ester is received.
Qb = Total liquid production value when peak of alcohol is received.
The retardation factor and partitioning coefficient are used to determine the residual oil saturation.
Sros = Residual oil saturation.
K = Partitioning coefficient.
β = Retardation factor.
Tracer interpretation or tracer concentration calculation is a subsection of the laboratory analysis phase. It depends on multiple scenarios and has various restrictions and parametric limitations. According to Xie et al. 2012, interpretation methods are broadly classified according to their consideration of the geological model. The method not taking geological model into account is termed as landmark, and their counterparts are further divided into three subsections, namely analytic method, numerical simulation method and semianalytic method. Application of these methods further depends upon the complexity of the well injection and production pattern and the migration mechanism adopted by the injected fluid. Table 2 depicts a review of different types of tracers used to study different areas of oil and gas industry along with their detection and or interpretation methods.
Conclusion
Tracers are a rapid, cost-effective way to gain insight into complex reservoirs where interactions of multiple variables make simulation or analytical description difficult. Chemical tracers have a proven track record of delivering a greater return on investment for operators by providing important flow insights into porous reservoirs. However, the success of a tracer study is dependent on using the correct tracer and a suitable technique. Similarly, data analysis through machine learning methods (Joshi et al. 2021; Khilrani et al. 2021) combined with the results obtained through the tracer test can be integrated with the knowledge of reservoirs gained from other sources to successfully plan secondary and tertiary O&G recovery methods.
-
1.
The radioactive and chemical tracers are the most common and prominent ones within various types of available tracers. A comprehensive delineation of subsurface and near-wellbore properties can be done by effectively selecting and utilizing these tracers. Although tracers are subjected to environmental criticism, constant technological developments and availability of novel tracers are making tracer testing a sought after tool for reservoir characterization.
-
2.
Different types of tracer testing techniques, IWTT and SWTT, make tracer testing adaptable for all kinds of field development programs. Present reservoir characterization tools, surveys and logs, characterize only the near wellbore region or give a generalized picture of the reservoir, whereas tracer testing gives an exhaustive insight into the reservoir and helps in the formulation of an effective and efficient secondary and tertiary development plan for the reservoir.
-
3.
Tracer analysis methods such as gas chromatography, spectrophotometer and liquid scintillation counter can be coupled with artificial intelligence/machine learning principles for generating and comprehending the results of tracer testing. AI/ML techniques and principles can also be used to develop an efficient plan for conducting and implementing an effective tracer test.
-
4.
Apart from reservoir characterization, with the advent of technology, tracers are being used for production profiling as well. Smart tracers not only help in characterizing the reservoir but they also detect, quantify and monitor phase breakthroughs (Samantray et al. 2018). Sustained-release tracers are one such example of smart tracers. They are cost-effective, sustainable, and relatively simpler than production logging tools to help develop the production profile of wells (Li et al. 2021). Nano-tracers are also used where quantitative subatomic detection is required.
-
5.
Tracer testing has also been proven to help in unconventional reservoirs. The unconventional reservoirs are subjected to multiple fracturing and EOR techniques (Joshi et al. 2022); tracers can also be utilized to study the effect of production while fracturing and EOR process are underway (Wu et al. 2022). Therefore, providing a real-time data analysis of the process helps in optimizing the same.
