Abstract
The volumetric sweep efficiencies of CO2 flooding for enhanced oil recovery (EOR) are generally low due to problems of viscous fingering and gravity override. This paper attempts to study a relatively new and promising method to reduce the mobility of CO2 flooding and increase oil recovery under reservoir conditions. Referred to as alkaline-surfactant-alternated-gas/CO2 (ASAG) flooding, this method is essentially the synergic combination of chemical and immiscible CO2 flooding. In this work, chemical formulations were identified through foam stability tests based on their foaming ability coefficients. The selected formulations were further tested for their capabilities to reduce oil–water interfacial tensions (IFT) to ultra-low value. With the best performing formulations, the laboratory-scale core flooding experiments were conducted to evaluate their EOR potential. The core flooding experiments were performed with sandstone reservoir core samples from two different depths of a major depleted oil field of Upper Assam Basin, India. This study reports the successful application of a natural anionic surfactant (black liquor) as a co-surfactant and foaming agent during ASAG flooding. It was observed that higher oil recovery of 14.26% original oil in place (OOIP) was obtained by surfactant-alternated-gas (SAG) flooding compared to 12.03% OOIP by immiscible CO2 alternated with brine (WAG) flooding. The highest residual oil recovery of 20% OOIP was obtained for ASAG flooding with the alkali, surfactant and black liquor in the chemical slug. Oil recovery performances during SAG and ASAG flooding were found to be better for core samples with lower porosity–permeability due to stronger foam formation in lower permeability cores.
Similar content being viewed by others
Explore related subjects
Find the latest articles, discoveries, and news in related topics.Avoid common mistakes on your manuscript.
Introduction
EOR processes include all those methods that mobilize and recover the oil left behind or that cannot be produced economically by the conventional use of reservoir energy and pressure maintenance schemes with gas or water. Chemical flooding is one of the major EOR methods which involve the injection of water containing chemical additives. Currently, alkaline-surfactant-polymer (ASP) is considered as the most promising chemical EOR method having the ability to improve the microscopic sweep as well as the macroscopic displacement efficiencies. Among the gas EOR methods, CO2 flooding is the leading EOR technique for light and medium crude oil (Yongmao et al. 2004; Gogoi 2013). Notwithstanding the successful application of CO2 EOR in many laboratory-scale and field-scale tests, CO2 cannot recover all of the oil in the formation because its low density and viscosity gives rise to a number of mobility and conformance issues (Enick and Olsen 2012; Farajzadeh et al. 2012; Talebian et al. 2013; Yang Zhang et al. 2015). The low density of CO2 gas relative to oil promotes gravity override, whereas low viscosity results in unfavorable mobility ratio leading to viscous fingering.
If the high mobility of CO2 compared to oil and water can be controlled, a large part of the residual oil can be recovered. The water-alternated-gas (WAG) technique had been accepted as the technology of choice to control CO2 mobility by the EOR industry (Enick and Olsen 2012). In WAG, both CO2 and water are injected into the formation alternately in the ratio of 0.5 to 4.0 volumes of water to 1.0 reservoir volume of gas to arrest the gas mobility. Injecting water with CO2 increases the water saturation and so decreases the CO2 saturation in the pore spaces. This leads to a reduction of CO2 relative permeability making the mobility ratio favorable with the resulting better sweep efficiency and improvement in the oil recovery efficiency (Zekr et al. 2011). Although the WAG method is the state-of-the-art technique for CO2 mobility control in porous media and is more efficient than CO2 injection alone, the process leaves behind 35–65% original oil in place (OOIP). Furthermore, the large volumes of brine injected along with CO2 prolong the life of the project, inhibit the contact of CO2 and oil, and result in the production of large amounts of water.
To overcome the limitations of WAG, a new technique in the form of chemically induced water-alternated-gas had been developed to take advantage of the synergic combination of chemical and gas EOR methods. The types of chemicals commonly used are surfactants, co-surfactants, co-solvents, polymers, alkalis, salts, nanoparticles, etc. depending upon the specific application (Talebian et al. 2013; Kumar and Mandal 2017). The technique is called surfactant-alternated-gas (SAG) flooding when surfactant solutions are alternately injected with gas to form foam in the porous media (Kibodeaux and Rossen 1997). The higher oil recovery obtained by SAG injection compared to WAG can be attributed to a number of factors like reduction of oil–water interfacial tension (IFT), better mobility control due to foam formation, mutual mass transfer between crude oil and injected gas, etc. Although foam can be formed by surfactant alternated with gas injection, clay minerals in rock matrix adsorb the surfactants and reduce the efficiency of the process. So alkali is added with the surfactant in the chemical slug, where alkali works as a clay stabilizer to reduce surfactant adsorption by rock grains. The addition of alkali also helps to convert naturally occurring naphthenic acids in crude oils to produce in-situ surfactant (soaps). It is assumed that a highly oil-soluble single pseudo-acid component (HA) is present in oil (Sheng 2015). This pseudo-acid component partitions into the aqueous phases upon contact with water, and due to subsequent hydrolysis of the alkali, OH− ions are produced which react with acid species in the aqueous phase to form natural soaps NaA (soluble anionic surfactant). The presence of synthetic surfactants and natural soaps assists in further lowering of IFT to ultra-low value (< 0.01 mN/m) resulting in the formation of more stable foam which in turn increases the oil recovery efficiency. The process of co-injection or alternate injection of gas and alkali-surfactant slug which results in the formation of foam in the porous media is termed in the literature as alkali surfactant gas (ASG) or alkaline-surfactant-alternated-gas (ASAG) or low-tension gas process (Srivastava et al. 2009, 2011; Cottin et al. 2012; Guo et al. 2012; Lashgari et al. 2015).
