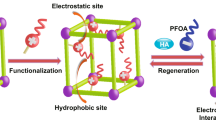
Abstract
Perfluorooctane sulfonic acid (PFOS), a perfluoroalkyl substance, has engendered alarm over its presence in water sources due to its intrinsic toxicity. Hence, there is a pressing need to identify efficacious adsorbents capable of removing PFAS derivatives from water. To achieve this, batch adsorption studies under various circumstances were employed to tune amorphous polymer networks regarding their morphological configuration, heat durability, surface area and capacity to adsorb PFOS in water. A facile, one-pot nucleophilic substitution reaction was employed to synthesize amorphous polymer networks using triazine derivatives as building units for monomers. Notably, POP-3 exhibited a superlative adsorption capacity, with a removal efficiency of 97.8%, compared to 90.3% for POP-7. POP-7 exhibited a higher specific surface area (SBET) of 232 m2 g−1 compared to POP-3 with a surface area of 5.2 m2 g−1. Additionally, the study emphasizes the importance of electrostatic forces in PFOS adsorption, with pH being a significant element, as seen by changes in the PFOS sorption process by both polymeric networks under neutral, basic and acidic environments. The optimal pH value for the PFOS removal process using both polymers was found to be 4. Also, POP-7 exhibited a better thermal stability performance (300 °C) compared to POP-3 (190 °C). Finally, these findings indicate the ease with which amorphous polymeric frameworks may be synthesized as robust and effective adsorbents for the elimination of PFOS from waterbodies.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Environmental safety and quality are crucial considerations subject to increasing regulation to ensure the consumption of healthy air and uncontaminated water (Han et al. 2023; Xu et al. 2022; Zhang et al. 2022). Nevertheless, the process of industrialization has persisted, leading to the contamination of water sources (Guo et al. 2022; Liu 2023; Wang, et al. 2020). Water sources face a significant challenge due to the proliferation of harmful contaminants including the presence of heavy metals, high ammonia levels, nitrates and so on (Zhao et al. 2022; Guo et al. 2023; Wang et al. 2021a). Among these harmful contaminants, per- and polyfluoroalkyl substances (PFAS) are of particular interest due to their totally or partially fluorinated alkyl groups that terminate with a variety of functionalities, such as phosphonic, carboxylic acids and sulfonic (Glüge et al. 2020). Perfluorooctane sulfonic acid (PFOS) is widely recognized as one of the most significant PFAS substances due to its unique physicochemical properties, including hydrophobicity and lipophobicity (Liu et al. 2020). This has resulted in its extensive use in industrial production, fibers and textiles, packaging materials, surfactants, insecticides, gene identification and firefighting foams (Liu et al. 2020; Yi et al. 2022). From the 1950s onwards, PFOS and its salts have been extensively employed in a wide range of consumer and industrial goods. These include the treatment of surfaces to enhance soil and stain resistance in textiles, paper, metals and pesticides (Geng et al. 2022; Zhang et al. 2021a; Song et al. 2023). Notably, PFOS has been utilized in aqueous film-forming foam (AFFF) (Buck et al. 2011; Ahrens and Bundschuh 2014). Although the use of PFOS has largely diminished since 2002, a few minor applications persist, such as AFFF and the suppression of mist in hard chrome plating (Ahrens and Bundschuh 2014). However, the persistence, bioaccumulation and toxicity of PFOS have significant adverse impacts on human health, including liver function, fertility and immunity. PFOS is also potentially carcinogenic, further highlighting the need for caution in its use (Brooke et al. 2004; Liu 2022; Shafqat et al. 2022).
Due to its widespread use, PFOS is commonly found in aqueous environments, including drinking water, groundwater, rainwater, lake water and wastewater (Brooke et al. 2004; Hosseinzadeh et al. 2022; Lin and Zhao 2022). Its prevalence in these environments has resulted in its detection at concentrations ranging from ng/L to μg/L. In 2018, the European Food Safety Authority established a weekly tolerance limit for PFOS at 13 ng/kg based on observed effects in humans (Zacs et al. 2023). However, many survey results have shown concentrations of PFOS to exceed the tolerance limit, underscoring the importance of effective treatment technologies for PFOS removal from aqueous environments.
The potential ecological and human health risks associated with contaminations, especially PFOS, are substantial, and thus, it is crucial to identify and implement efficient removal technologies to reduce the prevalence of PFOS in the environment (Zacs et al. 2023; Chen et al. 2020; Liu et al. 2018). Addressing this challenge is of paramount importance, and scientists and policymakers alike must work together to prioritize the development of solutions that can effectively remove PFOS from aqueous environments. While activated carbon adsorption is a widely used, eco-friendly and cost-effective approach, its slow adsorption rates and limitations have prompted scientists to explore alternative adsorbents and modifications (Zacs et al. 2023; Mohammadi and Mousazadeh 2022; Zhao and Ma 2022).
A variety of techniques have been proposed for the elimination of the compounds such as reverse osmosis, ion exchange, membrane filtration and electrochemical treatment (Zacs et al. 2023; Woodard et al. 2017; Lin et al. 2021; Wei et al. 2023). However, these methods are costly and only partially effective, making the large-scale elimination of PFAS a persistent challenge (Li et al. 2023; Wang et al. 2021b; Xia et al. 2022). Recently, covalent organic frameworks (COFs) have been increasingly investigated as potential sorbents for the elimination of PFAS derivatives due to their high porosity and pH stability, making them more favorable over metal–organic frameworks (MOFs) at severe water pH values (Ji et al. 2018). As a result of COFs' tunable crystallinity, functional groups can be optimized to promote interactions with PFAS derivatives with electrostatic and hydrophobic interactions being the primary driving forces for adsorption (Ji et al. 2018). Even though COFs have demonstrated favorable overall performance with increased adsorption capacities for a variety of PFAS substances, their ubiquitous application for large-scale PFAS separation is curtailed by their inherent limited yield, and the meticulous synthesis process demanded for the attainment of desired crystalline structure and morphological precision (Liu et al. 2022a).