References
Aanonsen SI, Eide AL, Holden L, Aasen JO (1995) Optimizing reservoir performance under uncertainty with application to well location. Proc SPE Annu Tech Conf Exhib Sigma. https://doi.org/10.2118/30710-MS
Abbaszadeh M (1995) Analytical flow model for design and analysis of tracer pulse tests. Dev Pet Sci 43:429–473. https://doi.org/10.1016/s0376-7361(06)80012-9
Abbaszadeh-Dehghani M, Brigham WE (1984) Analysis of well-to-well tracer flow to determine reservoir layering. J Petrol Technol 36(10):1753–1762. https://doi.org/10.2118/10760-PA
åberg G (1995) The use of natural strontium isotopes as tracers in environmental studies. Water Air Soil Pollut 79(1):309–322. https://doi.org/10.1007/BF01100444
Abernathy SE, Woods SE, Taylor JL (1994) Radioactive tracers in oil and gas production: practical considerations in the 1990’s. SPE Health Saf Environ Oil Gas Explor Prod Conf. https://doi.org/10.2118/27236-MS
Baldwin M Aem DE (1966) Prediction of tracer performance in a five-spot pattern. J Petrol Technol 18(04):513–517. https://doi.org/10.2118/1230-PA
Agency IAE (2004) Validation of tracers and software for interwell investigations
AlAbbad M, Balasubramanian S, Sanni M, Kokal S, Zefzafy I, Adam F, AlHajji A (2016) Single-well chemical tracer test for residual oil measurement: field trial and case study. In: Society of petroleum engineers - SPE Kingdom of Saudi Arabia annual technical symposium and exhibition. https://doi.org/10.2118/182811-MS
Alrumah M, Ertekin T (2019) Prediction of the water saturation around wells with bottom water drive using artificial neural networks. J Pet Gas Eng 10(2):14–22. https://doi.org/10.5897/JPGE2018.0299
Ashworth RJ (1964) An electron capture detector unit for gas chromatography. J Chromatogr Sci 2(8):252–253. https://doi.org/10.1093/CHROMSCI/2.8.252
Aydin H, Akin S (2001) Analysis of tracer tests with simple spreadsheet models. Comput Geosci 27(2):171–178. https://doi.org/10.1016/S0098-3004(00)00084-4
Berkeley C, Oak Ridge T (2009) Insight from simulations of single-well injection-withdrawal tracer tests on simple and complex fractures. Lawrence Berkeley National Laboratory, Berkeley
Bjornstad T, Garder K, Hundere I, Michelsen OB (1990) Tracer tests in oil appraisal and reservoir evaluation: state of the art. North Sea Oil Gas Reserv II:261–270. https://doi.org/10.1007/978-94-009-0791-1_22
Bjørnstad T, Haugen OB, Hundere IA (1994) Dynamic behavior of radio-labelled water tracer candidates for chalk reservoirs. J Petrol Sci Eng 10(3):223–238. https://doi.org/10.1016/0920-4105(94)90083-3
Braconnier B, Preux C, Douarche F, Bourbiaux B (2019) MUSCL scheme for single well chemical tracer test simulation, design and interpretation. Oil Gas Sci Technol Revue d’IFP Energies Nouvelles, Institut Français Du Pétrole. https://doi.org/10.2516/ogst/2018090ï
Brigham BE, Aime M, Smith DH, Member AIME, J. (1965) Prediction of tracer behavior in five-spot flow. In: Society of petroleum engineers - conference on production research and engineering, PRE 1965, pp 103–109. https://doi.org/10.2118/1130-MS
Bu PX, AlSofi AM, Liu J, Benedek L, Han M (2014) Simulation of single well tracer tests for surfactant–polymer flooding. J Pet Explor Prod Technol 5(4):339–351. https://doi.org/10.1007/S13202-014-0143-9
Bunert E, Kirk AT, Oermann J, Zimmermann S (2017) Electron capture detector with non-radioactive electron source. Proceedings 1(4):443. https://doi.org/10.3390/PROCEEDINGS1040443
Catrouillet C, Guenet H, Pierson-Wickmann A-C, Dia A, LeCoz MB, Deville S, Lenne Q, Suko Y, Davranche M, Catrouillet C, Guenet H, Pierson-Wickmann A-C, Dia A, LeCoz MB, Deville S, Lenne Q, Suko Y, Davranche M (2019) Rare earth elements as tracers of active colloidal organic matter composition. Environ Chem 17(2):133–139. https://doi.org/10.1071/EN19159
Chen C, Balhoff M, Mohanty KK (2014) Effect of reservoir heterogeneity on primary recovery and CO2 Huff ‘n’ Puff recovery in shale-oil reservoirs. SPE Reserv Eval Eng 17(03):404–413. https://doi.org/10.2118/164553-PA
Chen Y-C, Jolicoeur B, Chueh C-C, Wu K-T (2021) Flow coupling between active and passive fluids across water–oil interfaces. Sci Rep 11(1):1–16. https://doi.org/10.1038/s41598-021-93310-9
Choppin GR, Liljenzin J-O, Rydberg J (2002) Radiation biology and radiation protection. Radiochem Nucl Chem. https://doi.org/10.1016/B978-075067463-8/50018-2
Chrysikopoulos CV (1993) Artificial tracers for geothermal reservoir studies. Environ Geol 22(1):60–70. https://doi.org/10.1007/BF00775286
Clayton GC (1967) A survey of the application of radiation techniques in oil and mineral boreholes.
