Abstract
In the sight of the ever-increasing significance of green-based iron nanoparticles especially in wastewater treatment applications is a compelling reason for their use in a waste prevention opportunity, safer environment and benign precursor materials become the vital considerations. Hence, in the current investigation, an efficient co-precipitation technique was applied to prepare highly active chitosan-coated magnetic iron oxide that is applied for wastewater remediation. In the current investigation, chitosan coupled with magnetite nanoparticles namely CS-M was attained by coupling chitosan (CS) with magnetite nanoparticles via simple co-precipitation in different weight proportions and the attained samples labeled as CS-M-(2:1), CS-M-(3:1) and CS-M-(1:2). The structure, morphology and characteristics of the prepared samples were characterized using X-ray diffraction spectroscopy and transmission electron microscopy (TEM). The catalytic oxidation activity of the prepared samples was investigated to eliminate Basic Blue 9 (BB9) dye from aqueous effluent as simulated textile polluted stream. The experimental data exposed almost BB9 dye emanation. The system parameters revealed the maximal BB9 oxidation (99%) was attained within 2 h of irradiance time. Box–Behnken design factorial design based on response surface methodology was applied to optimize the Fenton’s system (CS-M-(2:1)/H2O2) parameters to maximize the efficiency 2.4 and 767 mg/L of CS-M and H2O2, respectively, at pH 7.0. The experimental data exposed that CS-M-(2:1) is signified as the optimal catalyst mixture. The kinetic data verify the oxidation system follows the second-order reaction kinetic model. Further, thermodynamic variables predicted that the reaction is endothermic and non-spontaneous in nature. Hence, the catalyst could be environmental benign and the evaluation introduces the role of engineers and chemists in a world for a sustainable material use.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Presently, the most challenge issue that facing our societies is water contamination and pollution, which needs reasonable solution. As an indispensable element for the sustainable modern communities, using environmentally benign materials plays a significant role in global awareness and economics. Noteworthy to mention, water is an exceptionally substance that persist on earth through the ecosystem to preserve the life-quality (Al et al. 2008; Ashour and Tony 2020). Although 71% of water is covered on the earth surface, due to the salty taste, merely 2.5% of freshwater is available. However, only 0.27% is clean water that is ready for use since the residual amounts is scattered in the underground, frozen areas and marshland areas (Thabet et al. 2022b). Recently, in the industrialized modern societies and the quick hike of human population, the result is depletion in water resources. Additionally, the scarce freshwater is not ample to meet up the necessities of massive population (Golka et al. 2004).
Among the various wastewater pollutants types, dyes are signified as key treacherous substances that detected in the industrial sewages since they used in several applications such as textile manufacturing, foodstuffs, paper printing, and leather industries (Ioannou and Fatta-Kassinos 2013; Tony and Lin 2020b). Azo dyes between the numerous dye species could only posses an inadequate degradability or may be non-degradable in the atmospheric conditions, besides their water solubility and toxicity (Thabet et al. 2020; Tony and Mansour 2020a). Moreover, some types of these azo dyes may be decomposed in the anaerobic environments into carcinogenic substances. Thus, they might posses the property to resist the oxidant or may be also sunlight. Therefore, the selection of their proper elimination technology is a problematic. From this regard, urgent searching for new technologies for such dyes elimination from aqueous streams is required (Tony and Mansour 2020b; Zubir et al. 2014).
In order to minimize freshwater consumption with the industrialized modern societies, wastewater reclamation and management is suggested to effectively improve water conservation in various ways. Physical, chemical and biological wastewater treatment opportunities are signified a viable wastewater treatment opportunity (Thabet et al. 2022a; Tony and Lin 2021; Yang et al. 2021). Although chemical treatments are a viable option for wastewater treatments among other suggested treatments, it incurs high operational costs due to the large cots of the coagulants, as well as the disposal of massive sludges after treatments which needs further treatments. Under such circumstances, the idea of using environmentally friendly, recoverable, recyclable and reusable material may be favorable. Many possible attempts have been recorded in the literature for using conservative materials in treatments (Laib et al. 2021). Among the possible routes suggested in the literature, using nanocomposite especially environmentally benign material has been gained immense attention in the photo-oxidation ability since of a variety of toxicity caused by the other materials. So, searching for attractive materials is an attractive research technology (Pourali et al. 2021; Thabet et al. 2022b; Tony and Lin 2020a). Choosing the appropriate material that possesses unique and attractive properties such as the catalytic activity, optical activity and also possesses chemical selectivity has a great attention in wastewater treatment applications. Biopolymers are suitable candidates that exhibit extended stability and possess a flexibility of blending or cross-linking with other materials (Ahmadi et al. 2016; Tony 2021a). Thus, they are a superior host compounds to prepare a various composite photocatalytic materials that plays a prominent role in eliminating toxic dyes from aqueous effluents. Moreover, metal augmentation with biopolymers displays a synchronous effect in enhancing the photocatalytic and catalytic oxidation activity (Tony and Eltabey 2022; Yang et al. 2021).
Advanced oxidation processes, AOPs, have been emerged and gained the researchers’ attention for their in situ remediation. Thus, AOPs are viable option due to the mineralization of a wide range of pollutants because of the presence of highly selective oxidative hydroxyl radicals, ·OH species. Commonly, the attractive use of ultraviolet illumination (UV) through AOPs applications is promising tool since it improves the treatment efficiency capabilities. Fenton-based AOPs are a promising process for their high efficiency. Such system is associated with the initiation of the active reaction intermediates by interacting H2O2 and Fen+ that is so-called classical Fenton’s or Fenton-Like reaction oxidation (Zhang et al. 2018). Although, some limitations are related to such classical reaction, i.e., working at acidic pH conditions and iron sludge after the catalytic cycle that might be separated still restricts the process practical applications (Shaheen and Emam 2018; Tony 2021b). Hence, searching for an advanced iron source to overcome such limitations is still gaining researchers’ interest.
Chitosan, chitin biopolymer is characterized by its high mechanical strength, non-toxic material, cost efficient, high water permeability besides the successful ability to chemical modification through the addition of metal oxides to be a nanocomposites. Chitosan is widely applied in wastewater treatment as adsorbent material since it possesses hydroxyl and amino functional groups. Such groups might protonated in acidic aqueous media, and the result is a massive electrostatic attraction for anionic molecules. However, according to the authors’ knowledge it is not applied so far as a photocatalytic material. Moreover, although it is efficient in water remediation, chitosan separation from reclaimed aqueous media is still an obstacle that limiting its real industrial applications.