ASAG flooding involves the alternate injection of chemical slug and CO2 gas leading to the formation of foam in the reservoir at the gas surfactant contact due to the alternating imbibition/drainage cycles. In fact, field experiences suggest that ASAG would be advantageous than direct foam injection as it will minimize contact between gas and water in the injection facilities. This can help reduce corrosion and also improve injectivity. The ASAG process is relatively new, and only a few experimental works have been reported. Srivastava (2010) observed increased oil recovery during alkali surfactant gas (ASG) core floods, conducted on Silurian dolomite and Berea sandstone rocks, due to the combined effect of foam and ultra-low IFT conditions (Srivastava 2010). ASG slug was injected by co-injecting chemicals and gas into the core followed by ASG drive, which consisted of an aqueous solution of low concentration surfactant and gas (CO2). The oil recoveries with ASG core floods were found to be comparable with ASP core floods, conducted under similar conditions. An important observation reported was that the negative salinity gradient concept, which is generally applied to chemical floods, increased foam strength during the ASG/CO2 flooding. When a negative salinity gradient is imposed, initial formation brine existing in the reservoir/core at a Winsor Type II salinity is followed by a surfactant slug at optimal salinity, which is finally followed by a drive at Winsor Type I salinity. With the negative salinity gradient design, over-optimum salinity injected ahead of chemical slug bank and less-than-optimal salinity in the drive causes the salinity to become optimal somewhere in the surfactant bank due to dispersive mixing (Hirasaki et al. 1983). A surfactant formulation at the optimal salinity exhibits the lowest oil–water IFT and consequently results in the best oil recovery (Romsted 2014; Torrealba and Johns 2017). Srivastava et al. (2011) conducted surfactant-alternated-gas (SAG) core flood experiments on Berea sandstone core and compared their displacement efficiencies with ASP core floods. They observed that incremental oil recoveries after waterflooding with single cycle and two-cycle SAG processes were 27.79 and 29.01%, respectively (Srivastava et al. 2011). Guo et al. (2012) reported a laboratory study of a novel alkaline/surfactant/foam (ASF) process using a specially designed alkaline/surfactant (AS) formulation and nitrogen gas. Bentheimer sandstone cores were used to perform the core flood experiments. They reported that alkali-surfactant (AS) flooding recovered only approximately half of the oil left after waterflooding, and ASF flooding without preflush of AS recovered slightly more than AS flooding. But with an AS preflush, ASF flooding recovered almost all the oil present in the core after waterflooding (Guo et al. 2012). Cottin et al. (2012) conducted ASG and surfactant gas (SG) laboratory core flood experiments on carbonate core samples of middle east reservoirs and obtained promising results in favor of ASG flooding (Cottin et al. 2012). Majidaie et al. (2015) studied experimentally and with numerical simulation the EOR potential of the novel chemically enhanced water alternating gas (CWAG) injection method. Alkaline, surfactant, and polymer additives were used in the chemical slug which was injected during the WAG process with CO2 gas. From the core flood experiments that were done using Berea sandstone cores, they observed that oil recovery using CWAG was better by 26% of the residual oil in place after waterflooding compared to the recovery using WAG. Their study also reported that attainment of ultra-low IFT (10−2 or 10−3 mN/m) during the CWAG process was critical for minimizing the water blocking effect (Majidaie et al. 2015).
In this study, the ASAG flooding was investigated by laboratory-scale core flood studies, using sandstone reservoir cores and crude oils from different depths of a depleted oil field of Upper Assam Basin, India, to evaluate its EOR potential. Earlier core flood experiments on ASG and ASAG were done on homogenous Berea and Bentheimer cores. In this work, actual reservoir cores from producing formations of the oil field were used to conduct the flooding experiments. So, the performances of ASAG flooding on reservoir cores which are non-homogeneous and not reported in the literature were investigated in this study. The use of optimum chemical formulation is of utmost importance for the success of ASAG flooding, so the application of black liquor (BL) as a co-surfactant for improving the performance of ASAG flooding was also studied in this work. Foam stability tests were carried out to identify optimum performing chemicals and their concentrations with the best foaming ability. The selected formulations were then tested for their ability to decrease the oil–water IFT to ultra-low value. Finally, the screened formulations were used in the SAG and ASAG core flooding experiments and their oil recovery results were compared with WAG flooding. The experimental works and their results are presented in four parts. In the first part, crude oil and reservoir rock characterization of a promising reservoir of the oil field are presented. In the second part, screening of chemical formulations for SAG and ASAG flooding are included. In the third and fourth parts, the IFT measurement of chemical formulations and the core flooding works, respectively, are presented.
Materials and methods
Materials
Anionic and nonionic surfactants together with a natural surfactant were used in the experiments. The nonionic surfactant Triton X-100 (TX-100), polyethyleneglycol 4-tert-octyphenolether (MW = 250.382), was purchased from Sisco Research Laboratories Pvt. Ltd., India. Anionic surfactants, sodium dodecyl benzene sulfonate (SDBS) (C18H29NaO3S, MW = 348.48), was procured from Sisco Research Laboratory Pvt. Ltd., Mumbai, India, and sodium dodecyl sulfate (SDS) (NaC12H25SO4, MW = 288.372) from Merck (India). Another anionic surfactant, alpha olefin sulfonate (AOS) (RCH = CH(CH2)15SO3Na, MW = 320) a 32% yellowish transparent liquid was purchased from Aman Enterprises, India. The natural surfactant used was black liquor (BL), an anionic water-soluble surfactant, whose main constituent was Na-lignosulphonate and an effluent of Nowgong paper Mill, Assam, India. Sodium carbonate (Na2CO3), the conventional alkali, was purchased from Merck Specialities Pvt. Ltd., Mumbai. Sodium chloride used for adjusting the brine salinity was purchased from Merck Specialities Pvt. Ltd., Mumbai. The porous media were conventional reservoir core samples brought with courtesy from a nearby oil exploration and production industry from two main producing reservoirs/sands of a major depleted oil field of Upper Assam Basin, India, from depths of (2500–2700) and (2900–3100) meters. The crude oil samples and formation water for the study were obtained from the same oil field.
Experimental methods
Reservoir rock and crude oil analysis
Porosities of the reservoir rock samples were determined by measuring the bulk volume of the core plugs with the help of calipers and grain volume by the Boyle’s law double-cell method. The instrument used for grain volume determination was the TPI-219 Helium Porosimeter, Coretest Systems, Inc, USA. The liquid permeabilities of the core plugs were measured with LiquidPerm, Vinci Technologies, France, using the brine (3800 ppm NaCl) as the pore fluid. For each core plug, three readings were taken and their averages were calculated.
The laboratory analysis of crude oil is important to characterize the crude of a particular reservoir and to make a preliminary assessment of the application of any EOR method. In this study, the crude oils from two selected formation/sands of the oil field were analyzed for their properties. All the samples were separated from water using separating funnel at a temperature of 35 °C before carrying the detailed analysis. The ASTM D1298-67 [IP 150/68] hydrometer method was followed for the determination of the density of crude oil samples. The dead oil viscosity of the oil samples was measured with the GRACE M3600 Viscometer at the reservoir temperature. The IP 15/67 method was followed to determine the pour points of the crude oil samples. The asphaltene content of oil samples was measured by asphaltene precipitation with n-heptane (ASTM D2007-80). Resin content was estimated according to Hubbard and Stanfield (1948) method, and the acid number of the crude oils was determined by titration method.
