Abstract
Injecting carbon dioxide into oil reservoirs has the potential to serve as an enhanced oil recovery (EOR) technique, mitigating climate change by storing CO2 underground. Despite the successful achievements reported of CO2 to enhance oil recovery, mobility control is one of the major challenges faced by CO2 injection projects. The objective of this work is to investigate the potential of using surfactant and a mixture of surfactant and nanoparticles to generate foam to reduce gas mobility and enhance oil recovery. A newly developed anionic surfactant and a mixture of the surfactant and surface-modified silica nanoparticles were used to assess the ability of generating a stable foam at harsh reservoir conditions: sc-CO2 and high temperature. Dynamic foam tests and coreflood experiments were conducted to evaluate foam stability and strength. To measure the mobility of injected fluids in sandstone rocks, the foam was generated by co-injection of sc-CO2 and surfactant, as well as a mixture of surfactant and nanoparticles at 90% quality. The coreflood experiments were conducted using non-fractured and fractured sandstone cores at 1550 psi and 50 °C. The use of surfactant and mixture was able to generate foam in porous media and reduce the CO2 mobility. The mobility reduction factor (MRF) for both cases was about 3.5 times higher than that of injecting CO2 and brine at the same conditions. The coreflood experiments in non-fractured sandstone rocks showed that both surfactant and a mixture of surfactant and nanoparticles were able to enhance oil recovery. The baseline experiment in the absence of surfactant resulted in a total recovery of 71.50% of the original oil in place. However, the use of surfactant was able to bring oil recovery to 76% of the OOIP. The addition of nanoparticles to surfactant, though, resulted in higher oil recovery, 80% of the OOIP. In fractured rocks, oil recoveries during secondary production mechanisms for the mixture, the surfactant alone, and sc-CO2 alone were 12.62, 8.41, and 7.21% of the OOIP, respectively. Huge amount of oil remains underground following the primary and secondary oil production schemes. CO2 has been widely used to enhance oil recovery. However, its high mobility might result in unfavorable and unsuccessful projects. The use of specially designed surfactants and the synergistic effect of surfactant and nanoparticles may provide a solution to stabilize CO2/brine foam at harsh reservoir conditions and, therefore, reduce the gas mobility and, consequently, enhance oil recovery.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The remaining oil underground following traditional recovery mechanisms is considerably huge (Hirasaki et al. 2011). Typically, fields can produce about 45–50% of the original oil in place (OOIP) following primary and secondary oil production mechanisms (Sandrea and Sandrea 2007). As a result, the oil produced is coming up short in meeting the ever-increasing global energy demand (EIA 2011). Enhanced oil recovery (EOR) techniques are needed to recover this huge amount of residual oil. CO2 is extensively applied for enhancing oil recovery. Technically, it can promote swelling, reduce oil viscosity, vaporize, and extract portions of crude oil. Moreover, the easy solubility of CO2 in oil makes it an ideal gas for EOR applications (Slobod and Koch 1953; Enick et al. 1988; Bayraktar and Kiran 2000). Despite the reported successes of CO2 injection, a major challenge faced by this technique is poor volumetric sweep efficiency. Major factors that contribute to this problem are the low density and viscosity of CO2 relative to reservoir fluids, as well as reservoir heterogeneity such as high permeability and heavily fractured zones (Campbell and Orr 1985; Chakravarthy et al. 2004; Masalmeh et al. 2010). To solve this issue, either conformance control or mobility control techniques might be applied (Seright 1997; Choi et al. 2010; Pu et al. 2018; Torrealba and Hoteit 2018; Enick et al. 2012). Some researchers recommended the use of both techniques to overcome the poor sweep efficiency challenge. They recommended to start first with the use of gel, as an example, for conformance control and then the CO2 foam for in-depth mobility control (Enick et al. 2012). The focus of this work will be on the mobility control of CO2 during EOR processes.
