Abstract
Chronic Kidney Disease of unknown aetiology (CKDu) is a major health concern in North Central Province (NCP) of Sri Lanka. Anuradhapura and Polonnaruwa are the two most affected districts in NCP. This research was designed to identify main suspected CKDu causative agents in drinking water in NCP. Drinking water samples were collected from shallow wells of CKDu patients and non-patients in Anuradhapura and Polonnaruwa districts. They were tested for chemical ions: chloride, fluoride, nitrate, phosphate, calcium, magnesium, sodium, cadmium and arsenic. An analytical framework was developed to analyse water quality data using statistical methods, namely univariate analysis of variance (ANOVA) and Dunnett’s T3 post hoc test, Kruskal–Wallis (KW) and Mann–Whitney’s post hoc test, discriminant analysis, factorial analysis followed by reliability tests. ANOVA, KW and their post hoc tests were applied to show the significant differences at p < 0.05 in mean and median values of chemical constituents between CKDu patient and non-patient samples. Discriminant analysis was applied to show the degree of accuracy in original sampling groups. Factorial analysis was applied to identify the ion combinations in each group. A secondary set of data obtained from drinking water samples of CKDu endemic and non-endemic areas were also analysed independently using the same analytical techniques to compare the results.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Chronic Kidney Disease (CKD) is a major health concern in Sri Lanka which is characterized by permanent loss of kidney function over time. In Sri Lanka, approximately 5000 patients had been treated for CKD in 2009 (WHO 2009), while in 2012 the number has escalated to 8000 (WHO 2012). A descriptive study conducted in Sri Lanka has shown the common causes of CKD to be diabetic nephropathy, hypertension, glomerulonephritis and obstructive uropathy while causes had been unknown in 25.6% of patients (Gooneratne et al. 2008). Due to the nature of unknown aetiology of this disease, it has been named CKD of unknown aetiology (CKDu) in Sri Lanka. The research area of North Central Province (NCP) in Sri Lanka reports the highest CKD patients in Sri Lanka with 10% of the adult population affected by this disease (Ileperuma 2012). Out of them, 27% have been related to unknown causes related to CKDu (Dissanayake et al. 2012). NCP is divided to two administrative areas, namely Anuradhapura district (745,700 persons in 6664 sq. km area) and Polonnaruwa district (359,000 persons in 3077 sq. km area). Anuradhapura district reports three times more CKDu patients and deaths compared to Polonnaruwa district (Poulter and Mendis 2009).
North Central Province (NCP) is the largest province out of nine provinces covering 15% of the total land area and 5.5% of the total population in Sri Lanka. NCP is situated in the dry zone of Sri Lanka with a mean annual rainfall of 1250 mm and a mean annual temperature of 25–30 °C (Department of Meteorology of Sri Lanka 2012). The main rainfall season in NCP is from October to January which brings around 80% of the annual rainfall. During May to September, this region is shadowed from southwest monsoons, which results in a protracted dry period with strong desiccating winds. The mean annual evaporation in NCP is between 1700 and 1900 mm, implying water stress during the dry period of the year. Gampaha district located far southeast from NCP was selected as a control area free from CKD in this research. Gampaha is in the wet zone receiving an average rainfall of 2000–2500 mm/year and a mean annual temperature of 27 °C (Department of Meteorology of Sri Lanka 2012).
Large-scale industrial mining or quarrying activities are nonexistent in NCP. Agriculture is the main sector of employment with more than 50% of the population involved in farming (Central Bank of Sri Lanka 2010). NCP has a cascade irrigation system where the agricultural fields are fed by irrigation canals from reservoirs. The reservoirs are fed by river diversions from the middle of the country which is free from CKDu. In cascade irrigation system, agrochemical washout tends to accumulate downstream (Gunatilake and Illangasekera 2015, in Jayasumana et al. 2017). However, the affected community does not obtain drinking water from irrigation canals or reservoirs. Pipe-borne drinking water is available to only 16% of the total population (Perera et al. 2008). The rest of the drinking water requirements of NCP are met by spring wells and shallow wells. Spring water is less likely to contaminate from irrigation canals as they originate from deeper quartzite formation but shallow wells are more contaminated by irrigation water (Jayasumana et al. 2017). A low prevalence of CKDu has been reported among consumers of spring water (1.5%), and high prevalence (7.7%) among consumers from shallow wells in the endemic area of Sri Lanka (Jayasekara et al. 2015).