Recently, with the advanced environmental remediation systems, iron oxides nanoparticles are encouraged due to their small size and high surface area (Tahoun et al. 2022; Thabet et al. 2022a).
However, for their better separation from aqueous media after usage, magnetic iron oxides application with polymers as a support system is preferred. Among iron oxides with magnetic properties, magnetite stands out since it possesses high surface area, chemical and temperature stability, great oxidation ability and superparamagnetic properties. Such composite could be synthesized via simple co-precipitation route to produce high yield and environmentally non-toxic products.
To the best of the authors’ knowledge, according to the articles cited, there is a shortage in the research literature cited on modulating chitosan/magnetite composite material as a source of Fenton-Like reaction. Herein, the present investigation is based on altering the source of Fenton reaction and replace iron system with chitosan/magnetite composite (CS-M) as a source of Fenton oxidation to be one of AOPs. The system is investigated for treating Basic Blue 9 (BB9) as a source of azo dye contaminated wastewater that simulated textile effluents polluted water. The influence of the operating parameters, i.e., catalyst and H2O2 reagents loadings, pH of the aqueous media, dye concentration, and wastewater temperature on the oxidation reaction is assessed.
Experimental section
Synthesis of nanostructures photocatalysts
Magnetic chitosan, chitosan/magnetite nanoparticles composite (CS-M) were synthesized via simple co-precipitation route followed by hydrothermal treatment (Swayampakula et al. 2009). Initially, one wt% of chitosan is dissolved in drops of CH3COOH solution prior it is dissolved in 50 mL of distilled water through magnetic stirring for 15 min. Fe2+ and Fe3+ in 1:2 molar ratio, i.e., FeSO4⋅7H2O (0.829 g) and FeCl3 (1.607 g) each was dissolved in 50 mL distilled water. Thereafter, the prepared three solutions, i.e., chitosan, Fe2+ and Fe3+, were mixed together to achieve a mixture of 2:1 of chitosan/magnetite, while a solution of NaOH is added to maintain the pH to 10. Then, the mixture was subjected for heating for 45 min to 90 °C ± 1 °C. The precipitate was then washed with distilled water until pH 7.0 is reached prior to drying in a vacuum oven at 70 °C, and the attained sample is labeled as CS-M (2:1). Furthermore, to obtain chitosan/magnetite nanoparticles composite with different mass ratios different proportions from the precursors were prepared at the same procedure to attain the samples of CS-M (3:1) and CS-M (1:2).
Wastewater
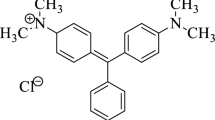
Azo dye namely Basic Blue 9, BB9, is supplied by Sigma-Aldrich and is used as a source of synthetic textile dye wastewater aqueous environment. The dye powder was used as received with no further treatment or purification. BB9 dye is dark blue thiazine dye. Primarily, a stock solution of 1000-ppm is prepared at room temperature, and thereafter, a successive dilution is done to attain the desired dye loadings as required.
Methodology and analytical determination
100 mL of BB9 dye-containing aqueous effluent is poured into a glass vessel to prepare the dye for catalytic oxidation for dye loading elimination. The initial pH of the effluent is adjusted, when needed, to the required values via diluted H2SO4 (1:9) and/or 1 M NaOH solutions using digital pH meter (AD1030, Adwa instrument, Hungary). Afterward, CS-M material is added at a definite amounts and then, the reaction is initiated using hydrogen peroxide (30% 30% w/v). Then, the sample is subjected to UV illumination which irradiated using ultraviolet lamp of 15 W, 230 V/50 Hz, with 253.7 nm wavelength to initiate the oxidation reaction. Consequently, the samples were withdrawn periodically after selected time intervals for spectrophotometric analysis to inspect the remaining concentration after they are filtered, to remove any remaining catalyst, using a micro-filter. The graphical scheme of representation of experimental set-up and reaction steps is displayed in Fig. 1.
Factorial design
Box–Behnken design model of experiments based on Response Surface Methodological analysis (RSM) is used to optimize the optimal operating conditions. The most effective operating factors are optimized using such model. Three factors run by the software SAS version (SAS 1990) was used to locate the optimum values of the selected experimental parameters according to preliminary results of experiments. The selected parameters are H2O2 and CS-M catalyst concentrations and pH value. Then, the experimental responses were attained from checking the process parameters on the oxidation efficiency. Thus, coded and original values of the process variables used in the matrix designed are displayed in Table 1. The full experimental matrix is designed according to SAS software, and the 15-run experiments are designed according to Table 2. Furthermore, to avoid the experimental errors, the experiments were accomplished in random order and all of them were conducted in duplicates. Then, the second-order polynomial quadratic model for the oxidation system is attained according to Eq. (1). Additionally, to assure the model significance, the statistical analysis using analysis of variance (ANOVA) was performed via SAS software and further, Mathematica software (V 5.2) is applied to locate the numerical values of the optimized variable and responses.
Results and discussions
Characterization of the prepared nanomaterials
XRD analysis
X-ray diffraction (XRD) pattern using a Bruker–Nonius Kappa CCD diffractometer equipped with CuKα radiation (λ = 1.5406 Å) with 2θ ranging from 10 to 80° was used to investigate the XRD pattern of chitosan-magnetite composite materials. The data shown in Fig. 2 exposed the main phases of the chitosan and iron oxide (Fe3O4) are attained in the three composite, i.e., CS-M-(2–1), CS-M-(3–1) and CS-M-(1–2). The diffraction peaks of magnetic nanoparticles which can be indexed to 2θ of 18.30, 30.05, 35.49, 43.22, 53.63, 57.06, 62.48 and 74.24° that corresponding to the planes [(111), (220), (311), (400), (422), (511), (440), (533)], respectively, such planes and relative intensities of all diffraction peaks are well corresponding to magnetite. According to the graphs, Fe3O4 phase is of highly crystalline nature. Further, magnetite nanoparticles have been coated by amorphous chitosan, which does not result in phase change of Fe3O4. Also, peak broadening is indicating small size of nanoparticles.