To address the drawbacks associated with COFs, porous organic polymers (POPs) might offer a practical and economical alternative for the removal of PFAS derivatives, even though they lack the delicate spatial configuration control of COFs (Liu et al. 2022a, 2022b). For instance, Liu et al. have synthesized a fluorinated triazine-based POP, demonstrating effective adsorption and desorption of PFOA, indicating the potential of amorphous POPs as an alternative adsorbent for PFAS removal (Liu et al. 2022b). Moreover, POPs find applications in diverse fields, including membrane technology for water treatment, renewable energy systems, proton exchange membrane (PEM) fuel cells, batteries and more (Li et al. 2020a, 2020b; Khan et al. 2022). An example of such research is the study conducted by Liu et al., which explored the incorporation of active adsorption sites into POPs for the efficient removal of perfluorooctanoic acid (PFOA) from water using a membrane system (Liu et al. 2022a). The study demonstrated a remarkable performance, with a removal efficiency of 99.99% achieved within a mere 2 min. Another study conducted by Wang et al. showcased the successful utilization of POPs in a PEM fuel cell system, demonstrating their exceptional proton exchange capabilities (Wang et al. 2023).
To design efficient POPs for PFAS sorption, the selection of monomer units and functionalities is of paramount importance (Wanninayake 2021). We have successfully fabricated two distinct types of porous organic polymers, each utilizing 2,4,6-trichloro-1,3,5-triazine and 2,5,8-trichloro-s-heptazine as building blocks, respectively. Using a nucleophilic substitution strategy, these monomers were linked utilizing 4,4′,4′′,4′′′-(pyrene-1,3,6,8-tetrayl) tetra-aniline (Py-TA). The Py-TA linker has an array of protonation-prone amine sites, potentially enhancing the adsorption of PFOS. Moreover, the carboxylate, the amine groups or the fluorinated alkyl chain of PFOS may interact to form hydrogen bonds, which might help the adsorption process even more (Mei et al. 2021). The utilization of heptazine and its subunit, triazine, as the building blocks, in conjunction with the Py-TA linker, contributes to the enhancement of the organic polymer's thermal stability and surface area, which are crucial factors for efficient PFOS adsorption (Wang et al. 2022a).
While triazine has been used as a building unit for the synthesis process of porous organic polymers (POPs) for some time, heptazine has only recently emerged as a promising alternative. The three combined triazine rings that make up heptazine linkers' π-conjugated structure not only provide increased stability but also more nitrogen, which can enhance the adsorption capacity of POPs (Xu et al. 2019). To synthesize stable POPs with improved adsorption capabilities for PFOS, we cross-linked triazine derivatives with Py-TA to introduce secondary nitrogen groups into the structure. These secondary nitrogen sites can potentially interact with PFOS through electrostatic interactions with the anionic head of the molecule, especially when a strong chemical like hydrochloric acid exists in the environment. Furthermore, protonation of the triazine and triazine-derivative linkers can create positively charged surface sites on the POPs that can facilitate electrostatic interactions with PFOS (Liu et al. 2022a, 2022b; Jia et al. 2022).
As such, we conducted a comprehensive investigation to compare the influence of heptazine and triazine linkers on the fabrication of two distinctive porous organic polymers (POPs). In order to determine the efficacy of these polymeric networks as potential adsorbents for perfluorooctane sulfonic acid (PFOS) removal, batch adsorption studies under various circumstances were employed to tune amorphous polymer networks with regard to their morphological configuration, heat durability, surface area and capacity to adsorb PFOS in water. This study provides valuable insights into the feasibility of using heptazine-based linkers for POPs and its potential advantages over traditional triazine-based structures in terms of adsorption capacity and stability. This work advances the development of cost-effective and easily synthesized alternatives to existing PFOS adsorbents, with potential implications for environmental remediation efforts. However, additional research is imperative to delve into the refinement of the process for the practical implementation of these systems.
Materials and methods
Chemicals
The following materials were utilized in the current research: phosphorus(V) oxychloride, phosphorus pentachloride, cyanuric chloride, melamine, palladium tetrakis(triphenyl phosphine), 1,4-dioxane, tetrahydrofuran (THF), N,N-diisopropylethylamine (DIPEA), potassium carbonate, potassium hydroxide and perfluorooctane sulfonic acid (PFOS). 4-(4,4,5,5-Tetramethyl-1,3,2-dioxaborolan-2-yl)-aniline as well as 2,5,8-trichloro-s-heptazine were also used in this work. The chemical reagents utilized in this study were acquired from several well-established suppliers, namely TCI Chemicals, Sigma-Aldrich, Chem-Impe, Fisher Chemical and AmBeed.
In order to synthesize 2,5,8-trichloro-s-heptazine, a modified strategy based on a previously described technique was used (Luebke et al. 2014). Briefly, melamine was dissolved in a solution consisting of water and hydrochloric acid and heated at reflux temperature. Phosphorus pentachloride was introduced dropwise to the solution, and after many hours of processing, the mixture turned thick. The reaction materials were transferred into cold water, and the resultant material was then recovered by filtration and rinsed using ethanol and water. The solid was then treated with potassium hydroxide to remove any remaining impurities, followed by recrystallization from ethanol to yield the desired product.
Synthesis procedure of Py-TA linker
Using 4-(4,4,5,5-tetramethyl-1,3,2-dioxaborolan-2-yl) aniline (2.54 g) as well as 1,3,6,8-tetrabromopyrene (1 g) as reactants, with palladium tetrakis (triphenyl phosphine) (0.12 g) as a catalyst and potassium carbonate (2.10 g), a reaction was carried out. The reaction materials were added to anhydrous dioxane (15 mL) and then refluxed for 3 days under argon. After dilution with dioxane (10 mL), the mixture was treated with DI water (10 mL). Vacuum filtration of the mixture yielded Py-TA precipitate, without any further purification. The yield was 82%.