Foam stability test
Foamability and foam stability are two important properties of foam that determine its propagation during foam-based EOR. Foamability is defined as the capability of the surfactants to form foam, and foam stability is described by the variations of foam height or volume with time, following foam generation (Belhaij and Al-Mahdy 2015). In foam stability test, surfactants were tested for their ability to form stable foam and to provide suitable concentration for preparation of formulation. In this work, the foamability and foam stability of different surfactants and their mixtures were studied to screen chemical formulations for core flood studies.
For the foam stability test, equal volumes (2 ml) of the surfactant solutions in distilled water/brine were dispensed in test tubes over which specific volumes of crude oil were added. The test tubes were then shaken in Tarsons Rotospin Rotary Mixer at a speed of 50 rpm for 6 h, which resulted in the formation of foam at the top of the liquid column. After foam generation, the initial foam volume (Vi) and the time for dewatering half volume from foam (t1/2) were noted by visual observation (Srivastava et al. 2009; Bera et al. 2013). To measure the foaming ability of a particular system, the foaming ability coefficient (Fq) was used, which was defined as (Wang et al. 2001):
The systems with best foaming ability exhibit the highest values of Fq. The most efficient foaming formulations were screened for core flood studies where their efficiencies in enhancing oil recovery were tested.
Interfacial tension measurements
The formulations screened through foam stability tests were further evaluated for their ability to reduce the oil–water IFT to a sufficiently low value. The interfacial tension (IFT) between crude oil and surfactants solutions was measured using a Grace M6500 Spinning Drop Tensiometer at a constant rotation of 6500 rpm. The IFT values were calculated using the following Vonnegut’s equation while maintaining drop length to diameter (L/D) greater than 4 (Kirubanandan 2015).
where \(\Delta \rho\) is the density difference between the crude oil and the surfactant solution, g/cm3; \(\omega\) is the angular velocity, rad/s; λ is the IFT, mN/m; r is the radius of the drop in the circular cylinder, cm.
Core flooding
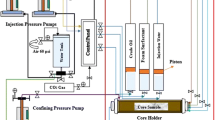
The core flood tests were conducted to determine the oil recovery efficiencies by injecting a fluid into a core plug containing reservoir fluid. The tests were done at various pressures and reservoir temperature. The schematic of the core flooding system is shown in Fig. 1. Gas and liquid enter a T-section right before the core inlet line. Pressures were measured at the inlet and at the outlet. The core flood system consisted of three liquid pumps, a CO2 cylinder with regulator and gas flow controller, back-pressure regulator (BPR), Hassler core holder, pressure transducers, data acquisition system, and oven. The ASG flooding experiments were conducted with the same procedures as followed by previous authors (Syahputra et al. 2000; Liu 2007; Srivastava et al. 2009; Yin et al. 2009; Samanta et al. 2012). After the core plug was prepared by cleaning and drying, its weight was taken and the pore volume (PV) of the core plug was determined from its dimensions and porosity. The core plug was placed in the Hassler core holder, and brine (3800 ppm NaCl) was injected by the liquid pump to saturate the core plug at room temperature (about 30 °C). Several PVs were injected into the core at a constant flow rate to completely saturate the core with brine. The absolute permeability of the core to brine was then determined using Darcy’s equation. After brine saturation, crude oil was injected into the core plug at a constant rate until the effluent was 100% oil. At the end of oil saturation, connate water saturation (Swc) was determined based on mass balance calculations and oil relative permeability (Ko) was determined at Swc. The oil-saturated core plugs were maintained at 70 °C for 24 h. The purpose of this aging procedure was to allow for possible wettability alteration. After aging, the original oil in place (OOIP) in the core plug was determined by mass balance calculations. The waterflooding was then carried out by injecting the same brine (3800 ppm NaCl) at the same constant rate. At the end of waterflood, the residual oil saturation (Sor) in the core was determined by measuring the volume of crude oil collected in the measuring cylinder and doing a mass balance calculation. After waterflooding, the tertiary recovery, i.e., EOR techniques were applied for determining the residual oil recovery potential of these methods. In case of CO2 alternated with brine (WAG) flooding, 0.25 PV of CO2 gas was injected continuously at the rate of 0.2 ml/min followed by injection of approximately 0.25 PV of brine. At least 6 cycles of WAG were continued, followed by a continuous brine flooding until no more oil was produced and the incremental oil recovery was noted. For SAG flooding, 0.25 PV of best performing surfactant slug formulation were alternately injected with 0.25 PV of CO2 gas (injected at the same rate). At least 6 cycles were repeated, followed by injection of extended chase water (brine) flooding till no more oil was produced in the effluent. The incremental oil recovery was noted. For ASAG flooding, the same injection method as SAG flooding was followed with the best performing AS formulation. The core flood system temperature throughout waterflooding, WAG, SAG, and ASAG flooding methods were maintained at reservoir temperature of 70 °C.
Results and discussion
Reservoir rock and crude oil analysis
Core analysis was done with 1.5 inch diameter cleaned and dried reservoir core plugs. The porosities and absolute permeabilities of the core plugs from depths of (2900–3100) meters were in the range of 17.62–18.62% and 2–3 md, respectively, whereas for the shallower depth (2500–2700 m) core plugs, the respective values of porosities and absolute permeabilities were in the range of 24.64–25.15% and 25–30 md respectively. An inverse correlation was observed between porosity–permeability and depth for the porous media under study. The most obvious reason for this inverse relationship was due to the combined effects of compaction and cementation (Nelson and Bird 2005).
Table 1 presents the results of the crude oil analysis. The API gravities of the two crude oil samples were found to be 23° and 31°API, indicating that the oil field produces medium gravity crudes. The dead oil viscosities of the crudes were 9.3 and 4.46 cP measured at reservoir temperature (70 °C). Viscosities of crude oil play an important role during its recovery. High viscosity leads to high remaining oil saturation after waterflooding and conformance problems during chemical and gas EOR processes. High pour point crudes generally have pour points in the range from 15 to 52 °C and may be solid at room temperature (27 °C). The higher value of pour point is generally associated with crude oils of high paraffin content and the lower value of pour point with crudes of more aromatic nature. The pour points of the crude oils were obtained as 24 and 27 °C, respectively. These values agree well with data reported in the literature (Kandwal et al. 2007; Pillon 2007).