Morphology and particle size of chitosan/magnetite composite
To investigate the morphology of chitosan/magnetite nanoparticles, Transmission Electron Microscope (TEM) analysis has been done (type Tecnai G20, FEI). Figure 3 displays the TEM micrograph of chitosan augmented iron oxide nanoparticles composite materials. The TEM analysis of the products provided information on the size and morphology of the nanoparticles. It can be seen from Fig. 3 that the magnetic particles have a spherical shape with a mean diameter of about 25 nm. The dark dense areas signify the crystalline Fe3O4, while the bright ones surrounding the iron particles are assigned for amorphous chitosan (CS) material. However, some particles are aggregated together due to the iron oxide dipole/dipole attraction force. To add up, the amount of spherical particles of magnetite appeared differs in the three composites Fig. 3A–C according to the ratio of chitosan in the sample.
Furthermore, the particle size distribution was examined using the digital TEM images that were analyzed using the, IMAGEJ 1.48 V program. CS-M composite materials’ images are signified to calculate their particle size distribution in the histogram in the inset of Fig. 3. The particle size distribution of CS-M-(1–2) composite is ranged from 10 to 40 nm, CS-M-(2–1) is in the range of 10–20 nm, and for CS-M-(3–1) is in the range of 10–40 nm. This signifies a reasonable size to offer a high surface area of the CS-M composite materials’ particles to be an efficient photocatalyst for the pollutant elimination through oxidation.
Application of CS-M composite in BB 9 wastewater oxidation
Comparison of various oxidation systems
Effect of reaction time on various oxidation systems namely H2O2/UV, CS-M/UV, photo-Fenton (CS-M/H2O2/UV) and dark-Fenton (CS-M/H2O2) is investigated to test the experimental condition for further research. The efficacy of such techniques was assessed in the terms of BB9 dye color removal, and the data are displayed in Fig. 4A. The results compare the BB9 oxidation using different systems at room temperature and pH 3.0. It is clear from the results that in the solo H2O2 oxidation system results in only 50% removal within 80 min of reaction time and the solo catalyst (CS-M/UV) system attains 7% removal (60 min). But, 82% for the photo-Fenton (CS-M/H2O2/UV) system within 170 min and 21% for the dark-Fenton (CS-M/H2O2) system during 60 min of reaction time are achieved. However, it is noteworthy to mention that after 10 min of illumination time the CS-M/H2O2/UV system achieving a higher initial removal compared to the other systems. So, after 10-min of reaction time, 19% dye removal is attained for CS-M/H2O2/UV system in comparison with only 4.8% and 7.5% for CS-M/UV and H2O2/UV, respectively. However, with time prolonging, the BB9 dye removal slowed down and the efficacy became stabilized. Interestingly, such results confirming the role of Fenton’s treatment are related to the ˙OH radicals generation that associated with the rapid initial stage of oxidation. The oxidation occurred utilizing the activation of H2O2 with Fe2+/3+ salt (Li et al. 2018a). However, with the exceeding of reaction time, a decline in the oxidation rate is attained that corresponding to the reduction in the H2O2 concentration, which is related to the hydroxyl radicals formation. The result is inhibition in the reaction rate rather than increasing the oxidation rate. Although, hydrogen peroxide is consumed after the initial reaction period, a complex reaction occurred and such reaction producing further radicals that is so-called hydroperoxyl radicals (HO2). But, such HO2 radical possesses a low oxidation speciation rather than the hydroxyl radical. Thus, BB9 oxidation rate is reduced with the reaction exceeds. Scattered results (Guan et al. 2005; Li et al. 2018a; Tony 2022a) have displayed the rapid initial reaction time due to the immediate formation of ˙OH radicals. Moreover, the less oxidation tendency of the dark reaction test is related to the absence of the UV illumination, which enhances the oxidation radiation through the production of more ˙OH radicals’ species. It is noteworthy to mention that such catalyst is easily recoverable since it is a magnetic catalyst that possesses the advances of recoverable, recyclable and reusable as a sustainable catalyst.
Figure 4B displays various composite CS-M-(2:1), CS-M-(3:1) and CS-M-(1:2) use in BB9 elimination. The time-profile of BB9 oxidation using various chitosan/magnetite-based Fenton composite mixture was studied at room temperature, and the data displayed in Fig. 4B revealed that the highest oxidation yield is associated with the CS-M-(2:1) system. The majority of the dye is oxidized in the initial 30 min of reaction time 93% using CS-M-(2:1) Fenton system compared to 85 and 88% for CS-M-(1:2) and CS-M-(3:1), respectively. Whereas the final removal rate reached to 95% for CS-M-(2:1) and CS-M-(3:1) Fenton systems and 94% for CS-M-(1:2) Fenton-based system. This could be illustrated by composition ratio of cross-linker that plays a significant role in affecting physical properties and surface morphology of chitosan-Fe3O4 nanoparticles composite materials, thus showing a different behavior in treatment. Also, the composition ratio of chitosan and Fe3O4 influenced the nanoparticles particles characteristic (Clark et al. 2016; Tony and Mansour 2019; Wulandari et al. 2018).
Effect of pollutant loading
According the industrial regard and practical point of view, it is essential to analyze and study different aqueous streams concentrations since it is also varied in the industrial discharge (Tony 2020). The initial BB9 dye load in aqueous effect on the dye elimination efficiency by chitosan/magnetite Fenton oxidation is displayed in Fig. 5. The initial H2O2 concentration was adjusted to 800 mg/L, and the CS-M-(2–1) catalyst loading is 2 mg/L at pH 6.0 and the catalytic oxidation in terms of dye removal efficiency undertaken for the dye ranging from 5 to 40 mg/L. Figure 5 illustrates the effect of reaction time on the profile of various BB9 dye concentrations. Whereas, the initial period of reaction is signified with the same rate of rapid oxidation for all BB9 dye concentrations treated. However, examination of Fig. 5 illustrates that the oxidation rate declined with increasing BB9 dye concentration. The removal efficiencies are 99, 99, 90 and 43% for 5, 10, 20 and 40 ppm BB9 dye loading in the aqueous stream, respectively. Moreover, more irradiance time is required to attain such dye elimination with increasing the dye loads, and the reaction time is increased from 110 to 160 min. This could be attributed by at higher BB9 loadings, and the concentration of ˙OH radicals generated is insufficient since the same amounts of reagents are used for reaction whereas the dye loading is increased. Thus, the result is incomplete BB9 dye oxidation. This finding of increasing the photo-oxidation activity with decreasing the initial load has also previously been observed by Najjar et al. (2001) who applied such Fenton’s reagent for the oxidation of nitrophenol and Tony et al. (2015) in oxidizing oil from wastewater.