The high mobility of injected gas may lead to early breakthrough of gas, leaving most of the residual/trapped oil untouched and increasing the gas-to-oil ratio (GOR). To solve the CO2 injection issues, several approaches have been tested. The most reported and applied approaches are: water alternating gas (WAG), generation of foams, and increasing gas viscosity by adding thickening agents (Christensen et al. 1998; Chakravarthy et al. 2004; Enick 1998; Dalland and Hanssen 1997, Enick et al. 2012; Dandge and Heller 1987; Heller 1994). The use of foam has the potential to reduce the gas mobility in a petroleum reservoir by increasing the gas apparent viscosity and reducing the gas relative permeability and, hence, improve the volumetric sweep efficiency (Falls et al. 1988; Kovscek and Radke 1994). However, the generation and stabilization of foam at reservoir conditions are major challenges. The major contributors to foam destabilization in porous media are the harsh conditions such as reservoir temperature and salinity, surfactant adsorption to the rock, and the presence of crude oil (Mannhardt et al. 1993; Al-Hashim et al. 1988; Figdore 1982; Grigg and Bai 2005).
Nanoparticles (NPs) were used to stabilize CO2/brine emulsion at reservoir conditions (Espinoza et al. 2010; Al Otaibi et al. 2013; Worthen et al. 2013a, b). Also, the use of specially designed surfactants and the synergistic effect of surfactant and NPs may provide a solution to stabilize CO2/brine foam at harsh reservoir conditions and, therefore, reduce the gas mobility and, consequently, enhance oil recovery (AlYousef et al. 2017a). Worthen et al. 2013a, b used non-modified silica NPs and caprylamidopropyl betaine (CAPB) surfactant. The mixture produced a stable and viscous CO2-in-water foam when neither of these materials could stabilize foam individually at experimental conditions. Singh et al. 2015 used fly ash powder and three types of surfactants: anionic, cationic, and nonionic. In the presence of NPs, anionic and nonionic surfactants produced foam with smaller bubble size. In porous media, NPs and anionic surfactant produced a stable foam. AlYousef et al. 2017a, b also reported a stable foam when they mixed anionic surfactant and coated silica NPs. Binks et al. 2015 reported a stable foam by mixing calcium carbonate (CaCO3) particles and sodium stearoyl lactylate surfactant (SSL). Xue et al. 2016 found that mixing silica NPs and laurylamidopropyl betaine (LAPB) surfactant produced a viscous foam with small bubble sizes.
The objective of this study is to investigate foam strength using a newly developed anionic surfactant and the mixture of the surfactant and surface-modified silica NPs. Importantly, this study reports the CO2 mobility reduction factor (MRF) and oil recovery factors as a result of using the surfactant and the mixture at 1550 psi and 50 °C.
Materials
The surfactant used in this study is a complex nanofluid (CNF) anionic surfactant. The NPs used are surface-modified silica nanoparticles received in aqueous form from Nyacol Chemicals (DP 9711). The size of the particles was measured using dynamic light scattering (DLS) and found to be 30 nm ± 1. Brine was prepared using deionized water (DI) (ASTM Type II, Lab Chem) and sodium chloride (99%, Cole-Parmer). The cores used in this study were non-fractured and fractured Bentheimer sandstone from Kocurek Industries. Table 1 summarizes the properties of these cores. The oil used in this study was North Burbank Unit (NBU) oil with an average viscosity of 3.2 cp at 50 °C.
Methodology
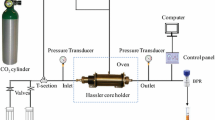
This study consists mainly of dynamic foam tests and coreflood experiments for CO2, surfactant, and the mixture of surfactant and silica NPs. The dynamic foam was generated using a coreflood apparatus, as shown in Fig. 1, and the CO2 mobility was evaluated in rock samples at 1550 psi, 50 °C, and 90% quality (the gas fractional flow in the co-injection process). At least five pore volumes (PVs) of 1 wt% brine were injected at 5 ft/day to ensure the sample was 100% saturated with brine. The BPR was set to be 1550 psi. The baseline experiment was conducted through a co-injection of sc-CO2 and brine at 90% quality. For other experiments, the samples were pre-flushed with a surfactant or a mixture of surfactant and NPs at 5 ft/day for 1 PV before starting the co-injection. Then, the co-injection of sc-CO2 and surfactant/mixture was conducted also at 90% quality and the drop in pressure was recorded for each case. The same setup at the same conditions, except that water was injected at 3 ft/d during waterflooding process, was used to conduct coreflood experiments to assess the ability of the generated foam to reduce gas mobility and enhance oil recovery. Non-fractured rocks were used to run the mobility tests, while fractured and non-fractured rocks were used to conduct the coreflood experiments. For fractured rocks, the sample was initially 100% saturated with crude oil. Fractures were created through the horizontal axis by cutting the rocks from the center.