Synthesis of heptazine-based ligands
Initially, to produce melon, 31.00 g of melamine was poured into a porcelain dish and then heated using a furnace to reach a temperature of 490 °C and kept for four days. A mortar and pestle were then used to grind the product, yielding a yellow powder with a mass of 5.80 g. Next, to synthesize potassium cyamelurate, 5.80 g of melon was introduced to a flask with a round bottom containing approximately 100 mL of 2.5 M KOH aqueous solution. After that, the reaction material was refluxed for four hours, followed by a cooling process. The supernatant liquid was collected by vacuum filtration and allowed to crystallize overnight, yielding white solids. The filtrate was also collected by vacuum filtration with multiple ethanol washes; then, the product was dehydrated in a vacuum oven at 50 °C yielding a white-colored powder with a mass of 1.964 g. Then, in an argon-filled glovebox, potassium cyamelurate (1.430 g) and PCl5 (3.097 g) were added to a round-bottomed flask. After purging the mixture with nitrogen for approximately 30 min, POCl3 (30 mL) was slowly added. The solution was then carefully concentrated using rotary evaporation after being refluxed for an entire night at 110 °C. To the resulting material, ice water was introduced and stirred for several minutes. The material was then vacuum-filtered and washed copiously using ice water. Finally, the resulting material was dehydrated in an oven under vacuum conditions at 40 °C, resulting in the formation of a yellow solid (0.769 g).
Preparation of porous organic polymer with triazine-based ligands (POP-3)
Into a 75-mL flask with a round bottom containing a stir bar, 113.2 mg of Py-TA and 278.08 μL of DIPEA was introduced, followed by introducing 8 mL of anhydrous THF. The flask was nitrogen-purged for 20 min before cooling in an ice bath. Subsequently, a mixture containing 2,4,5-trichloro-1,3,5-triazine (49.8 mg) and 2 mL THF was introduced slowly under continuous stirring. The mixture was agitated for 40 min while keeping the temperature at 0°C, next heated up to 25°C and was stirred for another 40 min, and finally refluxed overnight. The temperature of the solution was reduced to room temperature; then, the obtained solid was filtered using a Buchner funnel. The solid was subjected to washing sequentially with THF and water and then collected and dehydrated in an oven under vacuum conditions at 90°C, producing a dark yellow solid with a weight of 105.8 mg. A schematic demonstration of the synthesis process is presented in Fig. 1.
Preparation of porous organic polymer with heptazine-based ligands (POP-7)
29.6 mg of 2,5,8-trichloro-s-heptazine and 68.0 mg of Py-TA were combined with 8 mL of dry THF. The mixture was put in a 75-mL flask with a round bottom that contained a stir bar and then subjected to nitrogen purging for around 15 min. After cooling the mixture using an ice bath, DIPEA (104.8 μL) was slowly added dropwise with continuous stirring. The resulting mixture was kept under stirring conditions for 40 min maintaining the temperature at 0 °C, next left to reach room temperature being stirred during an extra 3 h, followed by refluxing during the night. Next, the temperature of the resultant material was reduced to room temperature before it was filtered through a Buchner funnel and subsequently subjected to washing using THF and water. Next, the resulting material was dehydrated in an oven under a vacuum at 90 °C. This process yielded a green solid product (58.2 mg). This procedure was followed to prepare the porous organic polymer with heptazine-based ligands (POP-7). A schematic demonstration of the synthesis process is presented in Fig. 1.
Characterization
The characterization of materials was performed using a variety of techniques, including thermogravimetric analysis (TGA), Fourier transform infrared spectroscopy (FTIR), nuclear magnetic resonance (NMR) spectroscopy, solid-state 13C NMR (126 MHz) cross-polarization magic angle (CP-MAS) NMR, X-ray photoelectron spectroscopy (XPS), Braeuer–Emmett–Teller (BET), particle size distribution (PSD) and X-ray diffraction (XRD).
Assessment of POP-7 and POP-3 efficiency in PFOS adsorption
The adsorption method involved preparing stock suspensions of POP-7 and POP-3 with a concentration of 1 g L−1 in ultrapure water, which was then sonicated for 60 min. An example sample contained 1 mL of the stock solution introduced to 4 mL of a solution in which the PFOS concentration was 10 mg L−1, and then, ultrapure water was added to reach a volume of 40 mL where the final PFOS concentration reached 1 mg L−1. The samples were agitated for 30 min at room temperature. In order to conduct an adsorption isotherm study, individual PFOS solutions were prepared with concentrations of 1–10 mg L−1, each with a final solution volume of 40 mL and containing 1 mL of the adsorbent stock solutions, which had a concentration of 1 g L−1. For the PFOS sorption study in the presence of NaCl, a sample containing PFOS with a concentration of 10 mg L−1 was mixed with 1 mg NaCl and 1 mL of the 1 g L−1 stock suspension. Following a final dilution with ultrapure water, the resultant mixture had a volume of 40 mL and a PFOS amount of 1 mg L−1. The samples were agitated for 30 min at room temperature. To conduct the kinetic experiments, 4 mL of a solution with a PFOS concentration of 10 mg L−1 was mixed with 1 mL of POPs stock suspensions prepared earlier. Then, the solution was diluted to reach a volume of 40 mL. During a period of 24 h, the samples were stirred while the remaining PFOS content in the samples was analyzed at regular time intervals. To investigate the impact of pH on PFOS uptake onto POPs, 1 mL of the adsorbent suspensions was combined with 4 mL of a solution containing a PFOS concentration of 10 mg L−1. To obtain a final volume of 40 mL, the solution was diluted with ultrapure water and its pH level was controlled using either NaOH (0.1 M) or HCl (0.1 M) while closely monitoring with a pH meter. The samples were then agitated for 30 min. After agitation, all samples in this work were filtered using a 0.22 μm nylon filter and analyzed using LC–MS.