Effect of chitosan/magnetite-based Fenton reaction parameters
Effect of chitosan/magnetite dosing
˙OH radical speciation that is well known as the strong oxidative radical is mainly the main responsible of oxidizing pollutant in the Fenton’s reaction, while such radicals’ generation is mainly dependent on various Fenton’s parameters. According to the previous investigations of scattered authors (Hilder et al. 2012; Muthukannan et al. 2015; Saad et al. 2021; Tony 2021a; Tony and Lin 2020a), Fenton’s reaction yield is highly determinate according to the reagent doses and the medium pH. Hence, studying such parameters is crucial.
Metal conversion in the Fenton’s reaction is critically vital for the oxidation system. Therefore, in order to confirm the job of the chitosan and magnetite catalysts in that such CS-M-(2–1) composite in such modified Fenton’s reaction, various doses of CS-M composite ranged from (1 to 40 mg/L) as a catalyst source were checked to investigate its role in the ˙OH radical production. The reaction is conducted, while keeping all other system parameters constant (pH 3.0 and H2O2 400 mg/L). As depicted in Fig. 6A, BB9 removal is enhanced to reach to 95% with elevating CS-M-(2–1) dose concentration up to 2 g/L and then, the BB9 dye elimination declined with further catalyst elevation. This could be illustrated by the hydroxyl radicals that are trapped by CS-M catalyst ions in excess (Tony and Mansour 2019). Interestingly, high doses of CS-M catalyst result in adverse effects of excess magnetite and chitosan doses on the solution as the excess catalyst specially iron species in the aqueous media decrees the reactive species of ˙OH radicals’ performance as given in Eq. (2, 3). Therefore, 2 mg/L is the signified as the catalyst concentration required for BB9 dye oxidation. According to the data cited by Cetinkaya et al. (2018), similar trend was recorded in treating wastewater using such Fenton technique.
Previous investigators recorded that variation of the hydrogen peroxide doses might affect significantly on the radicals’ generation and thus on the Fenton’s oxidation yield. From this regard, H2O2 dosage varied from100 to 1000 mg/L was selected to examine the effect of such reagent on the current studied system (at 10 mg/L of CS-M-(2–1) catalyst and pH of 3.0) and the results are displayed in Fig. 6B. The data in the Fig. 6B revealed that the BB9 oxidation is enhanced from 51 to 93% with the increase in hydrogen peroxide reagent from 100 to 800 mg/L, respectively. Although, the further increase in such reagent is resulting in a decline in oxidation system. Such data are in accordance with phenomena of the high concentration of hydrogen peroxide is predictable to attain a high yield of ˙OH radicals. Thus, an increment in BB9 oxidation is expected. However, in contrary the excess hydrogen peroxide reagent exploits as the ˙OH radicals’ hamper rather than a generator. The result in such case of excess H2O2 rather than the optimal, HO2 radical is produced, which is less active than the highly horsepower ˙OH reactive free species. This results in a negligible contribution in the oxidation reaction. Moreover, HO2 radicals react with the remaining ˙OH radicals (according to Eq. (5)) that result in further hindering the reaction rate (Amiri et al. 2017).
Not only hydrogen peroxide and CS-M doses affect the Fenton’s system, but also initial aqueous solution pH. Traditionally, Fenton reaction is signified as a strong dependent pH system. To investigate the optimal system pH, the initial pH of the aqueous medium is evaluated by changing the wastewater pH from 2.0 to 6.0 using the same doses of CS-M-(2–1) of 2 mg/L and H2O2 800 mg/L. Examination of the data displayed in Fig. 6C revealed that at the initial time period decreasing the pH to the acidic range from the original wastewater’s pH (6.0) consequences to higher BB9 dye elimination. However, with the prolonging time of irradiance, the higher reaction yield is attained to the aqueous media of pH 6.0 (reached to 99% within almost 2 h of reaction time). Although several authors recommended the acidic pH for Fenton’s oxidation, the current new system CS-M/H2O2/UV could work at various pH range with high yield reached to almost complete dye oxidation. However, it is noteworthy to mention that using pH in acidic range (2.0) shortening the reaction time. This could be illustrated by when pH dropped beyond 3.0, H3O2+ might be formed; hence, this leads to an enhancement and improvement stability of hydrogen peroxide, whereas the hydroxyl radicals had a strong scavenging effect by H+. In contrary, when the pH exceeded 3.0, iron is hydrolyzed and the hydroxyl radical is formed meanwhile FeOOH is precipitated from the solution. Thus, further experiments are conducted at more than acidic pH (El-Desoky et al. 2010).
RSM optimization strategy
From the preliminarily investigation data of single-variable tests, the selected most intensive variables effect on the Fenton system, namely, H2O2 concentration, CS-M dose and pH. Their levels were selected and determined. Then, the design of the response values was established. The optimization goal is to maximize BB9 removal efficiency as response values using the constraints explored in Table 1. Box–Behnken design optimization tool was applied for optimization, and the total 15 groups experimental design is tabulated in Table 2. Subsequently, after the experiments were conducted, the detailed test scheme is concluded in Table 2, which reveals the results of the corresponding responses. Then, polynomial model coefficients were processed by the regression equation in terms of coded variables for BB9 oxidation efficacy as stated as the quadratic model Eq. (6).
Figure 7A displays the plot that comparing the data of the predicted response attained via the regression model and experimental data accessed experimentally. The regression coefficient value of the residual showed the experimental data that well presented the quadratic model applied. The plot exposes just few deviations from normal representing well fitness of the subjected model.
Analysis of variance, ANOVA test for BB9 response, is commonly crucial applied to check the model adequacy. In order to investigate, the applied model fitness, ANOVA, is one of the primary numerical techniques to verify the model. ANOVA test is investigated, and the data are listed in Table 3. A t test is used to examine all the model terms, specifically called sum of squares (SS), mean squares (MS), estimated coefficient, standard error, and the corresponding F value and the portability (p values). In such study, the experimental results were suited to polynomial equation. F tests value is applied to check the suitability of the applied model. Also, lack of fit designates the data variation over the suggested model (Adesina et al. 2019; Setyono and Valiyaveettil 2014; Tony 2021a; Yin et al. 2020). Hence, when the model exhibitions lack of fit, it might not be used to evaluate the model response. As given in Table 3, the lack of fit leading to statistical implication as a large value of F value is recorded. F value is much larger than unity, besides a small probability (p value < 0.05) is attained for the regression model. Also, ANOVA revealed a well correlation between the proposed response and the operating parameters. Moreover, R2 (the coefficient of determination) is used to validate the fitness of the model that verifies the model is accepted if it is larger than (80%). The coefficient of determination of the fitted model is recorded 99%.