During sample preparation, the diluted surfactant and NPs solutions were stirred separately overnight to ensure homogeneity. The NPs were then added to the surfactant solution slowly, in a stepwise fashion, to avoid aggregation of NPs. The size of NPs was measured before and after the mixing to verify that no extensive aggregation occurs during mixing. The concentration of surfactant and NPs used was 0.50 wt%. The brine was prepared with 1 wt% NaCl.
Results and discussion
Dynamic foam tests
Comparisons here were based on recorded pressure drops across core samples and calculated MRF for the three cases: baseline, surfactant, and mixture of surfactant and NPs. Rock sample #1 was used to conduct the baseline experiment. The results, as shown in Fig. 2, showed that the steady-state pressure drop for the baseline experiment was about 0.29 psi. Bentheimer sample #2 was used to conduct the experiments in the absence and presence of NPs. In the absence of NPs, the foam behavior was excellent at the first PVs injected. After that, it had a sudden drop in the pressure values and it produced a foam with a steady-state pressure drop of 0.88 psi, as shown in Fig. 2. These results reflect the stability rather than the foamability. Surfactants have the ability to reduce the CO2/water IFT and generate foams, but the stability is challenging. In the presence of NPs, the behavior was similar to that in the absence of NPs. However, it had a lower foam generation ability in the first PVs injected. After 1.5 PVs of the co-injection process, as shown in Fig. 2, the mixture resulted in a slightly higher steady-state pressure drop, 1 psi, than the surfactant case. This is an indication of the ability of NPs to produce a more stable foam in porous media. The foamability (the ability of a material to generate foam) might be higher for the surfactant case; however, the foam stability is much better in the case of the mixture. The permeability of the rocks used here was about 1.5 Darcy, so these reported values are still acceptable. The MRF values calculated for both the surfactant and mixture were found to be 3.04 and 3.45, respectively. This means that both the surfactant and the mixture were able to reduce the CO2 relative permeability and increase the gas apparent viscosity, thereby reducing gas mobility.
Coreflood experiments
Two sets of experiments were conducted to assess the ability of foam to enhance oil recovery, one in non-fractured rocks (3–5) and the other in fractured rocks (6–8).
Non-fractured rocks
Coreflood experiments showed that both conditions, with and without NPs, improved oil recovery during foam injection processes, with higher recovery in the presence of NPs. Figure 3 shows the results of coreflood experiments following waterflooding and CO2 injection. Oil recovery following the waterflooding process was about 32.82% of the OOIP. At least 4 PVs of water were injected to ensure that no more oil could be recovered in this process and to diminish any capillary end effects that might exist. Then, CO2 was injected at 5 ft/d and total oil recovery reached 71.50% of the OOIP. This means that CO2 was able to produce about 38.68% of the OOIP and 57.58 of the remaining oil in place. The average pressure drop during CO2 injection was about 0.36 psi.
Figure 4 shows the results of coreflood experiments when surfactant was used. Oil recovery following the waterflooding process was about 36.15% of the OOIP. As before, at least 4.5 PVs of water were injected to ensure that no more oil could be recovered in this process and to diminish any capillary end effects that might exist. Then, 1 PV of surfactant was injected as a pre-flush step. The objective of this step was to minimize the adsorption that might occur during the co-injection processes. There was no significant amount of oil produced during the pre-flush step. The surfactant foam was able to produce about 39.90% of the OOIP and 62.50% of the remaining oil in place. This brought the total oil recovery to around 76.06% of the OOIP. This is 4.56% higher than injecting CO2 alone. The average pressure drop during the co-injection process of CO2 and surfactant was about 0.71 psi. This is almost double that of injecting only CO2.
