Abstract
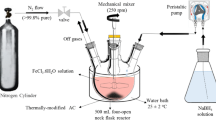
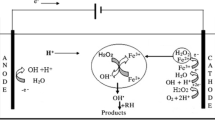
The accessibility of clean water and green environment are the major requirements for survival and sustainable development. In this study, iron-coated sand, derived from acid mine drainage effluent, has been applied as a heterogeneous catalyst. Treatment with H2O2, iron-coated sand, and iron-coated sand-Fenton processes is compared for the COD removals from municipal wastewater effluent. The results showed that the iron-coated sand catalyzed Fenton process could generate hydroxyl radicals (•OH) and oxidize the organic pollutants. Fenton process based on the iron-coated sand catalyst proved to be the most efficient process. The effect of operating conditions such as initial pH and Fenton’s reagent doses, i.e. initial H2O2 and iron on the organics oxidation from such wastewater was investigated. The results showed that 70% removal efficiency of COD was obtained within 30 min under optimized conditions (pH 3.0, H2O2 400 mg/L and iron 40 mg/L). The rate equation of iron-coated sand-Fenton system was simply expressed by the second-order equation and the model was found to fit well the data. Thermodynamic analysis of the results indicated that the iron-coated sand-Fenton oxidation is non-spontaneous and endothermic in nature. Regeneration of iron-coated sand was attempted and the catalyst had a good stability and reusability for successive treatments and reducing the quantity of sludge produced in Fenton reactions. Thus, expanding the sustainability scope of iron-coated sand based Fenton catalyst and offer new sustainable and inexpensive alternatives for the classical Fenton process.
Article Highlights
-
Iron recovered from acid mine drainage to prepare iron coated-sand as a Fenton source.
-
Novel Fenton reaction is proposed for treating polymer industry wastewater.
-
The system avoids iron sludge by-product by providing catalyst reusability.
-
The approach points the competitive novel iron waste source to applied as a Fenton technology.







Similar content being viewed by others
References
APHA (2005), Standard Methods for the Examination of Water & Wastewater (Port City Press, Baltimore, MD, 2005). American Water Works Association; Water Environment Federation
Ashour EA, Tony MA, Purcell PJ (2014) Use of agriculture-based waste for basic dye sorption from aqueous solution: kinetics and isotherm studies. Am J Chem Eng 2(6):92–98
Ashour A, Tony MA (2017) Equilibrium and kinetic studies on biosorption of iron (II) and iron (III) ions onto eggshell powder from aqueous solution. Appl Eng 1(3):65–73
Ashour A, Tony MA (2020) Eco-friendly removal of hexavalent chromium from aqueous solution using natural clay mineral: activation and modification effects. SN Appl Sci 2:2042. https://doi.org/10.1007/s42452-020-03873-x
Anabela MFM, Guedes AM, Madeira LM, Boaventura RA, Costa CA (2003) Fenton oxidation of cork cooking wastewater—overall kinetic analysis. Water Res 37:3061–3069
Argun ME, Karatas E (2011) Application of Fenton process for decolorization of reactive black 5 from synthetic wastewater: kinetics and thermodynamics. Environ Prog Sus Ener 30(4):540–548
Bradu C, Frunza L, Mihalche N, Avramescu S, Neata M, Udrea I (2010) Removal of reactive black 5 azo dye from aqueous solutions by catalytic oxidation using CuO/Al2O3 and NiO/Al2O3. Appl Catal B 96(3–4):548–556
Bwapwa JK, Jaiyeola AT, Chetty R (2017) Bioremediation of acid mine drainage using algae strains: a review. S Afr J Chem Eng 24:62–70
Cantinh P, Matos M, Trancoso MA, Correia dos Santo MM (2016) Behaviour and fate of metals in urban wastewater treatment plants: a review. Int J Environ Sci Technol 13:359–386
Deng D, Lin O, Rubenstein A, Weidhaas JL, Lin L-S (2019) Elucidating biochemical transformations of Fe and S in an innovative Fe(II)-dosed anaerobic wastewater treatment process using spectroscopic and phylogenetic analyses. Chem Eng J 358:1208–1217