To evaluate the effects of the parameters and locate the optimal operating variables of BB9 oxidation, the numerical optimization technology provided by Mathematica software (V 5.2) is used. The optimization goal is to maximize the BB9 oxidation rate. The optimal operating condition predicted at 30 min of irradiance time and the point at which the final BB9 oxidation is projected to be in its maximum value that reached to 89% after 30 min. The optimum reagent doses are examined at 767 mg/L and 2.4 mg/L for H2O2 and CS-M, respectively, and the pH is verified at 7.0. Therefore, in order to validate the quadratic model, experiments were conducted in replicate at the optimal data and the experimental responses were compared to the predicted one. The experimental data revealed a reasonable (65.5%) good settlement to the predicted model value. The result reveals a reasonable (90% after 30 min) good agreement to the predicted value. Also, almost complete BB9 oxidation (99%) is attained at the optimum operating value within 120 min of reaction time. Thus, the determined optimum point through the current proposed empirical model is efficiently verified the maximum dye removal via Box–Behnken design.
Following the model validation, further clarification of the three independent parameters was investigated through 3D surface and 2D contour plots to investigate the correlation between such parameters. The response surface analysis might explore an indication to the type of interactions between the selected variables As displayed in Fig. 7(B–D), the flexure nature of the surface plot explains the interaction between the parameters and response (Tony 2022b). As given in Fig. 7(B, C), BB9 oxidation efficiency is steadily enhanced by the addition of CS-M nanoparticles and H2O2 reagent. This might be attributed by the formation of hydroxyl radicals in the wastewater media that is related to the reagent doses (see Eq 7–9). This is true till a certain limit the oxidation efficiency is lessened. Such decline is due to the overdosing rate that might act as hydroxyl free radicals’ hinder rather than a generator (Eq. 10). Additionally, as seen in Fig. 7(C and D), BB9 oxidation efficiency is extremely more sensitive to pH change in comparison with the other investigated parameters.
Thermal effects on kinetics and thermodynamic profile
As previously stated in the literature (Abdou et al. 2018; Oyewo et al. 2020; Rezgui et al. 2021), there is an optimum temperature for conducting the Fenton’s reaction. From this regard, temperature effect on BB9 oxidation reaction on Fenton based CS-M reaction was conducted at four different temperatures, i.e., 26, 40, 50 and 60 °C and the data are displayed in Fig. 8. The purpose of investigating its effects on the oxidation reaction is to determine the overall kinetics and to allow the examination of the Arrhenius-type dependence of the global kinetic constant on temperature. As seen in Fig. 8, temperature increment had a negative impact on BB9 oxidation rate. Also, it is noted that a shorter oxidation reaction period is required, needed for the BB9 oxidation at low temperature (room temperature). The temperature elevation showed a markedly decline in the BB9 dye oxidation with a subsequently decrease in the reaction yield. This could be due to the significant decomposition of H2O2 reagent into O2 and H2O. Such rapid H2O2 at high temperatures exceeds than 26 °C results in a lessening in the overall reaction efficacy (Ashour and Tony 2017; Guedes et al. 2003; Soliman et al. 2020; Tony and Lin 2020c).
From the theoretical and practical point of view, it is crucial to investigate large-scale treatment capacity. Thus, understanding the oxidation kinetics for eliminating BB9 contaminating wastewater is an essential. Kinetic statistics provides estimation for: the optimal system parameter; reactor design; actual process control and a jointly examination for economic study of capital and operating budget (Al et al. 2008; Ashour et al. 2014; Tony 2021c). Table 4 presented the assessed zero-, first- and second-order kinetic models and their corresponding determination of the kinetic constants and parameters.
In light of R2 values (regression coefficient), the highest was chosen to select their corresponding model to be the most adequate one. By examining the data in Table 4, second-order reaction kinetics model was chosen to be the appropriate model that represents the data. k2, the second-order model rate constants are elevated from 0.0157 to 0.0028 L/mg.min with temperature increase. Further, t1/2, the half-life time increases with the temperature elevation. The lowest t1/2 for the BB9 oxidation is estimated at the temperature 26 °C. Hence, the second-order model proposed the oxidation yield is precise by the reaction temperature up to 26 °C. Scattered authors previously reported the Fenton’s reaction is controlled by the second-order model kinetics (Ahmadi et al. 2016; Al et al. 2008; Ashour et al. 2014; Tony et al. 2016).
To further understanding the oxidation of CS-M-based Fenton system, thermodynamic parameters were tested and the data are tabulated in Table 5. Based on the second-order kinetic rate constant, Arrhenius principle Eq. (11) might be written in the linearized form taking the natural logarithm yielding Eq. (12):
where A is the constant corresponding to pre-exponential factor; Ea is the energy of activation (kJ/mol); R is the gas constant (8.314 J/mol.K) and T is temperature (K). Further, the linear fit of lnk2 and − 1/T (Fig. 9) could leading to the calculation of energy of activation. Consequently, the energy of activation (Ea) for the system is 43.49 kJ/mol. Such minimal energy of activation significance confirms that the BB9 oxidation conducted at low energy barrier through CS−M-based Fenton system. Previous investigators Ahmadi et al. (2016) and Sun et al. (2007) recorded low energy barrier (53.96 and 45.84 kJ/mol, respectively) in treating dyes and p-nitroaniline from wastewater using such Fenton reaction.
Eyring’s relation (Bounab et al. 2015) (Eq. (13)), Gibbs free energy of activation (∆G`) was calculated to estimate the viability of the oxidation.
where kB and h are constants of Boltzmann and Planck’s. Also, the enthalpy of activation (∆H`) (Eq. (14)) and the entropy of activation (∆S`) (Eq. 15)) were estimated (Wahab and Hussain, 2016).