The next experiment, as shown in Fig. 5, was for the mixture of surfactant and NPs. The same procedures used in the previous experiment were used in this run. Oil recovery following the waterflooding process was about 35.73% of the OOIP. The pre-flush with the mixture was not able to significantly recover any additional oil. During the co-injection processes, the mixture was able to produce about 44.33% of the OOIP and 68.97% of the remaining oil in place. The total oil recovery following the mixture foam process was around 80.05% of the OOIP. This is around 4% higher than the previous experiment where only surfactant was used and 8.55% higher than CO2. The average pressure drop during the co-injection process of CO2 and the mixture was about 1.16 psi. This is higher than both the surfactant and CO2 cases.
A comparison between the three cases is presented in Fig. 6. The highest oil recovery was reported for the mixture, while the lowest was for CO2. The high oil recovery produced for CO2 was because the experiment was conducted at or near the minimum miscibility pressure (MMP) of CO2 in NBU oil. The higher oil recovery reported for surfactant compared to CO2 demonstrates the ability of foam flooding to reduce gas mobility and enhance oil recovery. Also, the higher recovery of the mixture compared to that of surfactant demonstrates the ability of the presence of NP to further reduce gas mobility, improving the gas sweep efficiency and, therefore, recovering more oil.
Fractured rocks
Similar to the previous experiments, the results of coreflood experiments here on non-fractured rocks showed improved oil recovery during the foam injection processes, with higher recovery when NPs were used. Figure 7 shows the results of coreflood experiments for the baseline case, surfactant, and the mixture.
For the baseline experiment, the oil recovery following the waterflooding process was about 59.71% of the OOIP. At least 4 PVs of water were injected at 3 ft/d to ensure that no more oil could be recovered in this process and to diminish any capillary end effects that might exist. Then, CO2 was injected at 5 ft/d and the total oil recovery reached 66.92% of the OOIP. This means that the CO2 was able to produce about 7.21% of OOIP and 17.90% of the remaining oil in place.
For the surfactant case, the oil recovery following the waterflooding process was about 54.01% of the OOIP. At least 5.5 PVs of water were injected at 3 ft/d to ensure that no more oil could be recovered in this process and to diminish any capillary end effects that might exist. Then, 1 PV of surfactant was injected at 1.5 ft/d as a pre-flush step. There was no significant amount of oil produced during the pre-flush step. The co-injection process was conducted at 5 ft/d and 90% quality. The surfactant foam was able to produce about 8.41% of the OOIP and 15.28% of the remaining oil in place. This brought the total oil recovery to be around 62.42% of the OOIP. Even though the total oil recovery of CO2 was higher than surfactant, the recovery factor during foam injection was higher than the CO2 case. The surfactant produced 8.41% following waterflooding, whereas the CO2 recovered 7.21% of the OOIP. Also, the recovery factor during the waterflooding for the CO2 case was higher than the surfactant case, 59.71% for CO2 versus 54.01% for surfactant. This resulted in a higher total recovery for CO2 compared to surfactant.
The next run, as shown in Fig. 7, was for the case where the mixture of surfactant and NPs was used. The same procedure as in the previous experiment was used in this run. The oil recovery following the waterflooding process was about 57.90% of the OOIP. A small amount of oil was produced during the pre-flush process. During the pre-flush and co-injection processes, the mixture was able to produce about 12.62% of the OOIP and 29.98% of the remaining oil in place. The total oil recovery following the mixture foam process was around 70.52% of the OOIP. This is around 8.10% higher than the previous experiment in which only surfactant was used and 3.60% higher than CO2.
A comparison between the three cases is presented in Fig. 8. The highest oil recovery was reported for the mixture, while the lowest was for surfactant. However, the results reported for surfactant compared to CO2 are already discussed above. The high oil recovery reported for all cases was because the experiments were conducted at or near the minimum miscibility pressure (MMP) of CO2 in NBU oil. Also, the rock samples were 100% saturated with oil. The higher oil recovery reported for surfactant compared to CO2, at the secondary recovery scheme, demonstrates the ability of foam flooding to reduce gas mobility, hence improving oil recovery. Also, the higher recovery of the mixture compared to that of surfactant and CO2 demonstrates the ability of NPs to further reduce gas mobility, improving the sweep efficiency and, therefore, recovering more oil. The summary of the performance of waterflooding and secondary recovery schemes can be found in Fig. 8.