Cobb WM, Marek FJ (1997) Determination of volumetric sweep efficiency in mature waterfloods using production data. In: SPE annual technical conference and exhibition, San Antonio, Texas, October, vol 56, pp 182–190. https://doi.org/10.2118/38902-MS
Cubillos H, Torgersen H, Chatzichristos C, Kleven R, Dugstad O, Galdiga C (2007) Integrated approach: key for successful interwell tracer project. In: Offshore Mediterranean conference and exhibition
Cuddeback JE, Burg WR, Birch SR (2002) Calibration of a gas sampling valve for gas chromatography. Anal Chem 47(2):355–356. https://doi.org/10.1021/AC60352A034
Davarpanah A, Mirshekari B, Razmjoo AA (2019) A simulation study of water injection and gas injectivity scenarios in a fractured carbonate reservoir: a comparative study. Pet Res 4(3):250–256. https://doi.org/10.1016/J.PTLRS.2019.02.001
Davis SN, Thompson GM, Bentley HW, Stiles G (1980) Ground-water tracers: a short review. Groundwater 18(1):14–23. https://doi.org/10.1111/J.1745-6584.1980.TB03366.X
Deans HA, Mut AD (1997) Chemical tracer studies to determine water saturation at prudhoe bay. SPE Reserv Eng (soc Pet Eng) 12(1):52–57. https://doi.org/10.2118/28591-PA
Ding M, Yuan F, Wang Y, Xia X, Chen W, Liu D (2017) Oil recovery from a CO2 injection in heterogeneous reservoirs: The influence of permeability heterogeneity, CO2-oil miscibility and injection pattern. J Nat Gas Sci Eng 44:140–149. https://doi.org/10.1016/J.JNGSE.2017.04.015
Du Y, Guan L (2005) Interwell tracer tests: lessons learned from past field studies. SPE Asia Pac Oil Gas Conf Exhib Proc 2005:211–219. https://doi.org/10.2523/93140-ms
Dugstad Ø (1992) An experimental study of tracers for labelling of injection gas in oil reservoirs, vol. 25, Issue 4) [University of Bergen]. http://inis.iaea.org/Search/search.aspx?orig_q=RN:25008965
Eiceman GA, Gardea-Torresdey J, And EO, Carney K, Dorman F (2002) Gas chromatography. Anal Chem 74(12):2771–2780. https://doi.org/10.1021/AC020210P
Ellis ES, Kosynkin D, Askar MN (2016) Transforming oil tracer studies. In: Society of petroleum engineers: SPE Kingdom of Saudi Arabia annual technical symposium and exhibition. https://doi.org/10.2118/182773-ms
Ermagambet BT, Abylgazina LD, Kholod AV, Bagnato G (2016) Petrochemistry and chemical engineering cobalt-based catalyst supported on modified shungite for fisher-tropsch synthesis water gas shift in membrane reactor: an experimental study. J Pet Environ Biotechnol. https://doi.org/10.4172/2157-7463.C1.026
Ferreira LEA, Descant FJ, Delshad M, Pope GA, Sepehrnoori K (1992) A single-well tracer test to estimate wettability. In: SPE/DOE enhanced oil recovery symposium, Tulsa, Oklahoma, April 1992. https://doi.org/10.2118/24136-MS