Asphaltenes are the polar, polyaromatic, high molecular weight hydrocarbon, and the wax-free fraction of crude oil that are soluble in toluene, but insoluble in alkanes (n-heptane to n-pentane) (Rabbani et al. 2012). Asphaltenes tend to remain in solution under reservoir temperature and pressure conditions stabilized by resins adsorbed on their surface. Asphaltenes may start to precipitate if the stability of crude oil is destabilized caused by changes in temperature and/or pressure during primary depletion. Asphaltenes may also become unstable as a result of the mixing of fluids as well as during gas injection for EOR operations (Ghedan 2009). During CO2 flooding, the asphaltene-to-resin ratio of crude oil is altered causing asphaltene precipitation and therefore its deposition (Srivastava and Huang 1997). This is one of the major problems that confront petroleum engineers during a CO2 flooding project. Resins have the effect of keeping asphaltenes in solution. A high resin-to-asphaltene ratio (R/A) indicates that asphaltenes are less likely to come out of solution (Bon and Sarma 2004). Leontaritis and Mansoori (1987) presented a condition for asphaltene stability as follows: R/A > 3.0 as steady state, 2.0 < R/A < 3.0 as meta-steady state and R/A < 2.0 as unsteady state (Leontaritis and Mansoori 1987; Sepulveda et al. 2015). The R/A for the present crudes was greater than 3, so based on the Leontaritis criterion, there were enough amounts of resins for asphaltene stabilization.
Acid number is a measure of the amount of acidic components present in a crude oil. It is expressed as the milligrams of KOH that is required to neutralize 1 gm of oil sample. In alkaline flooding, in-situ soap is generated by the reaction of alkali agents such as sodium carbonate with acids in crude oil. For a reservoir to be a good candidate for alkaline flooding, the acid number of the crude oil should be at least 0.5 mg/g. Crude oils with acid number greater than 0.5 mg KOH/g are known as acidic crudes (Ramirez-Corredores 2017). The acid numbers of the crude oil samples were above 0.5 mg KOH/g, indicating acidic nature of the crudes. Based on the above preliminary assessment of the crudes, the oil field was found to be suitable for the application of CO2 and ASAG flooding.
Chemical screening and formulation
The success of the SAG and ASAG process in recovering extra oil will depend on the use of correct chemical formulations. So it is desirable to screen optimal chemical formulations which will display the best foaming behavior to provide better mobility control during core flood experiments. The chemical formulations were screened based on the foam stability tests as described by previous researchers (Demin et al. 2001a, 2001b; Simjoo et al. 2013; Bageri et al. 2014; Belhaij and Al-Mahdy 2015).
Foam stability test
The ability of surfactants to form strong and stable foam under reservoir conditions in the presence of crude oil is the most crucial factor that will determine its applicability for foam-based EOR operation. A series of foam stability tests were performed to select suitable surfactants and their optimum concentrations based on their foaming ability coefficients. The effects of crude oil, alkali, and brine on foaming ability have also been investigated in this study.
Effect of surfactant type and concentration
The foaming ability of the different surfactants was initially tested with distilled water without crude oil. In Fig. 2, the foaming ability coefficients of different surfactants at different concentrations are presented. For a given surfactant, the value of Fq increased up to a certain concentration and then decreased or remained constant. With the increase in surfactant concentration, the surface tension was lowered resulting in better foaming ability; but after a certain surfactant concentration surface tension was constant so further increase in foaming behavior was not observed (Pu et al. 2017). The concentration with the highest value of Fq was taken as its economical and optimum concentration limit (Demin et al. 2001a, b; Farzaneh and Sohrabi 2015). For SDS, the optimum concentration was obtained at 0.7 wt% after which the value of Fq decreased with increasing surfactant concentration. Likewise, optimum concentrations of AOS and SDBS were 0.5 and 0.3 wt%, respectively. By comparing the highest Fq values, the surfactants were ranked in terms of their foaming ability as SDS > AOS > SDBS. It was noted that surfactants with smaller carbon number (SDS-12) have higher Fq value than surfactant with higher carbon number (SDBS-18) (Pu et al. 2017).
Effect of crude oil and its saturation
The foaming ability of surfactants in the presence of crude oil was also dependent on the nature of surfactant. In the presence of crude oil, both the foamability and foam stability at all concentration of surfactants were much lower than in the absence of oil, indicating that the oil phase lowered the foaming ability of surfactants (Simjoo et al. 2013). But the trend of Fq versus surfactant concentration remained the same in the presence of crude oil with the same optimum concentrations as shown in Fig. 2. The foaming ability of the surfactants in the presence of crude oil was ranked as SDS > AOS > SDBS. Since SDS exhibited the highest Fq in the presence of crude oil, subsequently foaming behavior of 0.7 wt% SDS with varying concentration of BL and alkali was investigated to observe the optimum point. The foam stability of TX-100 totally diminished in the presence of crude oil. In fact, TX-100 foam with crude oil decayed in less than 5 min. The electrostatic double-layer effect resulting from charge interactions at the film interface is suppressed in nonionic surfactants, which was why the stability of TX-100 was very low (Osei-Bonsua et al. 2015).
Figure 3a shows the effect of the crude oil saturation on Fq values at 0.7 wt% SDS. The presence of crude oil drastically reduced the foaming ability of SDS even at 10% volume fraction of crude oil. From 10 to 30% crude oil saturation Fq value slightly increased, but from 30% onwards, reduction in the value of Fq was observed. In other words, 30% crude oil saturation was the critical point beyond which foam performance dropped. As the oil saturation was increased, oil layers accumulate at the lamellas resulting in the transfer of surfactant molecules from the gas–water interface to the oil–water interface. This weakened the foam film strength and together with the Marangoni effect eventually leads to low foam performance (Pu et al. 2017). Henceforth, the experiments were conducted with 30% oil saturation.
Effect of mixed surfactants
The foaming behavior (in terms of Fq) of 0.7 wt% SDS at 30% crude oil saturation with varying concentration of BL is depicted in Fig. 3b. As can be seen, the foaming behavior of 0.7 wt% SDS improved with the addition of BL. The likely reason for this favorable foaming behavior is the further lowering of water–oil IFT due to BL addition. The Vi and t1/2 for BL alone were found to be considerably lower than the other anionic surfactants, reflecting BLs poor foaming ability. But when combined with other surfactants the foaming ability of the mixed surfactant system improved compared to individual surfactant system due to synergism. The Fq of 0.7 wt% SDS was observed to be maximum at 2 wt% BL concentration. This 2 wt% BL concentration was taken as the optimum BL concentration.