Conclusion
Anionic surfactant and a mixture of anionic surfactant and surface-modified silica nanoparticles were used in this study to assess the ability of the surfactant and the mixture to stabilize CO2/brine foam at reservoir conditions. Dynamic foam tests were conducted to test the ability of the surfactant and the mixture to generate foam in porous media and reduce CO2 mobility. Coreflood experiments were performed in Bentheimer non-fractured and fractured sandstone rocks to examine the ability of the generated foam to reduce gas mobility and enhance oil recovery. Based on the results of dynamic foam tests and coreflood experiments:
At harsh reservoir conditions, both surfactant and mixture were able to reduce the sc-CO2 mobility about 3–4 times.
Using non-fractured rocks, the mixture of surfactant and NPs recovered about 80.05% of the OOIP. This is around 4% higher than surfactant and 8.55% higher than sc-CO2.
Using fractured rocks, the presence of NPs was able to improve the oil recovery compared to the surfactant and pure sc-CO2 injection cases. The oil recoveries during secondary production mechanisms for CO2, surfactant, and mixture were 7.21, 8.41, and 12.62% of the OOIP, respectively.
References
Al Otaibi FM, Kokal SL, Chang Y et al (2013) Gelled emulsion of CO2-water-nanoparticles. In: SPE annual technical conference and exhibition, New Orleans, Louisiana, USA. SPE 166072
Al-Hashim HS, Celik MS, Oskay MM et al (1988) Adsorption and precipitation behaviour of petroleum sulfonates from Saudi Arabian limestone. J Pet Sci Eng 1(4):335–344
AlYousef ZA, Almobarky MA, Schechter DS (2017a) Enhancing the stability of foam by the use of nanoparticles. Energy Fuels 31(10):10620–10627. https://doi.org/10.1021/acs
AlYousef ZA, Almobarky MA, Schechter DS (2017b) The effect of nanoparticles aggregation on surfactant foam stability. J Colloid Interface Sci 511(2018):365–373
Bayraktar Z, Kiran E (2000) Miscibility, phase separation, and volumetric properties in solutions of poly(dimethylsiloxane) in supercritical carbon dioxide. J Appl Polym Sci 75(11):1397–1403
Binks BP, Campbell S, Mashinchi S, Piatko MP (2015) Dispersion behavior and aqueous foams in mixtures of a vesicle-forming surfactant and edible nanoparticles. Langmuir 31(10):2967–2978
Campbell BT, Orr FM Jr (1985) Flow visualization for CO2/crude-oil displacements. Soc Pet Eng J 25(5):665–678
Chakravarthy D, Muralidharan V, Putra E et al (2004) Application of X-ray CT for investigation of CO2 and WAG injection in fractured reservoirs. In: Canadian international petroleum conference, Calgary, Alberta. PETSOC-2004-232
Choi SK, Sharma MM, Bryant S et al (2010) pH-sensitive polymers for novel conformance-control and polymer-flood applications. SPE Reserv Eval Eng 13(06):926–939
Christensen JR, Stenby EH, Skauge A (1998) Review of WAG field experience. In: International petroleum conference and exhibition of mexico, Villahermosa, SPE-39883-MS
Dalland M, Hanssen JE (1997) Enhanced foams for efficient gas influx control. In: International symposium on oilfield chemistry, Houston, SPE-37217-MS
Dandge DK, Heller JP (1987) Polymers for mobility control in CO2 floods. In: SPE international symposium on oilfield chemistry, San Antonio, SPE-16271-MS
EIA (2011) Annual energy outlook 2011 with projections to 2035. US Department of Energy
Enick RM (1998) A literature review of attempts to increase the viscosity of dense carbon dioxide. University of Pittsburgh, Pittsburgh
Enick RM, Holder GD, Morsi BI (1988) A thermodynamic correlation for the minimum miscibility pressure in CO2 flooding of petroleum reservoirs. SPE Reserv Eng 3(1):81–92
Enick RM, Olsen DK, Ammer JR et al. (2012) Mobility and conformance control for CO2 EOR via thickeners, foams, and gels—a literature review of 40 years of research and pilot tests. In: SPE improved oil recovery symposium, Tulsa, SPE-154122-MS