Field MS (2003) A review of some tracer-test design equations for tracer-mass estimation and sample-collection frequency. Environ Geol 43(8):867–881. https://doi.org/10.1007/S00254-002-0708-7
Field MS (2002) Efficient hydrologic tracer-test design for tracer-mass estimation and sample-collection frequency. 2. Experimental results. Environ Geol 42(7):839–850. https://doi.org/10.1007/S00254-002-0592-1
Furuse H, Toda K (1999) Analysis of flow behavior of non-Newtonian fluids based on a concept of traveling force. J Biosci Bioeng 87(2):218–223. https://doi.org/10.1016/S1389-1723(99)89016-1
Gin J, Imwinkelried EJ (2018) Gas chromatography-mass spectrometer (GC/MS): in scientific evidence, even “gold standard” techniques have limitations. SSRN Electron J. https://doi.org/10.2139/SSRN.3245423
Gkatzelis GI, Coggon MM, McDonald BC, Peischl J, Aikin KC, Gilman JB, Trainer M, Warneke C (2020) Identifying volatile chemical product tracer compounds in U.S. cities. Environ Sci Technol 55(1):188–199. https://doi.org/10.1021/ACS.EST.0C05467
Gombert P, Biaudet H, Sèze R, de Pandard P, Carré J (2017) Toxicity of fluorescent tracers and their degradation byproducts. Int J Speleol 46(1):5. https://doi.org/10.5038/1827-806X.46.1.1995
Güyagüler B, Horne RN (2004) Uncertainty assessment of well-placement optimization. SPE Reserv Eval Eng 7(01):24–32. https://doi.org/10.2118/87663-PA
Hadi K, Saravana Kumar U, Al-Senafy M, Mukhopadhyay A, Al-Khalid A, Al-Fahad K, Bhandary H (2016) Multi-well and multi-tracer tests to characterize the groundwater aquifers in southern Kuwait. Environ Earth Sci 75(20):1–19. https://doi.org/10.1007/S12665-016-6143-Y
Hage DS (2018) Chromatography. Princ Appl Clin Mass Spectrom Small Mol Pept Pathog. https://doi.org/10.1016/B978-0-12-816063-3.00001-3
Harvey JW, Wagner BJ, Bencala KE (1996) Evaluating the reliability of the stream tracer approach to characterize stream-subsurface water exchange. Water Resour Res 32(8):2441–2451. https://doi.org/10.1029/96WR01268
Hematpour H., Parvazdavani M., Abbasi S., Mahmood SM (2016). Investigation of low saline water effects on relative permeability in carbonate reservoirs. J Teknol; 78(10): 93–99. https://doi.org/10.11113/JT.V78.8661
Hinshaw JV (2006) The flame ionization detector. LC GC Europe 19(4):206–216
Hirsch RL, Bezdek R, Wendling R (2005) Peaking of world oil production: Impacts, mitigation, and risk management. https://doi.org/10.2172/939271
Hou X, Dai X (2020) Environmental liquid scintillation analysis. In Handbook of radioactivity analysis. Vol 2. Academic Press. https://doi.org/10.1016/B978-0-12-814395-7.00002-7
Hou X (1989) Liquid scintillation counting for the determination of beta emitter: principle and application risø national laboratory for.