Effect of alkali
Na2CO3 alkaline solutions were tested with 0.7 wt% SDS and 2 wt% BL at 30% crude oil saturation with the aim of improving the foaming behavior. The addition of alkali can significantly decrease oil–water IFT to a very low value which would enhance the foamability and foam stability. Figure 3c shows that the value of Fq was effectively improved with alkali addition, and the best foaming behavior (with highest Fq value) was obtained at Na2CO3 concentration of 0.3 wt%.
Effect of salinity
Finally, the foaming ability of the chemical formulation (0.7 wt% SDS + 2 wt% BL + 0.3 wt% Na2CO3) was tested by varying brine salinity in the presence of 30% crude oil saturation. Brine salinities (0 to 3800 ppm NaCl) less than formation water salinity were tested for the optimum foaming condition. The purpose of using the salinity in this range was to maintain negative salinity gradient during ASAG core flood experiments. The salinity of formation water of the oil field was measured with Systronics Water Analyser Model No. 371 and was found to be 3800 ppm. As shown in Fig. 3d, the variation of brine salinity affects the foaming behavior of the formulation. The Fq increased with brine salinity up to 2000 ppm NaCl after which the trend was slightly decreasing. In the presence of NaCl, the anionic surfactants are adsorbed on the lamellae more tightly for which the foaming ability increased with the addition of NaCl (Bera et al. 2013).
AS slug formulation
As seen in the last subsection, the maximum foaming behavior of chemical formulations was observed at a certain optimum concentration of the chemicals in the presence of crude oil. With this information in mind, the chemical slug formulations for SAG and ASAG core flooding were designed.
Based on Fig. 2, SDS was observed to exhibit the highest foaming ability in the presence of crude oil, so this surfactant was selected for further investigation. SDS concentration was chosen as 0.7 wt% since this concentration displayed the maximum foaming ability both in the absence and presence of crude oil. With a fixed 0.7 wt% SDS concentration, the concentration of the co-surfactant BL was varied to obtain the maximum value of Fq as shown in Fig. 3b. The optimum concentration of BL with 0.7 wt% SDS and in the presence of crude oil was 2 wt%. Likewise, with fixed 0.7 wt% SDS and 2 wt% BL, the alkali concentration at which maximum Fq occurred was determined and was found to be 0.3 wt% Na2CO3 as in Fig. 3c.
It has been reported that best oil recovery in chemical floods occurs when a negative salinity gradient was imposed in injected fluids. A negative salinity gradient means the slug formulation is selected at an optimal salinity lower than the in situ reservoir brine salinity. The drive, which follows the slug, salinity is lower than the salinity of slug. With negative salinity gradient, the salinity in the reservoir/core changes from high salinity to low salinity through intermediate salinity. As a result, the benefits of high, moderate, and low salinities are taken during the flooding process. At high salinities, the oil microemulsion IFT is low while at low salinities the surfactant retention is low due to the phase behavior of surfactant/oil/brine systems. At some intermediate salinity, the system can have up to three phases and is called the Type III salinity. This salinity is the optimal salinity and is one of the significant parameters in surfactant flooding process because lowest IFT usually occurs near this salinity (Hirasaki et al. 1983; Lake et al. 2014). When the IFT decreases to the lowest, the capillary number will be large enough to move previously trapped oil leading to an increase in the oil recovery. A negative salinity gradient also assists foam-based EOR process by increasing foam stability (Srivastava 2010). Brine salinities less than formation brine salinity were tested with (0.7 wt% SDS + 2 wt% BL + 0.3 wt% Na2CO3) chemical solutions. The maximum foaming behavior was observed at a salinity of 2000 ppm NaCl as shown in Fig. 3d. Thus, 2000 ppm NaCl salinity was used in the chemical formulations, which was less than the salinity of injected brine (3800 ppm). The screened chemical slug formulations are presented in Table 2.
Interfacial tension (IFT) measurements
The selected surfactants and alkali formulations at different concentrations were evaluated for their ability to achieve ultra-low IFT (less than 10−2 mN/m) with crude oil. The solutions were prepared with brine of 2000 ppm NaCl salinity. The optimum concentrations of these surfactants and alkali formulations were determined based on the lowest value of IFT and correlated with the maximum foaming ability obtained for these formulations from the foam stability tests. For surfactant SDS and alkali Na2CO3, concentrations ranging from 0.05 to 1 wt% were selected for determining the IFT values with crude oil. For BL, concentrations ranging from 0.5 to 6 wt% were selected for the tests. As shown in Fig. 4, IFT values decreased with increasing surfactant/alkali concentration up to a certain concentration and then were almost constant. The concentration with the lowest value of IFT was taken as the optimum concentration. With the increase in surfactant concentration, surfactant molecules were adsorbed onto the oil–water interface and so the IFT decreased. After a certain surfactant concentration when the interface became saturated with surfactant molecules, no more decrease in the value of the IFT was observed. With only SDS surfactant in the solution, the lowest IFT was obtained at 0.7 wt% concentration. This concentration was also the same optimum concentration obtained from foam stability test where maximum foaming behavior was observed. Likewise, the lowest IFT for the compounded surfactants, SDS and BL, was obtained at 2 wt% BL concentration when the SDS concentration was kept constant at 0.7 wt%. With alkali addition to the compounded surfactant formulation, further lowering of IFT values was observed, with the lowest at 0.3 wt% Na2CO3 concentration. In all the cases, it was found that the concentrations with lowest IFT values were coincident with the concentrations at which maximum foaming behavior was observed. This behavior can be attributed to the fact that lowest IFT gives maximum foam stability. With the addition of BL to SDS, the lowest value of IFT decreased from 8.75 × 10−3 mN/m (with 0.7 wt% SDS alone) to 6.22 × 10−3 mN/m (with 0.7 wt% SDS + 2 wt% BL), indicating a synergistic effect. With addition of alkali, the oil–water IFT further decreased to 5.23 × 10−3 mN/m for the combined formulation (0.7 wt% SDS + 2 wt% BL + 0.3 wt% Na2CO3). Each of the lowest oil–water IFT values was in the ultra-low IFT range (i.e., less than 10−2 mN/m), indicating the EOR potential of the selected formulations.