Equation (1) was used to calculate the percentage of PFOS removed:
Here, \({C}_{i}\) (mg L−1) denotes the initial amount of PFOS, while \({C}_{f}\) (mg L−1) attributes the PFOS amount after the treatment. Equation 2 was used to determine the adsorption capacity:
Here, \({q}_{e}\) represents the adsorption capacity at equilibrium in mg/g, \({C}_{e}\) denotes the equilibrium amount of PFOS (mg L−1), \(v\) indicates the total volume of the samples (L), and \(m\) defines the weight of adsorbent used for treatment in g. The Langmuir isotherm model was employed and the corresponding equation; the data of PFOS adsorption onto POPs were examined using Eq. (3):
The equilibrium constant (\({K}_{1}\)) and the largest adsorption capacity (\({q}_{m}\)) were obtained using the Langmuir model. The Freundlich model was also used to evaluate the PFOS adsorption onto POPs, and it is determined using Eq. (4):
In addition, the kinetic data for the PFOS adsorption process onto adsorbents were evaluated via pseudo-first- and pseudo-second-order kinetic models. The corresponding equations [Eqs. (5) and (6), respectively] are as follows:
This equation involves several variables including \({q}_{m}\), which represents the maximum amount of PFOS that can be adsorbed per unit of adsorbent mass, \({q}_{t}\), which denotes the amount of PFOS adsorbed at a specific time point, \({K}_{1},\) which attributes the rate constant for the pseudo-first-order kinetic model, \({K}_{2},\) which refers to the rate constant for the pseudo-second-order kinetic equation, and \(t,\) which denotes the duration of the adsorption process in minutes.
The PZC (point of zero charge) values were obtained using POP-7 or POP-3 samples containing an amount of 1 mg mL−1 in ultrapure water. Prior to preparing 5 samples of each polymer, the suspensions were sonicated for 30 min to make sure that homogeneity was reached. The pH of the solutions was adjusted to various levels between 3 and 9 using either NaOH (0.1 M) or HCl (0.1 M), with careful monitoring utilizing a pH meter. The samples were agitated for 30 min, which corresponds to the typical duration of adsorption treatment. A pH meter was then used to determine the pH level, and the information was then utilized to plot ΔpH (the variation between the final and first pH readings) against the starting pH. The PZC was defined as the pH level at which ΔpH equals 0.
Results and discussion
Characterization
Upon analyzing the FTIR spectra, it was found that the Py-TA aniline groups exhibited an N–H stretch at 3380 cm−1 (Fig. 2A), indicative of primary amines. The observed bands at ~ 1585 and 1540 cm−1 in POP-3 and POP-7 suggest the presence of cross-linked heptazine- and triazine-based units, respectively, and are ascribed to the vibrational stretching of C–N. Furthermore, the almost negligible peak related to the C–Cl stretch in both POP-3 and POP-7 confirms the effective substitution of triazine- and heptazine-based units.
Confirmation of heptazine and triazine substitution in the Py-TA reactions was achieved through CCP-MAS NMR spectroscopy, which provides crucial insights into the chemical structure of the resulting polymers (Fig. 2B). As expected, the signal attributed to C–Cl ~ 175 ppm was absent in POP-7, indicating the successful substitution of heptazine-based units. POP-3, on the other hand, showed a minor intensity signal that could be attributed to triazine-based units, which may have lower reactivity than heptazine units in the cross-linking process (Sharma et al. 2021a). Heptazine and triazine carbons are reflected by the peaks at 162.9, 155.2 and 170.3 ppm, respectively (Kumar et al. 2022). The aliphatic region displayed residual DIPEA from the nucleophilic substitution reaction. The aromatic region showed a set of peaks ranging between 126.8 and 146.0 ppm, corresponding to Py-TA units in both POPs. The results suggest an effective cross-linking of triazine and heptazine units with Py-TA to form the resultant polymers.
The surface chemistry of POP-7 and POP-3 was investigated using X-ray photoelectron spectroscopy (XPS). The high-resolution C 1s spectra, presented in Fig. 3B, C, showed the characteristic peaks for carbon atoms in the polymer. The peak at ~ 285.0 eV refers to the C=N bond. The peak at ~ 288.0 eV is attributed to the N–C=N bond, indicating the presence of triazine moieties in the polymer network. The presence of these peaks confirms the successful synthesis of POP-7 and the incorporation of heptazine building blocks into the polymer network. Also, the peak at approximately 284.0 eV is related to the C=C bond. The N 1s XPS spectra of POP-7 exhibited characteristic peaks, including N-(C)3, N–H, C=C and π excitation peaks. The N-(C)3 peak at ~ 400 eV refers to nitrogen atoms in POP-7's heptazine rings, while the N–H peak at ~ 401 eV represented nitrogen–hydrogen bonds in the aniline component's amine groups. The π excitation peak at ~ 405 eV represented the excitation of electrons from the nitrogen atoms to the π* orbital of the heptazine rings. The N 1s XPS spectra of POP-3 also exhibited similar results with characteristic peaks of N-(C)3, N–H and C=C. The spectra for POP-3 exhibited characteristic peaks corresponding to N–C=N, C=N and C=C bonds. The C=C bond peak was observed at ~ 285.7 eV, which is consistent with the expected binding energy for a sp2-hybridized carbon atom. The C=N bond peak appeared at around 286.2 eV, which is also in agreement with the expected binding energy for a C=N bond. The N–C=N bond peak was seen at ~ 287.6 eV, demonstrating the presence of triazine units in the POP-3 structure. The C = C peak had a greater relative intensity than the C=N and N–C=N peaks, indicating a higher abundance of sp2 carbon atoms in both POP-3 and POP-7 structures. Overall, the XPS spectrum of POP-3 and POP-7 was consistent with their expected structure and confirms the results obtained from FTIR and NMR analysis (Fig. 4).