Hutchins RD, Dovan HT, Sandiford BB (1991) Aqueous tracers for oilfield applications. SPE Int Symp Oilfield Chem Anaheim, California. https://doi.org/10.2118/21049-ms
Joshi D, Patidar AK, Mishra A, Mishra A, Agarwal S, Pandey A, Dewangan BK, Choudhury T (2021) Prediction of sonic log and correlation of lithology by comparing geophysical well log data using machine learning principles. GeoJournal. https://doi.org/10.1007/S10708-021-10502-6
Joshi D, Prajapati P, Sharma P, Sharma A (2022) Past, present and future of coal bed methane (CBM): a review with special focus on the Indian scenario. Int J Coal Prep Util. https://doi.org/10.1080/19392699.2022.2051014
Khilrani N, Prajapati P, Patidar AK (2021) Contrasting machine learning regression algorithms used for the estimation of permeability from well log data. Arab J Geosci 14(20):1–14. https://doi.org/10.1007/S12517-021-08390-8
King AC, Mitchell CA, Howes T (1997) Hydraulic tracer studies in a pilot scale subsurface flow constructed wetland. Water Sci Technol 35(5):189–196. https://doi.org/10.2166/WST.1997.0195
Klett RD, Tyner CE, Hertel JES (1981) Geologic flow characterization using tracer techniques. https://doi.org/10.2172/6536587
Knackstedt MA, Latham S, Madadi M, Sheppard A, Varslot T, Arns C (2009) Digital rock physics: 3D imaging of core material and correlations to acoustic and flow properties. The Lead Edge 28(1):28–33. https://doi.org/10.1190/1.3064143
Kong X-Z, Deuber CA, Kittilä A, Somogyvári M, Mikutis G, Bayer P, Stark WJ, Saar MO (2018) Tomographic reservoir imaging with DNA-labeled silica nanotracers: the first field validation. Environ Sci Technol 52(23):13681–13689. https://doi.org/10.1021/ACS.EST.8B04367
Lasa J, Drozdowicz B, Śliwka I (1994) A theoretical model of the electron capture detector. Chromatographia 38(5):304–312. https://doi.org/10.1007/BF02269772
Li H, Liu Z, Li Y, Luo H, Cui X, Nie S, Ye K (2021) Evaluation of the release mechanism of sustained-release tracers and its application in horizontal well inflow profile monitoring. ACS Omega 6(29):19269–19280. https://doi.org/10.1021/ACSOMEGA.1C02748
Löfgren M, Crawford J, Elert M (2007) Tracer tests - possibilities and limitations. Experience from SKB fieldwork: 1977–2007. https://www.skb.com/publication/1563949/R-07-39.pdf
Mahmoud M, Janssen M, Haboub N, Nassour A, Lennartz B (2010) The impact of olive mill wastewater application on flow and transport properties in soils. Soil Tillage Res 107(1):36–41. https://doi.org/10.1016/J.STILL.2010.01.002
Matthiessen P (2000) Environmental impact of the offshore oil and gas industry. Environ Pollut 110(2):367. https://doi.org/10.1016/S0269-7491(00)00082-8
Mayerhöfer TG, Pipa AV, Popp J (2019) Beer’s law-why integrated absorbance depends linearly on concentration. ChemPhysChem 20(21):2748–2753. https://doi.org/10.1002/CPHC.201900787
McLeod WR, Rhodes DF, Day JJ (1971) Radiotracers in gas-liquid transportation problems-a field case. J Petrol Technol 23(08):939–947. https://doi.org/10.2118/3059-PA
Michael Shook G, Shannon L, Wylie A (2004) Tracers and tracer testing: design, implementation, tracer selection, and interpretation. Methods. https://doi.org/10.2172/910642
Michael Shook G, Sharma A, Pope GA (2017) Early-time analysis of tracers for use in enhanced-oil-recovery flood optimization. SPE Reserv Eval Eng 20(01):030–041. https://doi.org/10.2118/169109-PA
Mikutis G, Deuber CA, Schmid L, Kittilä A, Lobsiger N, Puddu M, Asgeirsson DO, Grass RN, Saar MO, Stark WJ (2018) Silica-encapsulated DNA-based tracers for aquifer characterization. Environ Sci Technol 52(21):12142–12152. https://doi.org/10.1021/ACS.EST.8B03285