The CKDu epidemic in NCP in Sri Lanka and the CKD epidemic in Central American countries (mainly El Salvador, Nicaragua and Costa Rica) have been found similar renal pathological characters and epidemiological characteristics with chronic tubule-interstitial nephritis characterized by tubular atrophy, interstitial fibrosis and variable interstitial mononuclear inflammatory infiltrate (Jayasumana et al. 2017; Orantes-Navarro et al. 2017). Similar risk factors found in both epidemics include pesticide use in farming, drinking untreated well water, a family history of renal dysfunction, taking traditional medical treatment and past history of snake bite (Jayasumana et al. 2017). CKD in both these regions has been termed Chronic Interstitial Nephritis in Agricultural Communities (CINAC) by these authors, and they have categorized the risk factors to two main groups: one related to the pesticides and heavy metals and the other being dehydration due to heat stress leading to drinking contaminated well water while working in agricultural fields. Dehydration, caused by inadequate fluid intake in a hot environment, is found to affect the elimination of toxic agents from the blood to increase their concentration in the kidney medulla, causing kidney injury (Orantes-Navarro et al. 2017). The dehydration hypothesis, however, cannot explain the CKDu patient prevalence in NCP where not all neighbours in the community and not all the family members engaged in farming are affected by the disease. Furthermore, CKDu is not observed in the adjacent northern part of Sri Lanka, where temperatures are warmer with drier conditions than the endemic NCP (Jayasumana et al. 2017). Localized nature and distribution patterns of CKDu patients in NCP impose deeper investigation into socioeconomic and environmental risk factors and their possible interactions in the eventual development of the disease.
The high groundwater fluoride zones overlap CKDu-prevalent regions in NCP which bring out the concerns of water fluoride in relation to CKDu (Dissanayake and Chandrajith 2007). Ramseyer et al. (1957) and Cittanova et al. (1996) have shown damage to kidney tissues by excessive and long-term exposure to fluoride. Furthermore, a dose–effect relationship between fluoride levels and CKDu has been reported as far back by Lantz et al. (1987). Undesirable effects of fluoride on cellular systems have been investigated by Agalakova and Gusev (2012) which reveals that fluoride can induce oxidative stress, intracellular redox homeostasis, lipid peroxidation, protein synthesis inhibition, gene expression alteration and apoptosis. Patients with reduced glomerular filtration rate have decreased ability to excrete fluoride in urine exposing them to increased risk of chronic fluoride toxicity (Schiffl 2018). A recent study by Dharma-wardana (2018) suggests F− in the presence of Mg2+ hardness to be more nephrotoxic than fluoride ions alone.
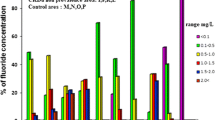
Fluoride can occur naturally in water above desirable levels. Fluoride contents above WHO recommended maximum limit (1.5 mg/L) have been reported in groundwater of NCP by Dissanayake and Weerasooriya (1985), Dissanayake (2005), Herath et al. (2005), Bandara et al. (2008), Perera et al. (2008), Chandrajith et al. (2010) and Dissanayake et al. (2012). Fluoride levels in the dry zone groundwater in NCP have been found to be in a higher range (0.2–8 mg/L) compared to those of the wet zone (0.2–0.8 mg/L) in Sri Lanka (Jayawardana et al. 2010). Water from nearly 50% of the wells in NCP has been reported with undesirable fluoride levels by Padmasiri et al. (2012). Some dug wells in Anuradhapura are found to have fluoride concentrations as high as 9 mg/L while some wells located 300–400 m from them were found with fluoride levels less than 1 mg/L having wide variability in some locations (Dissanayake and Weerasooriya 1985). Variation of fluoride levels in Anuradhapura according to various research findings is shown in Fig. 1.
According to World Health Organization (WHO 2006), the optimum fluoride level in drinking water for general good health is between 0.5 and mg/L and the maximum acceptable concentration is 1.5 mg/L. Maximum permissible limit (MPL) for tropical countries is 0.6 mg/L which is also applicable to Sri Lanka (Perera et al. 2008). Approximately 45% of shallow wells in NCP have been found to exceed the MPL for tropical countries according to Perera et al. (2008).