POP-3 and POP-7 were subjected to thermal analysis by TGA under a nitrogen gas ambient, using a heating rate of 10 °C min−1. POP-7 showed higher thermal stability than POP-3, with a degradation temperature of around 300 °C (Fig. 5). Because of the higher aromaticity provided by the peripheral nitrogen in the heptazine structure, POP-7 has higher heat stability. Moreover, TGA revealed that POP-7 displayed an initial weight loss of ∼4.00% in the range of 25–100 °C, which was ascribed to the discharge of entrapped solvents from the POP-7 framework. This low-temperature weight reduction, however, was less evident in POP-3, possibly due to its reduced surface area and intrinsic porosity, which resulted in fewer solvent entrapments. Overall, TGA data suggest that POP-7 has improved thermal stability compared to POP-3, which is favorable for its potential applications as a high-performance material. XRD analysis also indicated that both POP-3 and POP-7 were amorphous polymers.
An N2 adsorption–desorption isotherm at 77 K was used to probe the pore structure of both adsorbents. From Fig. 6, a type II reversible adsorption shape was identified in the N2 isotherms of POP-7. This adsorption profile demonstrates the presence of mesopores, which are described via monolayer adsorption at the pressure range of 0–0.2 p/p0, followed by multilayer adsorption in the range of 0.2–0.8 p/p0, before nitrogen condensation takes place in the pore structure. In contrast, the nitrogen isotherm of POP-3 exhibits a lower surface area, as well as a different adsorption profile. BET surface area analysis reveals that POP-7 possesses a dramatically higher surface area (232 m2 g−1) than POP-3 (5.2 m2 g−1). The more rigid fused-ring heptazine structure of POP-7, which likely prevents pore destruction under vacuum, is responsible for its mesoporous characteristic. Meanwhile, the lower surface area of POP-3 is likely due to its lower inherent porosity, resulting in fewer sites for N2 adsorption.
The mesoporous structure of POP-7 is supported by the pore size distribution displayed in Fig. 7, which reveals that a major proportion of the pore size diameters were smaller than 15 nm. Moreover, POP-7 exhibited a significantly higher pore volume of 0.61 cm3g−1 compared to POP-3's value of 0.03 cm3 g−1, further substantiating the mesoporous nature of the former. As expected, the SBET of POP-7 and POP-3 exhibited a substantial contrast, with values of 232 and 5.2 m2g−1, respectively. Notably, the SBET of other amorphous POPs reported in previous investigations vary widely from 423 to 116 m2g−1 (Sharma, et al. 2021b). The relatively low SBET of POP-3 may be attributed to its higher flexibility of the structure, which can be altered using rigid heptazine units.
Adsorption studies
In evaluating the adsorption capabilities of two novel porous organic polymers (POPs), POP-3 and POP-7, for the removal of perfluorooctane sulfonic acid (PFOS), an experiment was conducted wherein 1 mg of adsorbents was utilized to eliminate a PFOS solution with a concentration of 1 mg L−1 for 30 min. The residual PFOS amount in the samples was then measured to determine the PFOS removal percentage. Results indicated that POP-7 exhibited a PFOS removal percentage of 90.3% compared to POP-3's 97.8%. While both polymers demonstrated promising adsorption capabilities, POP-3 exhibited superior performance in terms of PFOS removal despite having a relatively lower nonporous SBET. This observation has been previously reported in the literature, and we hypothesize that the high level of cross-linking in POP-7 may restrict the accessibility of active adsorption sites, thus lowering PFOS removal. Notably, POP-3 and POP-7 showed competitive performance, particularly when compared to PFAS removal using electrochemical-assisted multi-walled carbon nanotubes (90% removal) and aluminum-based water decontamination (99.5% removal) (Zhang et al. 2021b).
Despite POP-3 exhibiting a higher PFOS removal percentage of 97.8%, compared to 90.3% for POP-7, the latter presents distinct advantages. Specifically, the high SBET of POP-7 allows for the effective adsorption of a wide range of pollutants, and its mesoporous structure provides a significant surface area for adsorption (Dang et al. 2015). Additionally, the high level of cross-linking in POP-7 may contribute to its durability and suitability for prolonged usage, resulting in its superior overall performance compared to POP-3 for various applications (Sharma, et al. 2021a; Wang et al. 2022b; Kumar et al. 2021). Also, its mesoporous structure allows for faster diffusion of adsorbates into the pores, resulting in shorter treatment times (Dang et al. 2015).
In this investigation, the Freundlich and Langmuir isotherm models were implemented to examine the PFOS adsorption behavior of the amorphous polymer networks toward PFOS. The Langmuir model presumes a unimolecular adsorption process with homogenous distribution of adsorption nodes on the surface of the sorbent, while the Freundlich isotherm suggests a heterogeneous surface with uneven adsorption energies (Mousazadeh et al. 2021; Sun et al. 2023). The findings revealed that both models aptly depicted the adsorption behavior of the amorphous polymer networks toward PFOS. However, the Langmuir model (\({R}^{2}= 0.9831\)) better portrayed the adsorption of PFOS onto POP-7, while the Freundlich model (\({R}^{2}= 0.9811\)) exhibited better prediction of PFOS uptake onto POP-3. The largest PFOS uptake for POP-3 was found to be 185.18 mg g−1, indicating its superior adsorption efficacy in comparison with POP-7 with a maximum removal performance of 144.93 mg g−1. The findings imply that the amorphous polymer networks possess desirable adsorption features for the removal of PFOS from water and have great potential for practical use in water treatment operations. Table 1 clearly demonstrates that the as-synthesized adsorbents, POP-3 and POP-7, exhibit outstanding performance in comparison with previously studied materials. For instance, MSNs/NIP, a molecularly imprinted polymer, has the best performance of only 21.10 mg g−1, thus highlighting the exceptional performance of the tailored amorphous polymer networks.