Mohammadi Z, Behrouj Peely A, Raeisi E (2021) Breakthrough curves of dye tracing tests in karst aquifers: review of effective parameters based on synthetic modeling and field data. J Hydrol. https://doi.org/10.1016/J.JHYDROL.2021.126604
Na X, Yin H, Liang H, Fu C, Wen G, Zhou M (2020). Pressure buildup test analysis for hydraulic fractured horizontal well with stimulated reservoir volume in tight oil reservoirs. In: Proceedings of the international field exploration and development conference 2019. IFEDC 2019. Springer Series in Geomechanics and Geoengineering, Singapore, 522–533. https://doi.org/10.1007/978-981-15-2485-1_48
Norman C, De Lucia JP, Turner BO (2006) Improving volumetric sweep efficiency with polymer gels in the cuyo basin of Argentina. In: SPE/DOE symposium on improved oil recovery, Tulsa, Oklahoma, USA, April 2006. https://doi.org/10.2118/99379-MS
Pang L, Abeysekera G, Hanning K, Premaratne A, Robson B, Abraham P, Sutton R, Hanson C, Hadfield J, Heiligenthal L, Stone D, McBeth K, Billington C (2020) Water tracking in surface water, groundwater and soils using free and alginate-chitosan encapsulated synthetic DNA tracers. Water Res 184:116192. https://doi.org/10.1016/J.WATRES.2020.116192
Pasco RW, Schwarz JA (1983) Temperature-ramp resistance analysis to characterize electromigration. Solid-State Electron 26(5):445–452. https://doi.org/10.1016/0038-1101(83)90101-6
Pédrot M, Dia A, Davranche M (2010) Dynamic structure of humic substances: rare earth elements as a fingerprint. J Colloid Interface Sci 345(2):206–213. https://doi.org/10.1016/J.JCIS.2010.01.069
Peralta Gomes C, Fossen H, de Almeida RP, Salmoni B (2018) Subseismic deformation in the Vaza-Barris Transfer Zone in the Cretaceous Recôncavo-Tucano-Jatobá rift system, NE Brazil. J Struct Geol 117:81–95. https://doi.org/10.1016/J.JSG.2018.09.007
Perez-Romero R, Espinosa-Leon C, Pinto-Rambauth K, Gutierrez-Benavides M. (2020). Practical methodology for interwell tracer applications. CT&F - Ciencia, Tecnologia y Futuro, 10, 27–38. https://doi.org/10.29047/01225383.261
Quang NH, Tri BQ, Thai H, Ngan K, Nguyen D, Duy T (2007) Establishment of tracer technologies in gas phase, vol 40, issue 23. http://inis.iaea.org/Search/search.aspx?orig_q=RN:40057554
Sallam S, Ahmad MM, Nasr M (2015). The effect of water injection on oil well productivity. Flex Autom Intell Manuf. https://doi.org/10.13140/RG.2.1.2892.8486
Samantray A, Kaiping C, Al Neyadi T, Almazrouei S, Al Marzouqi M, Alshmakhy A, Al Hammadi Y, Al Hosani F, Al Dhanhani H, Chitre S, Tippit J, Chatterjee M, Ford P (2018) First application of controlled release smart tracer technology for inflow monitoring in offshore abu dhabi – towards cost optimization and de-risking operation. In: Society of petroleum engineers - Abu Dhabi international petroleum exhibition and conference 2018, ADIPEC 2018. https://doi.org/10.2118/193246-MS
Sanni ML, Al-Abbad MA, Kokal SL, Hartvig S, Olaf H, Jevanord K (2015) A field case study of inter-well chemical tracer test. Proc SPE Int Symp Oilfield Chem 2:753–769. https://doi.org/10.2118/173760-MS
Santos FJ, Galceran MT (2002) The application of gas chromatography to environmental analysis. Trends Anal Chem 21(9–10):672–685. https://doi.org/10.1016/S0165-9936(02)00813-0
Schümann H, Fossen M (2018) Oil-water dispersion formation, development and stability studied in a wheel-shaped flow loop. J Petrol Sci Eng 162:567–576. https://doi.org/10.1016/J.PETROL.2017.10.066