Fluoride in water may cause long-term adverse effects on human health, such as severe dental fluorosis, skeletal fluorosis and weakened bones (Fawell et al. 2006). Patients with reduced glomerular filtration rate are known to develop skeletal fluorosis even at 1 part per million of fluoride in drinking water (Schiffl 2018). The community fluorosis index (CFI) has been found to have a zonal variation in Sri Lanka with 1.81 in Anuradhapura and 1.66 in Polonnaruwa, leading the fluorosis situations (Dissanayake 1991). In NCP, drinking water wells having undesirable fluoride levels have been found to cause dental fluorosis, especially in children (Padmasiri et al. 2012). However, the actual levels of fluoride intake are not only from drinking water, but also from fluoride in the diet (Schiffl 2018).
The variability of groundwater fluoride and the incidence of higher fluoride levels in shallow wells have been attributed to a range of geological factors including different dissolution rates of fluoride-bearing rocks and residence time (Herath et al. 2005). The minerals, namely charnockitic, granitic, hornblende and biotitic gneisses, enhance fluoride levels in NCP groundwater (Herath et al. 2005; Dissanayake 1991). Other fluoride-bearing minerals found in NCP include micas, pyroxene, fluorite, tourmaline, topaz, sphene and apatite (Young et al. 2011). High evaporation influenced by prevailing warm climate brings the soluble fluorides upward (Dissanayake 1991). This may be associated with groundwater extraction where Anuradhapura district reports the highest number of irrigation tanks and agro-wells in the country (Karunaratne 1983). Gampaha control area selected in this research is underlain by undifferentiated metasediments of compositions different from NCP (Dissanayake 1991).
According to Ozsvath (2006), dissolved fluoride in water is usually controlled by the solubility of fluorite (CaF2), with high fluoride concentrations often associated with soft, alkaline and calcium-deficient waters. This association has also been explained by Edmunds and Smedley (2005) where high dissolved fluoride in groundwater depletes calcium. Fluoride richness in groundwater in CKDu endemic areas is described to be associated with alkalinity of the soils by Chandrajith et al. (2011). However, according to Dissanayake et al. (1982), groundwater hardness in Northern, NCP and Central Province in Sri Lanka exceeds the maximum desirable level of 250 mg/L.
Many other researchers have suggested other factors to cause CKDu in NCP. Triple superphosphate (TSP) fertilizer imported for paddy farming in Sri Lanka has been found to contain cadmium as an impurity (Bandara et al. 2010). Cadmium is a known nephrotoxin and urinary excretion of cadmium is considered to be a reliable indicator of cumulative long-term exposure to cadmium (WHO 1992). Jayatilake et al. (2013) indicate a significantly higher cadmium concentration in the nails of CKDu cases compared to controls from the endemic area in Sri Lanka. Jayasumana et al. (2017) indicate that mean concentration of cadmium in urine in CKDu patients was significantly higher (1.039 mg/g) compared to controls in the endemic (0.646 mg/g) and non-endemic areas (0.345 mg/g). Levels of cadmium in lotus and tobacco have been found to be higher in CKDu endemic area in Sri Lanka compared to non-endemic areas by Jayatilake et al. (2013). However, cadmium levels in drinking water of CKDu patients in NCP has been found to be below WHO recommended maximum tolerance limit of 5 μg/L (WHO 2012; Wasana et al. 2012; Jayatilake et al. 2013). Dharma-wardana (2018) explains contaminated fertilizer cannot be considered the origin of higher levels of cadmium found in agricultural soils due to run-off during monsoons and conversion of impurities (Cd or As) to bio-unavailable forms held in the soil. According to Dharma-wardana (2018), the toxicities of cadmium and fluorides are both enhanced by their mutual presence with three times of joint toxicity than MAL of cadmium in soft water.
Mercury, uranium, selenium, lead and arsenic are some other metallic nephro-toxicants which have been known to cause progressive renal disease in other parts of the world (Navas-Acien et al. 2009). Bandara et al. (2010) have reported triple superphosphate (TSP) fertilizer imported for paddy farming in Sri Lanka to contain arsenic, lead, mercury and aluminium as impurities. Arsenic levels in hair have been significantly higher in CKDu cases compared to controls from the endemic area (Jayatilake et al. 2013). However, Jayawardana et al. (2012), Wasana et al. (2012) and WHO (2012) have shown arsenic, lead, aluminium, zinc, copper and nickel levels in the shallow wells of CKDu endemic areas were within WHO allowable limits. Furthermore, Jayatilake et al. (2013) have revealed lead and uranium levels in drinking water sources used by individuals with CKDu were within maximum allowed levels. Same authors have shown no significant dose–effect relationship between the concentration of arsenic, lead or selenium in urine and the stage of CKDu but the hardness of water and high fluoride content on top of inadequate intake of water to influence the excretion of heavy metals and oxidative damage to the kidneys caused by heavy metals (Jayatilake et al. 2013).