As exhibited in Table 5, ∆G` (Gibbs free energy) showed positive values and its values increased with the temperature elevation and the minimum Gibbs free energy value is corresponding to the temperature of 26 °C, this reveals that the reaction is non-spontaneous in nature. Besides, ∆H` values (enthalpy of activation) that is attained also positive values indicating the reaction is endothermic. But, ∆S` (entropy of activation) is negative sign that is confirming the non-spontaneity nature of such oxidation system. This illustrates a decline in the degree of freedom of the BB9 molecules and supported a high hydroxyl radicals that maximizing the oxidation yield. Such results in accordance with previously stated in the literature (Argun and Karatas 2011; Tony 2022c).
It is essential to compare the current study with the other investigated articles cited in the literature to demonstrate the importance of the current composite material application. Table 6 displays a comparison of various combinations composite materials in the last decades for the elimination of different types of wastewater polluted effluents. The Table are comparing Fenton-based catalyst which compromises of iron composed with other materials or Fenton-Like-based system such as Cu-based system. Tony and Mansour (2020a) applied Cu/Cu2O/CuO as a Fenton-Like active system, whereas Thabet et al. (2021b), Wang et al. (2019), Thabet et al. (2021a) and Guan et al. (2020) applied magnetite-based composite as a source of Fenton’s catalyst which possess the merit of recoverable, recyclable and the principle of reusable catalyst. Furthermore, Tony (2022a) and Thabet et al. (2022c, d) applied alum sludge waste as a source of catalyst combined with other materials to be a source of Fenton’s reaction. Generally, the use of waste materials is superior due to their advantages for industrial ecology aspects. Such regard appeals the contribution of reducing cost disposal and sustains the environment. From this regard, the current study satisfies such view since CS-M catalyst compromising of chitosan which is attained from waste materials and magnetite that is a recyclable catalyst. It is noteworthy to mention that almost a complete pollutant elimination is attained through the current investigation. Moreover, the catalyst used is a minimum amount compared to the other amounts of catalysts used. Thus, such advantages make the current system more environmentally green and economically efficient compared to other systems.
Conclusion
The co-precipitation of magnetite with chitosan is a promising treatment method when augmented with H2O2 as a Fenton method for the elimination of BB9 dye from aqueous stream. Crystalline magnetite nanoparticles embedded in the chitosan polymer showed a superior oxidation efficiency. The oxidation parameters demonstrated that the CS-M dosing and H2O2 concentration limiting the oxidation reaction. However, the reaction might work in a wide pH values that introduces the advantage of such system. The photo-catalytic oxidation efficiency increased with lessening the initial dye concentration in aqueous effluent. Further, elevating the temperature from ambient temperature declines the pollutant oxidation. The kinetic data showed the reaction following the second-order kinetics with global activation energy of 43.49 kJ/mol. Hence, it might be signified that the system is a promising technology for treating aqueous effluent in a green efficient way.
References
Abdou KA, Mohammed AN, Moselhy W, Farghali AA (2018) Assessment of modified rice husk and sawdust as bio-adsorbent for heavy metals removal using nano particles in fish farm. Asian J Anim Vet Adv 13:180–188
Adesina OA, Abdulkareem F, Yusuff AS, Lala M, Okewale A (2019) Response surface methodology approach to optimization of process parameter for coagulation process of surface water using Moringa oleifera seed. S Afr J Chem Eng 28:46–51
Ahmadi M, Behin J, Mahnam AR (2016) Kinetics and thermodynamics of peroxydisulfate oxidation of reactive yellow 84. J Saudi Chem Soc 20:644–650
Al MF, Mo’ayyad S, Ahmad S, Mohammad A-S (2008) Impact of Fenton and ozone on oxidation of wastewater containing nitroaromatic compounds. J Environ Sci 20:675–682
Amiri SA, Mohseni Bandpei MA, Javanshir K, Rezasoltani A, Biglarian A (2017) The effect of different exercise programs on size and function of deep cervical flexor muscles in patients with chronic nonspecific neck pain. Am J Phys Med Rehabil 96(8):582–588. https://doi.org/10.1097/PHM.0000000000000721
Argun ME, Karatas M (2011) Application of Fenton process for decolorization of reactive black 5 from synthetic wastewater: kinetics and thermodynamics. Environ Prog Sustain Energy 30:540–548
Ashour EA, Tony MA (2017) Equilibrium and kinetic studies on biosorption of iron (II) and iron (III) Ions onto eggshell powder from aqueous solution. Appl Eng 1:65–73
Ashour EA, Tony MA (2020) Eco-friendly removal of hexavalent chromium from aqueous solution using natural clay mineral: activation and modification effects. SN Appl Sci 2:1–13
Ashour A, Tony MA, Purcell PJ (2014) Use of agriculture-based waste for basic dye sorption from aqueous solution: kinetics and isotherm studies. Am J Chem Eng 2:92–98
Bounab L, Iglesias O, González-Romero E, Pazos M, Sanromán MÁ (2015) Effective heterogeneous electro-Fenton process of m-cresol with iron loaded actived carbon. RSC Adv 5:31049–31056
Cetinkaya S, Morcali M, Akarsu S, Ziba C, Dolaz M (2018) Comparison of classic Fenton with ultrasound Fenton processes on industrial textile wastewater. Sustain Environ Res 28(4):165–170
Clark JH, Farmer TJ, Herrero-Davila L, Sherwood J (2016) Circular economy design considerations for research and process development in the chemical sciences. Green Chem 18:3914–3934
Di L, Yang H, Xian T, Liu X, Chen X (2019) Photocatalytic and photo-Fenton catalytic degradation activities of Z-scheme Ag2S/BiFeO3 heterojunction composites under visible-light irradiation. Nanomaterials 9:399