Serres-Piole C, Preud’homme H, Moradi-Tehrani N, Allanic C, Jullia H, Lobinski R (2012) Water tracers in oilfield applications: guidelines. J Pet Sci Eng 98–99:22–39. https://doi.org/10.1016/J.PETROL.2012.08.009
Serres-Piole C, Commarieu A, Garraud H, Lobinski R, Preud’homme H (2011) New passive water tracers for oil field applications. Energy Fuels 25(10):4488–4496. https://doi.org/10.1021/EF2007485
Silin DB, Tsang CF (2003) A well-test analysis method accounting for pre-test operations. SPE J 8(01):22–32. https://doi.org/10.2118/83644-PA
Smith AL (1987) Radioactive-scale formation. J Petrol Technol 39(06):697–706. https://doi.org/10.2118/14589-PA
Sprunger C, Muther T, Syed FI, Dahaghi AK, Neghabhan S (2021) State of the art progress in hydraulic fracture modeling using AI/ML techniques. Model Earth Syst Environ 2021:1–13. https://doi.org/10.1007/S40808-021-01111-W
Stockinger MP, Bogena HR, Lücke A, Diekkrüger B, Cornelissen T, Vereecken H (2016) Tracer sampling frequency influences estimates of young water fraction and streamwater transit time distribution. J Hydrol 541(Part B):952–964. https://doi.org/10.1016/j.jhydrol.2016.08.007
Straub CP, Hagee GR (1957) Radioactive tracers in sanitary engineering. J Am Water Works Assoc. https://doi.org/10.1002/j.1551-8833.1957.tb16844.x
Sun Q, Ertekin T (2020) Screening and optimization of polymer flooding projects using artificial-neural-network (ANN) based proxies. J Petrol Sci Eng 185:106617. https://doi.org/10.1016/J.PETROL.2019.106617
Sun Q, Ertekin T, Zhang M, On T (2021) A comprehensive techno-economic assessment of alkali–surfactant–polymer flooding processes using data-driven approaches. Energy Rep 7:2681–2702. https://doi.org/10.1016/J.EGYR.2021.05.003
Swinehart JH, Schmidt WG (1967) Reaction of pentacyanonitrosylferrate(II) with bases. III. Propanone-, butanone-, and 3-pentanone-hydroxide solutions. Inorg Chem 6(2):232–237. https://doi.org/10.1021/IC50048A009
Gesell TF (1975). Occupational radiation exposure due to 222Rn in natural gas and natural gas products. Health Phys. https://inis.iaea.org/search/search.aspx?orig_q=RN:7223736
Tang JS, Harker B (1991) Interwell tracer test to determine residual oil saturation in a gas-saturated reservoir. Part II: field applications. J Can Pet Technol. https://doi.org/10.2118/91-04-01
Tang R, Bai Y, Wang T (2011) Research on GIS application system of environmental risk for hazardous chemicals enterprises. Procedia Environ Sci 10(PART B):1011–1016. https://doi.org/10.1016/J.PROENV.2011.09.162
Tayyib D, Al-Qasim A, Kokal S, Huseby O (2019) Overview of tracer applications in oil and gas industry. In: Society of petroleum engineers - SPE kuwait oil and gas show and conference 2019, KOGS 2019. https://doi.org/10.2118/198157-MS
Vértes A, Nagy S, Klencsár Z, Lovas RG, Rösch F (2003) Tracer technique. Handb Nucl Chem 2:1267–1297. https://doi.org/10.1007/0-387-30682-x_29
Vora M, Sanni S, Flage R (2021) An environmental risk assessment framework for enhanced oil recovery solutions from offshore oil and gas industry. Environ Impact Assess Rev 88:106512. https://doi.org/10.1016/J.EIAR.2020.106512
Wagner OR (1977) The use of tracers in diagnosing interwell reservoir heterogeneities: field results. J Petrol Technol 29(11):1410–1416. https://doi.org/10.2118/6046-PA
Wallick GC, Jenkins R (2004) Analysis of short-time tracer injection in underground formations. J Appl Phys 25(12):1491. https://doi.org/10.1063/1.1702370