Devi RR, Umlong IM, Das B, Borah K, Thakur AJ, Raul PK, Banerjee S, Singh L (2014) Removal of iron and arsenic (III) from drinking water using iron oxide-coated sand and limestone. Appl Water Sci 4:175–182
Divyapriya G, Nidheesh PV (2020) Importance of graphene in the electro-Fenton process. ACS Omega 5(10):4725–4732
Dutta B, Jana S, Bhattacharjee A, Gutlich P, Iijima S, Koner S (2010) γ-Fe2O3 nanoparticle in NaY-zeolite matrix: preparation, characterization and heterogeneous catalytic epoxidation of olefins. Inorg Chim Acta 363:696–704
Goto JJ, Gralla E, Valentine JS (1998) Reactions of hydrogen peroxide with familial amyotrophic lateral sclerosis mutant human copper-zinc superoxide dismutases studied by pulse radiolysis. J Bio Chem 273(46):30104–30109
He F, Lei L (2004) Degradation kinetics and mechanisms of phenol in photo-Fenton process. J Zhejiang Univ 5(2):198–205
House DA (1962) Kinetics and mechanism of oxidations by peroxydisulfate. Chem Rev 62:185–203
Huang K-C, Couttenye RA, Hoag GE (2002) Kinetics of heat assisted persulfate oxidation of methyl tert-butyl ether (MTBE). Chemosphere 49:413–420
Huling S, Arnold R, Sierka R, Jones P, Fine D (2000) Contaminant adsorption and oxidation via Fenton reaction. J Environ Eng 126(7):595–600
Janik I, Bartels DM, Jonah CD (2007) Hydroxyl radical self-recombination reaction and absorption spectrum in water up to 350 °C. J Phys Chem A 111(10):1835–1843
Klamerth N, Malato AS, Aguuera A, Fernandez-Alba A, Mailhot G (2012) Treatment of municipal wastewater treatment plant effluents with modified photo-fenton as a tertiary treatment for the degradation of micro pollutants and disinfection. Environ Sci Technol 46(5):2885–2892
Lin SH, Lin CM, Leu HG (1999) Operating characteristics and kinetic studies of surfactant wastewater treatment by Fenton oxidation. Water Res 33(7):1735–1741
Lin SH, Lo CC (1997) Fenton process for treatment of desizing wastewater. Water Res 31(8):2050–2056
Lu H, Zhang W, Yang Y, Huang X, Wang S, Qiu R (2012) Relative distribution of Pb2+ sorption mechanisms by sludge-derived biochar. Water Res 46:854–862
Liu Q, Qian K, Qi J, Li C, Yao C, Song W, Wang Y (2018) Improving the efficiency of Fenton reactions and their application in the degradation of benzimidazole in wastewater. RSC Adv 8:9741–9748
Mohan D, Rajput S, Singh VK, Steele PH, Pittman CU Jr ((2011) Modeling and evaluation of chromium remediation from water using low cost bio-char, a green adsorbent. J Hazard Mater 188: 319–333.
Najjar W, Chirchi L, Santosb E, Ghorhel A (2001) Kinetic study of 2-nitrophenol photodegradation on Al-pillared montmorillonite doped with copper. J Environ Monit 3:697–701
Nitoi I, Oncescu T, Oanc P (2013) Mechanism and kinetic study for the degradation of lindane by photo-Fenton process. J Ind Eng Chem 19:305–309
Peng G, Tian G, Liu J, Bao Q, Zang L (2011) Removal of heavy metals from sewage sludge with a combination of bioleaching and electrokinetic remediation technology. Desal 271:100–104
Rasheed R, Meera V (2016) Synthesis of iron oxide nanoparticles coated sand by biological method and chemical method. Procedia Technol 24:210–216
Rodrıguez-Chueca J, Polo-Lopez MI, Mosteo R, Ormad MP, Fernandez-Ibanez P (2014) Disinfection of real and simulated urban wastewater effluents using a mild solar photo- Fenton. Appl Catal B 150–151:619–629
Rule KL, Comber SDW, Ross D, Thornton A, Makropoulos CK, Rautiu R (2006) Diffuse sources of heavy metals entering an urban wastewater catchment. Chemosphere 63:64–72
Sidhu PS, Gilkes RJ, Cornell RM, Posner AM, Quirk JP (1981) Dissolution of iron oxides and oxyhydroxides in hydrochloric and perchloric acids. Clay Clay Miner 29(4):269–276