Figure S-1 (in Supplementary Material) shows the effect of salinity on oil–water IFT and foaming ability coefficient for the combined formulation of 0.7 wt% SDS + 2 wt% BL + 0.3 wt% Na2CO3. The IFT values undergo a decrease with increased salinity up to around 2000 ppm NaCl, and then, a slightly increasing trend of IFT values was observed with further increase in salinity. The decrease in IFT values with salinity occurred due to the fact that with an increase in salinity surfactants are adsorbed more strongly at the oil–water interface causing the lowering of IFT (A-Sahhaf et al. 2005; Salehi et al. 2017). However, at higher salinity, the coexistence of water-in-oil microemulsion with excess water resulted in surfactant retention due to phase trapping because of which the IFT values had an increasing trend with an increase in salinity (Ruckenstein and Rao 1987). The IFT value was found to be least at 2000 ppm NaCl and was taken as the optimal salinity following the relationship between phase behavior and interfacial tension phenomenon of surfactant flooding. From the figure, it could be observed that the IFT behavior was related to the foaming behavior for the selected formulation. The results indicated that the optimal salinity, where oil–water IFT is lowest, also corresponds to the point of maximum foaming behavior.
Core flooding
Eight core flooding experiments were performed details and results of which are summarized in Table 3. The cumulative oil recovery as a function of the injection pore volume is shown in Fig. 5. In the first four core flood experiments, viz. WAG1, SAG1, SsAG1 and ASAG1, oil recovery by conventional brine flooding was near about 27% OOIP, implying that the residual oil saturations were similar for all the four experiments prior to tertiary oil recovery.
Effect of WAG and SAG injection
In Experiment WAG1, the oil-saturated core plug was subjected to waterflooding until no more oil was produced. 24.96% OOIP was produced after 3 PV of brine injection. Further, the core with residual oil saturation was flooded with CO2 and brine in the immiscible WAG mode until no more oil was produced. Additionally, 12.03% OOIP was produced by injection of 2.5 PV of CO2 and brine alternately. This increase in oil recovery was apparently due to oil swelling and reduction of oil viscosity occurring when injected CO2 interacted with residual crude oil. The brine association with CO2 also contributed to the improvement in oil recovery through better mobility control and contact of unswept zones. In Experiment SAG1, after waterflooding the core plug was alternately injected with (0.7 wt% SDS + 2000 ppm NaCl) chemical solution and CO2 gas in the SAG mode. During the first cycle of SAG flooding, no oil recovery was observed as the oil bank breakthrough occurred only in the second SAG cycle. The additional residual oil recovery was 14.26% OOIP after 2 PV of total fluid injection. No more oil production was observed after 4 SAG cycles and during the extended chase waterflooding. The enhancement in oil recovery was due to the formation of foam in the core plug by the alternate injection of surfactant solution and CO2 gas. Foam propagation had resulted in improved sweep efficiency displacing residual oil from parts of the core plug that could not be swept by CO2/brine (WAG) injection. There was an improvement of 18.54% in the oil recovery by SAG flooding over the WAG flooding which demonstrated the benefits of SAG compared to WAG.
Effect of BL used as a co-surfactant
Experiment SsAG1 involved the use of compounded surfactant (SDS + BL) in the chemical slug where SDS was used as the primary surfactant and BL as co-surfactant. Following waterflooding, the chemical slug consisting of (0.7 wt% SDS + 2 wt% BL + 2000 ppm NaCl) solution was injected with CO2 gas alternately in cycles. Each cycle included 0.25 PV of chemical slug and 0.25 PV of CO2 gas. After 4 cycles of SAG injection, no more oil was produced and also during extended chase waterflooding. The oil recovery was 16.04% OOIP after 2 PV of total fluid injection. This amounts to a betterment of 12.49% in oil recovery obtained during SAG flooding in experiment SAG1 with single surfactant in the chemical slug. This higher recovery may be associated with BL working as a co-surfactant. Addition of BL had been reported to decrease IFT between oil and aqueous phase sufficiently to increase oil recovery from porous rock (Gogoi 2014). From the foam stability test done in this work, it has also been observed that BL has the ability to improve the foaming ability when mixed with another surfactant. With better foam performance, the residual oil in unswept zones was displaced leading to an improvement in oil recovery. Na-lignosulphonate, the main constituent of BL, has been known to act as sacrificial agent during CO2 foam flooding process (Hong et al. 1987). Injecting BL with SDS had most likely reduced SDS adsorption by rock grains, maintaining the foaming ability of the SAG process. All these beneficial factors of BL addition were responsible for the betterment of oil recovery.
Effect of ASAG flooding
Experiment ASAG1 describes the ASAG flooding process where combined alkali surfactants (AS) solutions were alternated injected with CO2 gas into the core plugs. After 3.5 PV of waterflooding when no more oil was produced, the recovery was 27.18% OOIP. Subsequent to waterflooding, the AS slug (0.3 wt% Na2CO3 + 0.7 wt% SDS + 2 wt% BL + 2000 ppm NaCl) and CO2 gas were alternately injected in cycles. Again each cycle included 0.25 PV of chemical slug and 0.25 PV of CO2 gas. Maximum oil recovery was achieved after 4 cycles of ASAG injection after which no more oil was produced and even during extended chase waterflooding. The residual oil recovery by ASAG flooding improved to 19.83% OOIP after 2 PV of total fluid injection—a betterment of 23.63% in oil recovery compared to experiment SaAG1. The oil recovery by ASAG flooding had improved for the obvious reason of alkali addition to the liquid slug. It is a well-known fact that injected alkali forms in-situ surfactant after reacting with crude oil which lowers the oil–water IFT to ultra-low values. It has also been found in this study that alkali improves foamability and foam stability of surfactant systems. Moreover, alkali also plays the role of a clay stabilizer reducing adsorption of foaming agents by reservoir rocks.