Wang M, Fan Z, Dong X, Song H, Zhao W, Xu G (2018) Analysis of flow behavior for acid fracturing wells in fractured-vuggy carbonate reservoirs. Math Probl Eng. https://doi.org/10.1155/2018/6431910
Wrona T, Magee C, Fossen H, Gawthorpe RL, Bell RE, Jackson CAL, Faleide JI (2019) 3-D seismic images of an extensive igneous sill in the lower crust. Geology 47(8):729–733. https://doi.org/10.1130/G46150.1
Wu L, Zhang J, Jia D, Wang S, Yan Y (2022) Performance evaluation of multistage fractured horizontal wells in tight gas reservoirs at Block M, Ordos Basin. Energies 15(2):613. https://doi.org/10.3390/EN15020613
Xie X, Liu T, Zhang X (2012) One fast resolution and application of mathematical model for tracer movement. Procedia Eng 37:85–89. https://doi.org/10.1016/J.PROENG.2012.04.207
Zemel B (1995a) Chapter 1 radioactivity basics. In: Developments in petroleum science, vol 43, issue C, pp 1–38. Elsevier. https://doi.org/10.1016/S0376-7361(06)80004-X
Zemel B (1995b) Chapter 2 measurements and applications. In: Developments in petroleum science, vol 43, issue C, pp 39–87. Elsevier. https://doi.org/10.1016/S0376-7361(06)80005-1
Zemel B (1995c) Chapter 3 interwell water tracers. In: Developments in petroleum science, vol 43, issue C, pp 89–135. Elsevier. https://doi.org/10.1016/S0376-7361(06)80006-3
Zemel B (1995d) Chapter 4 field examples and data analysis. In: Developments in petroleum science, vol 43, issue C, pp 137–189. Elsevier. https://doi.org/10.1016/S0376-7361(06)80007-5
Zemel B (1995e) Chapter 5 unconventional waterflood tracing. In: Developments in petroleum science, vol. 43, issue C, pp 191–242. Elsevier. https://doi.org/10.1016/S0376-7361(06)80008-7
Zemel, B. (1995f). Chapter 6 interwell gas tracing. In: Developments in petroleum science, vol. 43, issue C, pp 243–291. Elsevier. https://doi.org/10.1016/S0376-7361(06)80009-9
Zemel B (1995g) Chapter 7 downhole tracers. In: Developments in petroleum science, vol 43, issue C, pp 293–369. Elsevier. https://doi.org/10.1016/S0376-7361(06)80010-5
Zemel B (1995h). Chapter 8 tracers in facility operations. In: Developments in petroleum science vol 43, issue C, pp 371–426. Elsevier. https://doi.org/10.1016/S0376-7361(06)80011-7
Zhang R, Zhu Z, Wang C, Guan Z (2021) Research on an lost circulation zone location method based on transient pressure wave. ACS Omega 6(39):25807–25818. https://doi.org/10.1021/ACSOMEGA.1C04359
Zhang Z, Ertekin T (2019) Proxy models for evaluation of permeability, three-phase relative permeability, and capillary pressure curves from rate-transient data. SIMULATION 97(2):109–133. https://doi.org/10.1177/0037549719857137
Zuo H-L, Yang F-Q, Huang W-H, Xia Z-N (2013) Preparative gas chromatography and its applications. J Chromatogr Sci 51(7):704–715. https://doi.org/10.1093/CHROMSCI/BMT040
Acknowledgements
All the authors are gratefully acknowledged to the University Of Petroleum and Energy Studies (UPES), Dehradun. This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Author information
Authors and Affiliations
Contributions
All authors equally contributed to the preparation of this review paper.
Corresponding authors
Ethics declarations
Conflict of interest:
Authors state that there is no conflict of interest in the publication of this work.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Patidar, A.K., Joshi, D., Dristant, U. et al. A review of tracer testing techniques in porous media specially attributed to the oil and gas industry. J Petrol Explor Prod Technol 12, 3339–3356 (2022). https://doi.org/10.1007/s13202-022-01526-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13202-022-01526-w