El-Desoky HS, Ghoneim MM, El-Sheikh R, Zidan NM (2010) Oxidation of levafix CA reactive azo-dyes in industrial wastewater of textile dyeing by electro-generated Fenton’s reagent. J Hazard Mater 175:858–865
Golka K, Kopps S, Myslak ZW (2004) Carcinogenicity of azo colorants: influence of solubility and bioavailability. Toxicol Lett 151:203–210
Guan X-H, Chen G-H, Shang C (2005) Re-use of water treatment works sludge to enhance particulate pollutant removal from sewage. Water Res 39:3433–3440
Guan S, Yang H, Sun X, Xian T (2020) Preparation and promising application of novel LaFeO3/BiOBr heterojunction photocatalysts for photocatalytic and photo-Fenton removal of dyes. Opt Mater 100:109644
Guedes AMFM, Madeira LMP, Boaventura RAR, Costa CAV (2003) Fenton oxidation of cork cooking wastewater—overall kinetic analysis. Water Res 37:3061–3069
Hilder M, Winther-Jensen O, Winther-Jensen B, MacFarlane DR (2012) Graphene/zinc nano-composites by electrochemical co-deposition. Phys Chem Chem Phys 14:14034–14040
Ioannou LA, Fatta-Kassinos D (2013) Solar photo-Fenton oxidation against the bioresistant fractions of winery wastewater. J Environ Chem Eng 1:703–712
Laib S, Rezzaz-Yazid H, Yatmaz HC, Sadaoui Z (2021) Low cost effective heterogeneous photo-Fenton catalyst from drinking water treatment residuals for reactive blue 19 degradation: preparation and characterization. Water Environ Res 93:1097–1106
Li X, Cui J, Pei Y (2018a) Granulation of drinking water treatment residuals as applicable media for phosphorus removal. J Environ Manag 213:36–46
Li Y, Jiang J, Fang Y, Cao Z, Chen D, Li N, Xu Q, Lu J (2018b) TiO2 nanoparticles anchored onto the metal–organic framework NH2-MIL-88B (Fe) as an adsorptive photocatalyst with enhanced Fenton-like degradation of organic pollutants under visible light irradiation. ACS Sustain Chem Eng 6:16186–16197
Muthukannan V, Praveen K, Natesan B (2015) Fabrication and characterization of magnetite/reduced graphene oxide composite incurred from iron ore tailings for high performance application. Mater Chem Phys 162:400–407
Najjar W, Chirchi L, Santos E, Ghorhel A (2001) Kinetic study of 2-nitrophenol photodegradation on Al-pillared montmorillonite doped with copper. J Environ Monit 3:697–701
Oyewo OA, Adeniyi A, Sithole BB, Onyango MS (2020) Sawdust-based cellulose nanocrystals incorporated with ZnO nanoparticles as efficient adsorption media in the removal of methylene blue dye. ACS Omega 5:18798–18807
Pourali P, Behzad M, Arfaeinia H, Ahmadfazeli A, Afshin S, Poureshgh Y, Rashtbari Y (2021) Removal of acid blue 113 from aqueous solutions using low-cost adsorbent: adsorption isotherms, thermodynamics, kinetics and regeneration studies. Sep Sci Technol 56:3079–3091
Rezgui S, Díez AM, Monser L, Adhoum N, Pazos M, Sanromán MA (2021) ZnFe2O4-chitosan magnetic beads for the removal of chlordimeform by photo-Fenton process under UVC irradiation. J Environ Manag 283:111987
Saad RA, Younes G, El-Dakdouki MH, Al-Oweini R (2021) Vanadium-substituted polyoxomolybdates for methylene blue adsorption from aqueous solutions. J Cluster Sci 33:2077–2083
SAS (1990) SAS/STAT user’s guide. SAS Institute Inc, Cary, NC
Setyono D, Valiyaveettil S (2014) Chemically modified sawdust as renewable adsorbent for arsenic removal from water. ACS Sustain Chem Eng 2:2722–2729
Shaheen TI, Emam HE (2018) Sono-chemical synthesis of cellulose nanocrystals from wood sawdust using acid hydrolysis. Int J Biol Macromol 107:1599–1606
Soliman EM, Ahmed SA, Fadl AA (2020) Adsorptive removal of oil spill from sea water surface using magnetic wood sawdust as a novel nano-composite synthesized via microwave approach. J Environ Health Sci Eng 18:79–90
Sun J-H, Sun S-P, Fan M-H, Guo H-Q, Qiao L-P, Sun R-X (2007) A kinetic study on the degradation of p-nitroaniline by Fenton oxidation process. J Hazard Mater 148:172–177
Swayampakula K, Boddu S, Nadavala S, Abburi K (2009) Competitive adsorption of Cu (II), Co (II) and Ni (II) from their binary and tertiary aqueous solutions using chitosan-coated perlite beads as biosorben. J Hazard Mater 170(2–3):680–689
Tahoun BA, Mansour S, Tony MA (2022) Development and characterization of conjugated polyaniline/co-doped ZnO nanocomposites for enhanced dye oxidation from wastewater. ERJ Eng Res J 45:101–110
Thabet RH, Tony MA, El Sherbiny SA, Ali IA, Fouad MK (2020) Catalytic oxidation over nanostructured heterogeneous process as an effective tool for environmental remediation. IOP Conf Ser 975:012004
Thabet RH, Fouad MK, Ali IA, El Sherbiney SA, Tony MA (2021a) Magnetite-based nanoparticles as an efficient hybrid heterogeneous adsorption/oxidation process for reactive textile dye removal from wastewater matrix. Int J Environ Anal Chem. https://doi.org/10.1080/03067319.2021.1896716
Thabet RH, Fouad MK, Ali IA, El Sherbiny SA, Tony MA (2021b) Synthesis, characterization and potential application of magnetized nanoparticles for photocatalysis of levafix CA reactive azo-dye in aqueous effluent. Water Environ J 36:245–260
Thabet RH, Fouad MK, El Sherbiney SA, Tony MA (2022a) Solar assisted green photocatalysis for deducing carbamate insecticide from agriculture stream into water reclaiming opportunity. Int J Environ Anal Chem. https://doi.org/10.1080/03067319.2022.2027930
Thabet RH, Fouad MK, Sherbiny SAE, Tony MA (2022b) Zero-waste approach: assessment of aluminum-based waste as a photocatalyst for industrial wastewater treatment ecology. Int J Environ Res 16:1–19
Thabet RH, Tony MA, El Sherbiny SA, Ali IA, Fouad MK (2022c) A circular economy use of waste drinking water treatment plant sludge for magnetic photocatalyst composite production into wastewater treatment. Int J Environ Anal Chem. https://doi.org/10.1080/03067319.2022.2097009
Thabet RH, Tony MA, ElSherbiny SA, Ali IA, Fouad MK (2022d) Construction of a hetero-junction recyclable composite photocatalyst from aluminum-based waste/magnetite for efficient carbamate insecticide oxidation. Environ Sci 8:1874–1894