To optimize the treatment period for efficient perfluorooctane sulfonic acid (PFOS) removal, we conducted a comparative analysis of PFOS adsorption kinetics using two different types of porous organic polymers (POPs). The maximal adsorption performance was reached in less than 30 min, and both adsorbents showed rapid kinetics, a characteristic of sorption processes in which electrostatic interactions play a dominant role. Therefore, our synthesis approach has a distinct advantage, as surface optimization can assist future performance enhancements (Tables 2, 3).
Our investigation revealed that the adsorption of PFOS on both POPs followed a pseudo-second-order kinetic model, with excellent R2 values of 0.9952 and 0.9970 for POP-3 and POP-7, respectively. The observed trend of PFOS adsorption over time can be attributed to the existence of functionalities within the adsorbents to act as active sorption sites through the PFOS uptake onto POPs. In the initial stages of PFOS adsorption, an abundant number of free sorption sites are accessible for interaction. Nevertheless, with the occupation of active sorption sites, the other sites become increasingly confined. This results in a surge of interactions between solutes, encompassing weak hydrophobic and repulsion interactions (Hussain et al. 2022; Wang et al. 2022c; Mohammadi et al. 2021). This elucidates the observed phenomenon of hysteresis in adsorption, where certain levels of desorption occur with the passage of additional treatment time. The time-dependent desorption observed in this phenomenon highlights the dynamic and complex nature of adsorption processes.
The rate constant k2 for PFOS uptake onto POPS was calculated to be 0.65 and 2.25 g mg−1 h−1 for POP-3 and POP-7, respectively, indicating that POP-7 reaches maximum adsorption faster with superior PFOS removal efficiency. Overall, our results demonstrate that our tailored porous organic polymers with enhanced capacity for PFOS elimination have the potential to serve as efficient and tunable adsorbents for PFOS removal applications.
Our experimental investigation involved exploring the influence of pH on the underlying mechanisms that determine the sorption of PFOS and our customized POPs. When using POP-7 at a pH range of 3–9, it was observed that under basic conditions minimum PFOS removal occurred, as depicted in Fig. 4A. A similar trend was also observed when utilizing POP-3. This pH-dependent behavior of PFOS adsorption is likely attributed to the surface charge characteristics of the adsorbent, which was characterized by conducting a point of zero charge investigation. Our findings revealed that POP-7 and POP-3 exhibit pHPZC values of around pH 7.6 and pH 6.8, respectively. It is inferred from the experimental observations that the as-synthesized adsorbents exhibit a negative charge on their surface when pH value is 9, as pH > pHPZC (Figs. 8, 9, 10).
Our experimental investigation involved exploring the influence of pH on the underlying mechanisms that determine the sorption of PFOS and our customized POPs. When using POP-7 at a pH range of 3–9, it was observed that under basic conditions minimum PFOS removal occurred, as depicted in Fig. 11. A similar trend was also observed when utilizing POP-3. This pH-dependent behavior of PFOS adsorption is likely attributed to the surface charge characteristics of the adsorbent, which we characterized by conducting a point of zero charge investigation. Our findings revealed that POP-7 and POP-3 exhibit pHPZC values of around pH 7.6 and pH 6.8, respectively. It is inferred from experimental observations that the as-synthesized adsorbents exhibit a negative charge on their surface when pH value is 9, as pH > pHPZC.
The investigation of PFOS removal from natural drinking water sources was performed using a typical lake water sample to simulate real-world scenarios. The physicochemical properties of the lake water are presented in Table 4. POP-3 and POP-7 exhibited 86 and 79% PFOS removal from the lake water sample, respectively. As expected, a decrease in PFOS removal was observed in lake water samples in comparison with ultrapure water, owing to the high mineral content of the former. This could potentially lead to electrostatic interactions between the sorbent particles and the mineral ions, leading to interaction with the surfaces of the sorbents, ultimately resulting in a lower rate of PFOS uptake. These findings validate that the primary mode of PFOS adsorption by the POPs is owing to electrostatic interactions.
The water stability and leaching of both POPs were assessed through prolonged water contact. Figure 12 illustrates the results, showing no weight reduction for POP-7 until day 24 and then a negligible weight reduction of 0.4% until day 30. Also, POP-3 exhibited no weight reduction until day 22 and then a light decrease of 0.5%. These results indicate the high stability of the POPs in water, with no leaching from the polymer matrix into the water media. This finding assures that the use of these POPs is unlikely to have any adverse impact on water quality during the PFOS elimination process.
Conclusion
In brief, a one-pot nucleophilic substitution strategy was utilized to fabricate triazine- and heptazine-enriched POPs. POP-7 exhibited superior physical characteristics in comparison with POP-3, including higher SBET at 232 m2 g−1 and heat durability up to 300 °C. Nevertheless, POP-3 demonstrated greater efficacy in adsorbing PFOS, with 97.8% removal via electrostatic interactions that favor pH values below the pHPZC. The maximum PFOS adsorption capacities for POP-7 and POP-3 were found to be 144.94 mg g−1 and 185.18 mg g−1, indicating the superior performance of the as-synthesized polymers for PFOS removal from water. When testing both POPs in lake water, the presence of additional minerals resulted in competitive electrostatic interactions, thereby impeding PFOS adsorption. Prospective research initiatives will prioritize the meticulous refinement of conditions to facilitate efficacious PFOS adsorption and reversible desorption in aqueous media. The inquiry into these adsorbents illustrates an optimistic prospect for the elucidation of small SBET amorphous materials in the practical ambit of PFOS eradication applications.