Espinoza DA, Caldelas FM, Johnston KP et al. (2010) Nanoparticle-stabilized supercritical CO2 foams for potential mobility control applications. In: SPE improved oil recovery symposium, Tulsa, SPE-129925-MS
Falls AH, Hirasaki GJ, Patzek TW et al (1988) Development of a mechanistic foam simulator: the population balance and generation by snap-off. SPE Reserv Eng 3(3):884–892
Figdore PE (1982) Adsorption of surfactants on kaolinite: NaCl versus CaCl2 salt effects. J Colloid Interface Sci 87(2):500–517
Grigg RB, Bai B (2005) Sorption of surfactant used in CO2 flooding onto five minerals and three porous media. In: SPE international symposium on oilfield chemistry, The Woodlands, SPE-93100-MS
Heller JP (1994) Foams: fundamentals and applications in the petroleum industry. In: CO2 foams in enhanced oil recovery, ACS advances in chemistry (Reprint), vol 242. Washington, pp 201–234
Hirasaki G, Miller CA, Puerto M (2011) Recent advances in surfactant EOR. SPE J 16(4):889–907
Kovscek AR, Radke CJ (1994) Fundamentals of foam transport in porous media. In: Foams: fundamentals and applications in the petroleum industry, Chap. 3, Advances in Chemistry, American Chemical Society, 115–163
Mannhardt K, Schramm LL, Novosad JJ (1993) Effect of Rock type and Brine composition on adsorption of two foam-forming surfactants. SPE Adv Technol Ser 1(1):212–218
Masalmeh SK, Hillgartner H, Al-Mjeni RA et al. (2010) Simultaneous injection of miscible gas and polymer (SIMGAP) to improve oil recovery and sweep efficiency from layered carbonate reservoirs. In: SPE EOR conference at oil & gas West Asia, Muscat, SPE-129645-MS
Pu J, Bai B, Alhuraishawy A et al. (2018) A novel re-crosslinkable preformed particle gel for conformance control in extreme heterogeneous reservoirs. In: SPE annual technical conference and exhibition, 24–26 September, Dallas
Sandrea I, Sandrea R (2007) Global oil reserves—recovery factors leaves vast target for EOR technologies. Oil Gas J 105(41):1–8
Seright RS (1997) Use of preformed gels for conformance control in fractured systems. SPE Prod Facil 12(01):59–65
Singh R, Gupta A, Mohanty KK et al. (2015) Fly ash nanoparticle-stabilized CO2-in-water foams for gas mobility control applications. In: SPE annual technical conference and exhibition, Houston, SPE-175057-MS
Slobod RL, Koch HA Jr (1953) High-pressure gas injection- mechanism of recovery increase. Drilling and Production Practice, New York, p API-53-082
Torrealba VA, Hoteit H (2018) Conformance improvement in oil reservoirs by use of microemulsions. In: SPE reservoir evaluation & engineering preprint, 1–19
Worthen AJ, Bagaria HG, Chen Y et al (2013a) Nanoparticle-stabilized carbon dioxide-in-water foams with fine texture. J Colloid Interface Sci 391:142–151
Worthen AJ, Bryant SL, Huh C et al (2013b) Carbon dioxide-in-water foams stabilized with nanoparticles and surfactant acting in synergy. AIChE J 59(9):3490–3501
Xue Z, Worthen A, Qajar A et al (2016) Viscosity and stability of ultra-high internal phase CO2-in-water foams stabilized with surfactants and nanoparticles with or without polyelectrolytes. J Colloid Interface Sci 461:383–395
Acknowledgement
The authors would like to acknowledge the chemical companies for providing the chemicals to conduct this study. We would like also to thank laboratory assistant, Rodolfo Marquez Vivas, for help with experiments.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Al Yousef, Z.A., Almobarky, M.A. & Schechter, D.S. Surfactant and a mixture of surfactant and nanoparticles to stabilize CO2/brine foam, control gas mobility, and enhance oil recovery. J Petrol Explor Prod Technol 10, 439–445 (2020). https://doi.org/10.1007/s13202-019-0695-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13202-019-0695-9