Sylwan I, Thorin E, Zambrano J (2016) Biochar adsorption for separation of heavy metals during municipal wastewater treatment. Inter J Environ Sci Technol 13(1):359–386
Sun J, Su SP, Fan MH, Guo HQ, Qiao IP, Sun RX (2007) A kinetic study on the degradation of p-nitroaniline by Fenton oxidation process. J Hazard Mater 148:172
Tatsi AA, Zouboulis AI, Matis KA, Samaras P (2003) Coagulation flocculation pretreatment of sanitary landfill leachates. Chemosphere 53:737–744
Tony MA, Lin LS (2020a) Iron recovery form acid mine drain sludge as a Fenton source for municipal wastewater treatment. Inter J Environ Anal Chem. https://doi.org/10.1080/03067319.2020.1734196
Tony MA, Lin LS (2020b) Attenuation of organics contamination in polymers processing effluent using iron-based sludge: process optimization and oxidation mechanism. Environ Technol. https://doi.org/10.1080/09593330.2020.1803417
Tony MA, Parker HL, Clark JH (2019) Evaluating Algibon adsorbent and adsorption kinetics for launderette water treatment: towards sustainable water management. Water Environ J 33(3):401–408
Tony MA, Parker HL, Clark JH (2016) Treatment of Laundrette wastewater using Starbon and Fenton’s reagent. J Environ Sci Health A 23(11):974–979
Tony MA, Purcell PJ, Zhao Y (2012) Oil refinery wastewater treatment using physicochemical Fenton and photo-Fenton oxidation processes. J Environ Sci Health A 47(3):435–440
Tony MA, Zhao YQ, El-Sherbiny MF (2011) Fenton and Fenton-like AOPs for alum sludge conditioning: effectiveness comparison with different Fe2+ and Fe3+ salts. Chem Eng Commun 98(3):442–452
Tony MA, Purcell PJ, Zhao YQ, Tayeb AM, El-Sherbiny MF (2015) Kinetic modeling of diesel oil wastewater degradation using photo-Fenton process Kinetic modeling of diesel oil wastewater degradation using photo-Fenton process. Environ Eng Manag J 14(1):11–16
Tony MA (2019) An industrial ecology approach: green cellulose based bio-adsorbent from sugar industry residue for treating textile industry wastewater effluent. Inter J Environ Anal Chem. https://doi.org/10.1080/03067319.2019.1661397
Tony MA (2020a) Zeolite-based adsorbent from alum sludge residue for textile wastewater treatment. Inter J Environ Sci Technol 17:2485–2498. https://doi.org/10.1007/s13762-020-02646-8
Tony MA (2020b) Central composite design optimization of Bismarck Dye oxidation from textile effluent with Fenton’s reagent. Appl Wat Sci 10(5):108
Xu H, Lu G (2013) On-line spectrophotometric method for decolourizing reaction kinetics of reactive black 5 by Fenton oxidation. Asian J Chem 25(14):7989–7992
Villegas-Guzmana P, Giannakis S, Rtimi S, Grandjeanc D, Bensimonc M, Alencastro I, Torres-Palma R, Pulgarin C (2017) A green solar photo-Fenton process for the elimination of bacteria and micropollutants in municipal wastewater treatment using mineral iron and natural organic acids. Appl Cat 219:538–549
Vlyssides AG, Loukakis H, Karlis PK (2003) Small sewage treatment works using a Fenton oxidation method. Environ Technol 24:931. https://doi.org/10.1080/09593330309385631
Wei X, Jr CV, Buzby K (2005) Recovery of Iron and Aluminium from Acid Mine Drainage by Selective Precipitation. Environ Eng Sci, 22(6):745-755
Zhao YQ, Keogh C, Tony MA (2009) On the necessity of sludge conditioning with non-organic polymer: AOP approach. Res Sci Technol 6(3):151–155
Acknowledgements
The author Maha Tony acknowledges the financial support by Ministry of Higher Education, Mission Department, Egypt grant through the postdoctoral research fund.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Rights and permissions
About this article
Cite this article
Tony, M.A., Lin, LS. Iron Coated-Sand from Acid Mine Drainage Waste for Being a Catalytic Oxidant Towards Municipal Wastewater Remediation. Int J Environ Res 15, 191–201 (2021). https://doi.org/10.1007/s41742-020-00309-7
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s41742-020-00309-7