References
Ahrens L, Bundschuh M (2014) Fate and effects of poly-and perfluoroalkyl substances in the aquatic environment: a review. Environ Toxicol Chem 33(9):1921–1929
Brooke D, Footitt A, Nwaogu T (2004) Environmental risk evaluation report: perfluorooctanesulphonate (PFOS), Building Research Establishment Ltd, Risk and Policy Analysts Ltd, Environment Agency publication, UK. https://assets.publishing.service.gov.uk/media/5a7b984540f0b645ba3c5549/scho1009brbl-e-e.pdf
Buck RC et al (2011) Perfluoroalkyl and polyfluoroalkyl substances in the environment: terminology, classification, and origins. Integr Environ Assess Manag 7(4):513–541
Chen D et al (2020) A general linear free energy relationship for predicting partition coefficients of neutral organic compounds. Chemosphere 247:125869
Dang Q-Q et al (2015) Heptazine-based porous framework for selective CO2 sorption and organocatalytic performances. ACS Appl Mater Interfaces 7(51):28452–28458
Du L et al (2018) Preparation of mesoporous silica nanoparticles molecularly imprinted polymer for efficient separation and enrichment of perfluorooctane sulfonate. J Sep Sci 41(23):4363–4369
Elanchezhiyan SS et al (2021) Efficient and selective sequestration of perfluorinated compounds and hexavalent chromium ions using a multifunctional spinel matrix decorated carbon backbone N-rich polymer and their mechanistic investigations. J Mol Liq 326:115336
Geng C et al (2022) Enhancing the permeability, anti-biofouling performance and long-term stability of TFC nanofiltration membrane by imidazole-modified carboxylated graphene oxide/polyethersulfone substrate. J Membr Sci 664:121099
Glüge J et al (2020) An overview of the uses of per-and polyfluoroalkyl substances (PFAS). Environ Sci Process Impacts 22(12):2345–2373
Guo W et al (2022) Ge-doped cobalt oxide for electrocatalytic and photocatalytic water splitting. ACS Catal 12(19):12000–12013
Guo Z et al (2023) Innovative and green utilization of zinc-bearing dust by hydrogen reduction: recovery of zinc and lead, and synergetic preparation of Fe/C micro-electrolysis materials. Chem Eng J 456:141157
Han Y et al (2023) Hydrogen peroxide treatment mitigates antibiotic resistance gene and mobile genetic element propagation in mariculture sediment. Environ Pollut 328:121652
Hassan M et al (2022) Magnetic biochar for removal of perfluorooctane sulphonate (PFOS): interfacial interaction and adsorption mechanism. Environ Technol Innov 28:102593
Hosseinzadeh A et al (2022) Machine learning-based modeling and analysis of PFOS removal from contaminated water by nanofiltration process. Sep Purif Technol 289:120775
Hussain FA et al (2022) Adsorption of perfluorooctanoic acid from water by pH-modulated Brönsted acid and base sites in mesoporous hafnium oxide ceramics. Iscience 25(4):104138
Ji W et al (2018) Removal of GenX and perfluorinated alkyl substances from water by amine-functionalized covalent organic frameworks. J Am Chem Soc 140(40):12677–12681
Jia Y et al (2022) An attention-based cascade R-CNN model for sternum fracture detection in X-ray images. CAAI Trans Intell Technol 7(4):658–670
Khan SB et al (2022) 3D printed nanofiltration membrane technology for waste water distillation. J Water Process Eng 49:102958
Kumar S et al (2021) Understanding the role of soft linkers in designing hepatzine-based polymeric frameworks as heterogeneous (photo) catalyst. J Colloid Interface Sci 588:138–146
Kumar S et al (2022) Triazine based nanoarchitectonics of porous organic polymers for CO2 storage. Mater Lett 313:131757
Li Z et al (2020a) Sulfonated triazine-based porous organic polymers for excellent proton conductivity. ACS Appl Polym Mater 2(8):3267–3273
Li Z et al (2020b) Simple and universal synthesis of sulfonated porous organic polymers with high proton conductivity. Mater Chem Front 4(8):2339–2345
Li X et al (2023) Characterizing molecular transformation of dissolved organic matter during high-solid anaerobic digestion of dewatered sludge using ESI FT-ICR MS. Chemosphere 320:138101
Lin K, Zhao H (2022) The impact of green finance on the ecologicalization of urban industrial structure—based on GMM of dynamic panel system. J Artif Intell Technol 2(3):123–129
Lin X et al (2021) Membrane inlet mass spectrometry method (REOX/MIMS) to measure 15N-nitrate in isotope-enrichment experiments. Ecol Ind 126:107639
Liu X (2022) Real-world data for the drug development in the digital era. J Artif Intell Technol 2(2):42–46
Liu Z, et al. (2023) Remote sensing and geostatistics in urban water-resource monitoring: a review. Mar Freshwater Res
Liu W et al (2018) Effective extraction of Cr (VI) from hazardous gypsum sludge via controlling the phase transformation and chromium species. Environ Sci Technol 52(22):13336–13342
Liu L et al (2020) Removal of perfluorooctanoic acid (PFOA) and perfluorooctane sulfonate (PFOS) from water by carbonaceous nanomaterials: a review. Crit Rev Environ Sci Technol 50(22):2379–2414
Liu X et al (2022a) Installation of synergistic binding sites onto porous organic polymers for efficient removal of perfluorooctanoic acid. Nat Commun 13(1):2132
Liu G et al (2022b) Novel fluorinated nitrogen-rich porous organic polymer for efficient removal of perfluorooctanoic acid from water. Water 14(7):1010
Luebke R et al (2014) Microporous heptazine functionalized (3,24)-connected RHT-metal–organic framework: synthesis, structure, and gas sorption analysis. Cryst Growth Des 14(2):414–418