Effect of porosity–permeability variation
First four experiments (WAG1, SAG1, SsAG1, and ASAG1) were performed on core plugs with lower porosity–permeability (17–18% and 2.5–3 mD). Another set of the same experiments (WAG2, SAG2, SsAG2, and ASAG2) were performed on core plugs from a shallower depth of the oil field with higher porosity–permeability (24–25% and 25–30 mD). The purpose was to study the effect of porosity–permeability on WAG, SAG, and ASAG performances. From Table 3 and Fig. 6, it was observed that the performance of the WAG process declined from 12.03% recovery to 6.34% with an increase in porosity and permeability of the core plug. The plausible explanation for this negative behavior may be the comparatively higher mobility of the injected CO2 gas passing through core plugs which were more porous and permeable. The higher mobility had led to the early gas breakthrough and consequently higher residual oil. The oil recovery performance of the SAG and ASAG experiments also dwindled with core plugs of higher porosity–permeability as shown in Fig. 6. The residual oil recoveries for SAG experiments with lower and higher porosity–permeability values were respectively, 14.26 and 12.39% OOIP. For the SsAG experiments, oil recoveries were slightly higher than SAG experiments but still the recoveries were higher for the core plugs with lower porosity–permeability. For cores with lower values of porosity–permeability, 19.83% OOIP was produced during ASAG1 experiment compared to 13.10% OOIP for more permeable cores. The stronger foam formed in the less porous and permeable cores contributed to better mobility control leading to the efficient displacement of trapped oil (Bageri et al. 2014). The reverse was the case with more porous and permeable rocks, so the oil recovery decreased during the SAG and ASAG floodings. Higher recoveries from less permeable cores also indicated the ability of ASAG flooding to flush out residual oil from tight zones generally untouched by conventional recovery methods in heterogeneous reservoirs.
Conclusions
The results of the experimental work highlighted the successful application of the synergic combination of alkali, surfactant and CO2 gas for improving recovery of medium gravity crudes. From the foam stability tests, it was observed that optimum concentrations of surfactants, alkali, crude oil, and salinities exist which corresponds to maximum foaming behavior. One interesting fact observed was the coincidence of the optimum concentrations with best foaming behavior and lowest oil–water IFT values. The natural surfactant, black liquor, demonstrated good foaming and IFT behaviors with other anionic surfactant in the presence of crude oil. The core flooding experiments performed with reservoir rocks samples and crude oils indicated the effectiveness of SAG flooding over WAG injection and ASAG flooding over SAG flooding. The residual oil recovery efficiencies for WAG, SAG, and ASAG with CO2 gas were of the order of 12.03, 16.04, and 19.83% OOIP after conventional waterflooding. The higher recoveries by SAG and ASAG could be attributed to the reduction of IFT, better mobility control due to foam formation, mutual mass transfer between crude oil and injected gas, etc. The higher recoveries from less porous-permeable core plugs demonstrated the EOR potential of SAG and ASAG flooding in tight sands and heterogeneous reservoirs.
References
A-Sahhaf T, Elkamel A, Ahmed AS, Khan AR (2005) The influence of temperature, pressure, salinity, and surfactant concentration on the interfacial tension of the n-octane-water system. Chem Eng Commun 192(2005):18
Bageri AS, Sultan AS, Kandil ME (2014) Evaluation of novel surfactant for nitrogen-foam-assisted EOR in high salinity carbonate reservoirs. SPE EOR conference at oil and gas West Asia Muscat, Oman, Society of Petroleum Engineers. SPE-169717-MS: 12
Belhaij A, Al-Mahdy O (2015) Foamability and foam stability of several surfactants solutions: the role of screening and flooding. Pet Environ Biotechnol 6(4):6
Bera A, Ojha K, Mandal A (2013) Synergistic effect of mixed surfactant systems on foam behavior and surface tension. J Surfactants Deterg 16:10
Bon J, Sarma HK (2004) A technical evaluation of a CO2 flood for EOR benefits in the Cooper Basin, South Australia. SPE Asia Pacific oil and gas conference and exhibition Perth, Australia, SPE. 88451
Cottin C, Morel D, Levitt D, Cordelier P, Pope G (2012). Alkali surfactant gas injection—attractive laboratory results in carbonates under harsh salinity and high temperature. Abu Dhabi international petroleum exhibition & conference Abu Dhabi, UAE, Society of Petroleum Engineers. SPE 161727: 13
Demin W, Jiecheng C, Qun L, Junzheng W, Wenxiang W, Yanqi Z (2001a) First ultra-low interfacial tension foam flood field test is successful. 2001 SPE annual technical conference and exhibition. New Orleans, Louisiana, Society of Petroleum Engineers. SPE 71491
Demin W, Jiecheng C, Zhenyu Y, Qun L, Wenxiang W, Huiyu Y (2001b) Successful field test of the first ultra-low interfacial tension foam flood. SPE Asia Pacific improved oil recovery conference. Kuala Lumpur, Malaysia, Society of Petroleum Engineers. SPE 72147: 8
Enick RM, Olsen DK (2012). Mobility and conformance control for carbon dioxide enhanced oil recovery (CO2-EOR) via thickeners, foams, and gels—a detailed literature review of 40 years of research. J. Ammer, National Energy Technology Laboratory. DOE/NETL-2012/1540: 267
Farajzadeh R, Andrianov A, Krastev R, Hirasaki GJ, Rossen WR (2012) Foam–oil interaction in porous media: implications for foam assisted enhanced oil recovery. Adv Coll Interface Sci 183(184):13
Farzaneh SA, Sohrabi M (2015) Experimental investigation of CO2-foam stability improvement by alkaline in the presence of crude oil. Chem Eng Res Des 94:15
Ghedan S (2009) Global laboratory experience of CO2-EOR flooding. SPE/EAGE reservoir characterization and simulation conference held Abu Dhabi, UAE, SPE
Gogoi SB (2013) Carbon-dioxide for EOR in Upper Assam basin. In: Hou MZ, Xie H, Were P (eds) Clean energy systems in the subsurface: production, storage and conversion. Springer, Berlin, p 13
Gogoi SB (2014) Effluent as surfactant for enhanced oil recovery. Innov Energy Res 3(1):109
Guo H, Zitha PLJ, Faber R, Buijse (2012) A Novel alkaline/surfactant/foam enhanced oil recovery process. SPE J SPE 145043:10
Hirasaki GJ, Domselaar HR, Nelson RC (1983) Evaluation of the salinity gradient concept in surfactant flooding. Soc Pet Eng J 23(03):15