Tony MA (2020) Central composite design optimization of bismarck dye oxidation from textile effluent with Fenton’s reagent. Appl Water Sci 10:1–9
Tony MA (2021a) Nexus approach: ZSM-12 derived from industrial waste into microencapsulated in organic wax for solar energy storage system. Energy Sour Part A. https://doi.org/10.1080/15567036.2021.2001119
Tony MA (2021b) Recent frontiers in solar energy storage via nanoparticles enhanced phase change materials: succinct review on basics, applications and their environmental aspects. Energy Storage 3:e238
Tony MA (2021c) An industrial ecology approach: green cellulose-based bio-adsorbent from sugar industry residue for treating textile industry wastewater effluent. Int J Environ Anal Chem 101:167–183
Tony MA (2022a) Pattern, forms and bibliometric analysis for systematic study of silica-supported heterogeneous solar photocatalyst for lannate insecticide abatement from aqueous stream. Arabian J Sci Eng. https://doi.org/10.1007/s13369-022-06853-y
Tony MA (2022b) Valorization of undervalued aluminum-based waterworks sludge waste for the science of “The 5 Rs’ criteria”. Appl Water Sci 12:1–30
Tony MA (2022c) Aquananotechnology: oriented-sawdust waste valorization into magnetic nanocellulosic particles for synozol red K-HL sorption prospect. Appl Water. https://doi.org/10.1007/s13201-022-01725-0AWSC-D-22-00108R1
Tony MA, Eltabey MM (2022) End-of-life waste criteria: synthesis and utilization of Mn–Zn ferrite nanoparticles as a superparamagnetic photocatalyst for synergistic wastewater remediation. Appl Water Sci 12:1–17
Tony MA, Lin L-S (2020a) Iron recovery from acid mine drainage sludge as Fenton source for municipal wastewater treatment. Int J Environ Anal Chem 102:1245–1260
Tony MA, Lin LS (2020b) Attenuation of organics contamination in polymers processing effluent using iron-based sludge: process optimization and oxidation mechanism. Environ Technol 43:718–727
Tony MA, Lin LS (2020c) Performance of acid mine drainage sludge as an innovative catalytic oxidation source for treating vehicle-washing wastewater. J Dispers Sci Technol 43:50–60
Tony MA, Lin L-S (2021) Iron coated-sand from acid mine drainage waste for being a catalytic oxidant towards municipal wastewater remediation. Int J Environ Res 15:191–201
Tony MA, Mansour SA (2019) Removal of the commercial reactive dye procion blue MX-7RX from real textile wastewater using the synthesized Fe2O3 nanoparticles at different particle sizes as a source of Fenton’s reagent. Nanoscale Adv 1:1362–1371
Tony MA, Mansour SA (2020a) Microwave-assisted catalytic oxidation of methomyl pesticide by Cu/Cu 2 O/CuO hybrid nanoparticles as a Fenton-like source. Int J Environ Sci Technol 17:161–174
Tony MA, Mansour SA (2020b) Solar photo- Fenton reagent with nanostructured iron oxide for Bismarck dye oxidation: an Egyptian apparel case study. Int J Environ Sci Technol 17:1337–1350
Tony MA, Purcell PJ, Zhao Y, Tayeb AM, El-Sherbiny MF (2015) Kinetic modeling of diesel oil wastewater degradation using photo-Fenton process. Environ Eng Manag J (EEMJ) 14:11–16
Tony MA, Tayeb A, Zhao YQ (2016) An alternative arrangement for the alum sludge management: minimising waste with low-cost solar techniques. Am J Chem Eng 4:30–37
Wahab HS, Hussain AA (2016) Photocatalytic oxidation of phenol red onto nanocrystalline TiO2 particles. J Nanostruct Chem 6(3):261–274
Wang L, Wang W, Liu M, Ge H, Zha W, Wei Y, Fei E, Zhang Z, Long J, Sa R (2019) Understanding structure-function relationships in HZSM-5 zeolite catalysts for photocatalytic oxidation of isopropyl alcohol. J Catal 377:322–331
Wang S, Long J, Jiang T, Shao L, Li D, Xie X, Xu F (2021) Magnetic Fe3O4/CeO2/g-C3N4 composites with a visible-light response as a high efficiency Fenton photocatalyst to synergistically degrade tetracycline. Sep Purif Technol 278:119609
Wulandari IO, Mardila VT, Santjojo DJDH, Sabarudin A (2018) Preparation and characterization of chitosan-coated Fe3O4 nanoparticles using ex-situ co-precipitation method and tripolyphosphate/sulphate as dual crosslinkers. IOP Conf Ser 299:012064
Xu Z, Zhang M, Wu J, Liang J, Zhou L, Lǚ B (2013) Visible light-degradation of azo dye methyl orange using TiO2/β-FeOOH as a heterogeneous photo-Fenton-like catalyst. Water Sci Technol 68:2178–2185
Yang Y, Ma C, He X, Li J, Li M, Wang J (2021) Calcined aluminum sludge as a heterogeneous Fenton-like catalyst for methylene blue degradation by three-dimensional electrochemical system. Electrocatalysis 12:698–714
Yin Z, Li Y, Song T, Bao M, Li Y, Lu J, Li Y (2020) Preparation of superhydrophobic magnetic sawdust for effective oil/water separation. J Clean Prod 253:120058
Zhang Y, Sivakumar M, Yang S, Enever K, Ramezanianpour M (2018) Application of solar energy in water treatment processes: a review. Desalination 428:116–145
Zubir NA, Yacou C, Motuzas J, Zhang X, Diniz da Costa JC (2014) Structural and functional investigation of graphene oxide–Fe3O4 nanocomposites for the heterogeneous Fenton-like reaction. Sci Rep 4:1–8
Funding
Open access funding provided by The Science, Technology & Innovation Funding Authority (STDF) in cooperation with The Egyptian Knowledge Bank (EKB). The authors confirm that there is no funding applied through this work. The author(s) received no specific funding for this work.
Author information
Authors and Affiliations
Contributions
All authors designed and performed the experiments, analysis and calculations, helped shape the research and final manuscript representation.
Corresponding author
Ethics declarations
Conflict of interest
The authors confirm that there is no conflict of interest to declare.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Elsayed, S.A., El-Sayed, I.E.T. & Tony, M.A. Impregnated chitin biopolymer with magnetic nanoparticles to immobilize dye from aqueous media as a simple, rapid and efficient composite photocatalyst. Appl Water Sci 12, 252 (2022). https://doi.org/10.1007/s13201-022-01776-3
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s13201-022-01776-3