Mei W et al (2021) Per-and polyfluoroalkyl substances (PFASs) in the soil–plant system: sorption, root uptake, and translocation. Environ Int 156:106642
Mohammadi N, Mousazadeh B (2022) Carbon capture and utilization as an alternative for renewable energy storage. Synergy development in renewables assisted multi-carrier systems. Springer, pp 229–254
Mohammadi N, Mousazadeh B, Hamoule T (2021) Synthesis and characterization of NH 2-SiO 2@ Cu-MOF as a high-performance adsorbent for Pb ion removal from water environment. Environ Dev Sustain 23:1688–1705
Mousazadeh B, Mohammadi N, Hamoule T (2021) Removal of phosphate from the aqueous environment using iron oxide/activated carbon composites: activated carbon derived from Ziziphus nuts as a new precursor. Iran J Chem Eng (IJChE) 18(3):52–62
Shafqat S et al (2022) Standard ner tagging scheme for big data healthcare analytics built on unified medical corpora. J Artif Intell Technol 2(4):152–157
Sharma N et al (2021a) A tailored heptazine-based porous polymeric network as a versatile heterogeneous (photo) catalyst. Chem—A Eur J 27(41):10649–10656
Sharma N et al (2021b) Metal-free heptazine-based porous polymeric network as highly efficient catalyst for CO2 capture and conversion. Front Chem 2021:743
Song Z et al (2023) Formic acid formation via direct hydration reaction (CO+ H2O→ HCOOH) on magnesia-silver composite. Appl Surf Sci 607:155067
Sun S et al (2023) Application of a novel coagulant in reservoir water treatment in Qingdao. Desal Water Treat 284:49–60
Wang Z et al (2020) Enhanced denitrification performance of Alcaligenes sp. TB by Pd stimulating to produce membrane adaptation mechanism coupled with nanoscale zero-valent iron. Sci Total Environ 708:135063
Wang Z et al (2021a) Nano zero-valent iron improves anammox activity by promoting the activity of quorum sensing system. Water Res 202:117491
Wang Z et al (2021b) Enhanced adsorption and reduction performance of nitrate by Fe–Pd–Fe3O4 embedded multi-walled carbon nanotubes. Chemosphere 281:130718
Wang W et al (2022a) Preparation of magnetic covalent triazine frameworks by ball milling for efficient removal of PFOS and PFOA substitutes from water. Environ Sci Nano 9(4):1466–1475
Wang X et al (2022b) Design of porous organic polymer photocatalysts based on heptazine for efficient photocatalytic aerobic oxidation. Chem Eng J 431:134051
Wang Z et al (2022c) Bamboo charcoal fused with polyurethane foam for efficiently removing organic solvents from wastewater: experimental and simulation. Biochar 4(1):28
Wang S et al (2023) Porous organic polymer with high-density phosphoric acid groups as filler for hybrid proton exchange membranes. J Membr Sci 666:121147
Wanninayake DM (2021) Comparison of currently available PFAS remediation technologies in water: a review. J Environ Manage 283:111977
Wei S et al (2023) Recent advances in electrochemical sterilization. J Electroanalyt Chem 2023:117419
Woodard S, Berry J, Newman B (2017) Ion exchange resin for PFAS removal and pilot test comparison to GAC. Remediat J 27(3):19–27
Xia G et al (2022) Fabrication of ZnAl-LDH mixed metal-oxide composites for photocatalytic degradation of 4-chlorophenol. Environ Sci Pollut Res 29(26):39441–39450
Xu C et al (2019) Heptazine-based porous polymer for selective CO2 sorption and visible light photocatalytic oxidation of benzyl alcohol. Microporous Mesoporous Mater 282:9–14
Xu D et al (2022) Advances in continuous flow aerobic granular sludge: a review. Process Saf Environ Prot 163:27–35
Yan T et al (2013) Adsorption of perfluorooctane sulfonate (PFOS) on mesoporous carbon nitride. RSC Adv 3(44):22480–22489
Yi T, Shi M, Zhu H (2022) Medical data publishing based on average distribution and clustering. CAAI Trans Intell Technol 7(3):381–394
Zacs D et al (2023) Application of nano-LC–nano-ESI–Orbitrap-MS for trace determination of four priority PFAS in food products considering recently established tolerable weekly intake (TWI) limits. Analyt Chim Acta 2023:341027
Zhang G et al (2021a) Impacts of biochars on bacterial community shifts and biodegradation of antibiotics in an agricultural soil during short-term incubation. Sci Total Environ 771:144751
Zhang Z et al (2021b) Adsorption of perfluorooctanoic acid (PFOA) and perfluorooctanesulfonic acid (PFOS) by aluminum-based drinking water treatment residuals. J Hazardous Mater Lett 2:100034
Zhang X et al (2022) Plutonium reactive transport in fractured granite: multi-species experiments and simulations. Water Res 224:119068
Zhao H, Ma L (2022) Several rough set models in quotient space. CAAI Trans Intell Technol 7(1):69–80
Zhao Y et al (2022) Nitrogen recovery through fermentative dissimilatory nitrate reduction to ammonium (DNRA): carbon source comparison and metabolic pathway. Chem Eng J 441:135938
Acknowledgements
The authors extend their appreciation to the Deanship of Scientific Research at King Khalid University for funding this work through Large Groups Project under Grant Number RGP.2/133/44.
Funding
Open access funding provided by Lulea University of Technology.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Elboughdiri, N., Amari, A., Harharah, H.N. et al. Tailoring porous organic polymers with enhanced capacity, thermal stability and surface area for perfluorooctane sulfonic acid (PFOS) elimination from water environment. Appl Water Sci 13, 211 (2023). https://doi.org/10.1007/s13201-023-02014-0
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s13201-023-02014-0