Hong SA, Bae JH, Lewis GR (1987) An evaluation of lignosulfonate as a sacrificial adsorbate in surfactant flooding. SPE Reservoir Engineering 11
Hubbard V, Stanfield KE (1948) Determination of asphaltenes, oils, and resins in asphalt. Anal Chem 20(5):460–465
Kandwal VC, Agrawal KM, Nautiyal SP, Khan HU (2007) Paraffin deposition and viscosity temperature behaviour of assam crude oil. Pet Sci Technol 18:15
Kibodeaux KR, Rossen WR (1997) Coreflood study of surfactant-alternating-gas foam processes: implications for field design. SPE Western Regional Meeting. Long Beach, California, Society of Petroleum Engineers. SPE-38318-MS: 11
Kirubanandan S (2015) Influence of interfacial tension on dynamics of multiphase flow in mini-channel. Int J Eng Innov Technol 5(6):6
Kumar S, Mandal A (2017) A comprehensive review on chemically enhanced water alternating gas/CO2 (CEWAG) injection for enhanced oil recovery. J Petrol Sci Eng 157:20
Lake LW, Johns R, Rossen B, Pope G (2014). Fundamentals of enhanced oil recovery, Society of Petroleum Engineers
Lashgari HR, Sepehrnoori K, Delshad M (2015). Modeling of low-tension surfactant-gas flooding process in a four-phase flow simulator. SPE annual technical conference and exhibition, Houston, Texas, USA, Society of Petroleum Engineers. SPE-175134-MS: 21
Leontaritis KJ, Mansoori GA (1987). Asphaltene flocculation during oil production and processing: a thermodynamic colloidal model. SPE international symposium on oilfield chemistry. Texas SPE. 16258
Liu S (2007) Alkaline surfactant polymer enhanced oil recovery process. PhD, Rice University
Majidaie S, Onur M, Tan IM (2015) An experimental and numerical study of chemically enhanced water alternating gas injection. Pet Sci 12:470–482
Nelson PH, Bird KJ (2005) Porosity-depth trends and regional uplift calculated from Sonic Logs, National Petroleum Reserve in Alaska. Scientific Investigations Report 2005. U.S. Department of the Interior & U.S. Geological Survey, USA
Osei-Bonsua K, Shokria N, Grassiab P (2015) Foam stability in the presence and absence of hydrocarbons: from bubble- to bulk-scale. Colloids Surf A 481:13
Pillon LZ (2007) Interfacial properties of petroleum products. CRC Press
Pu W, Pang S, Wang C (2017) Experimental investigation of foam performance in the presence of crude oil. J Surfactants Deterg 20:9
Rabbani AR, Hassanzadeh G, Dehyadegari E (2012) Asphaltene deposition under CO2 injection: an experimental study of the Bangestan Reservoir of Ahwaz Oilfield, SW Iran. Pet Sci Technol 30(1):8
Ramirez-Corredores MM (2017) The science and technology of unconventional oils: finding refining opportunities. Academic Press, London
Romsted LS (2014) Surfactant science and technology: retrospects and prospects. CRC Press, Boca Raton
Ruckenstein E, Rao IV (1987) Interfacial tension of oil—brine systems in the presence of surfactant and cosurfactant. J Colloid Interface Sci 117(1):16
Salehi MM, Omidvar P, Naeimi F (2017) Salinity of injection water and its impact on oil recovery absolute permeability, residual oil saturation, interfacial tension and capillary pressure. Egypt J Pet 26(2017):12
Samanta A, Bera A, Ojha K, Mandal A (2012) Comparative studies on enhanced oil recovery by alkali–surfactant and polymer flooding. J Pet Explor Prod Technol 2:8
Sepulveda JA, Pizon-Torres C, Galindo JM, Charry C, Chavarro JI (2015) Effect of high CO2 content on formation damage of oil fields: a field case in a South Western Colombian field. ARPN J Eng Appl Sci 10:9
Sheng JJ (2015) Investigation of alkalineecrude oil reaction. Petroleum 1(2015):9
Simjoo M, Rezaei T, Andrianov A, Zitha PLJ (2013) Foam stability in the presence of oil: effect of surfactant concentration and oil type. Colloids Surf A 438:11
Srivastava M (2010) Foam assisted low interfacial tension enhanced oil recovery. PhD, The University of Texas at Austin
Srivastava RK, Huang SS (1997) Asphaltene deposition during CO2 flooding: a laboratory assessment. SPE production operations symposium Oklahoma, SPE. 37468
Srivastava M, Zhang J, Nguyen QP, Pope GA (2009) A systematic study of alkaline-surfactant-gas injection as an EOR technique. SPE 124752:15
Srivastava JP, Negi DS, Jain AK, Dhawan AK (2011) Surfactant-alternate-gas (SAG) injection process as a novel EOR technique—a laboratory investigation. 2nd South Asain geoscience conference and exhibition, GEO India 2011. New Delhi, India, Geo India, p 7
Syahputra AE, Tsau JS, Grigg RB (2000) Laboratory evaluation of using lignosulfonate and surfactant mixture in CO2 flooding. SPE/DOE improved oil recovery symposium. Tulsa, Oklahoma, SPE
Talebian SH, Masoudi R, Tan IM, Zitha PLJ (2013) Foam assisted CO2-EOR; concepts, challenges and applications. SPE enhanced oil recovery conference Kuala Lumpur, Malaysia, Society of Petroleum Engineers. SPE 165280: 14
Torrealba VA, Johns RT (2017) Coupled interfacial tension and phase behavior model based on micellar curvatures. Langmuir 33(2017):11
Wang D, Cheng J, Yanging Z, Li Q, Wu J, Wu W (2001) First ultra-low interfacial tension foam flood field test is successful. 2001 SPE annual technical conference and exhibition SPE, New Orleans, Louisiana, SPE, vol 71491, p 6
Yang Zhang YW, Xue Fangfang, Wang Yanqing, Ren Bo, Zhang Liang, Ren Shaoran (2015) CO2 foam flooding for improved oil recovery: reservoir simulation models and influencing factors. J Petrol Sci Eng 133:13
Yin G, Grigg RB, Svec Y (2009) Oil recovery and surfactant adsorption during CO2-foam flooding. Offshore Technology Conference Houston, Texas, USA
Yongmao H, Zenggui W, Yueming JBC, Xiangjie L (2004) Laboratory investigation of CO2 flooding. SPE 88883:6
Zekr AY, Nasr MS, AlShobakyh A (2011) Evaluation of oil recovery by water alternating gas (WAG) injection—oil-wet & water-wet systems. SPE Enhanced Oil Recovery Conference. Kuala Lumpur, Malaysia, Society of Petroleum Engineers. SPE-143438-MS: 8
Acknowledgements
The authors gratefully acknowledge Department of Petroleum Technology, Dibrugarh University Assam, India, and Indian Institute of Technology, Guwahati, for the facilities and materials provided for carrying out the experimental works.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Phukan, R., Gogoi, S.B. & Tiwari, P. Enhanced oil recovery by alkaline-surfactant-alternated-gas/CO2 flooding. J Petrol Explor Prod Technol 9, 247–260 (2019). https://doi.org/10.1007/s13202-018-0465-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13202-018-0465-0