Glyphosate is a widely used herbicide in the CKDu endemic area of NCP in paddy farming. Glyphosate has strong metal-chelating properties which are found to cause CKD by complexation with hardness of water by forming nephrotoxic metallic compounds (Jayasumana et al. 2014). These authors explain glyphosate–metal complexation to play a major role in the CKDu. However, according to Dharma-wardana (2018), glyphosate–metal complexation cannot explain toxicity as these complexes are insoluble forming precipitates in water. This can be explained by other sources of entry of toxicants to body as occupational and environmental toxins may enter humans through food, inhalation as well as direct contact. For example, farmers in the disease endemic area are seen spraying pesticides and working in the fields without protective clothing which enable exposure by inhalation and dermal absorption (Jayasumana et al. 2017).
The use of aluminium utensils for cooking and water storage has been suspected to cause CKDu in NCP by Herath et al. (2005). They have reported drinking water samples from CKDu prevalence areas having aluminium contents above WHO standard (10 μg/L). Later research by WHO (2012) has shown aluminium levels in serum of CKDu patients to be within the normal range. Dharma-wardana (2018) found aluminium in fluoride-rich water to form Al–F complex which has no toxic effect.
Blue-green algae (Cyanobacteria) have been identified to produce cyanotoxins (algal toxins), namely microcystin, cylindrospermopsin (CYN) and deoxy-cylindrospermopsin (DCYN), which are known to have acute nephrotoxic effects in animals (Liyanage and Magana-Arachchi 2012). Algae growing in open water in NCP were speculated to cause CKDu (Pethiyagoda 2011). However, Padaviya reservoir providing water to some CKDu-affected areas in NCP areas has shown low levels of DCYN (< 0.05 μg/L) and nonexistence of CYN and microcystin (Dissanayake et al. 2012). Jayasumana et al. (2017) report ground water in shallow wells and tube wells in CKD endemic area was not contaminated by cyanobacterial toxins.
Chandrajith et al. (2011) have shown CKDu endemic locations in NCP having comparatively lower Na+/Ca2+ ratios in drinking water in the range of 1.6–6.6 compared to a range of 34–469 in the non-endemic areas. According to Chandrajith et al. (2011), higher Ca2+ levels in relation to Na+ in drinking water favour CKDu while higher Na+ levels compared to Ca2+ favour non-CKDu situations. However, there are no other findings that suggest higher Na+/Ca2+ ratio favouring CKDu.
Methodology
In accordance with the existing information and theories on CKDu prevalence in Sri Lanka, this research was designed to carry out a comparative analysis of drinking water of CKDu patients and non-patients in Anuradhapura and Polonnaruwa districts to investigate possible CKDu-causing agents. Drinking water sample locations were identified based on the distribution of CKDu patients and non-patients in Anuradhapura and Polonnaruwa districts. Table 1 shows the sampling groups from NCP and Gampaha with number of samples in each group. A total of 102 patient and 60 non-patient samples were collected from Medawachchiya and Padaviya village divisions (subadministrative divisions) in Anuradhapura district, and 90 patient samples and 60 non-patient samples were collected from Medirigiriya village division in Polonnaruwa district. A number of 20 samples were collected from Gampaha district as controls.
A map of sampling locations is given in Fig. 2. Random sampling method was applied in this study. SPSS software was used for statistical analyses. The results were further compared with a secondary data source from Chandrajith et al. (2011) to draw conclusions. Table 2 shows the secondary data set consisting of 183 samples from CKDu endemic and non-endemic areas.
CKDu patient home addresses were identified with the help of Government Village Officers (Grama Niladhari). Hand-dug shallow drinking water wells of average 5 m depth were selected for water sample collection. When a drinking water well has been used for more than 10 years by a CKDu patient as the only drinking water source, it was selected as a patient water sample source. When a drinking water well has been used for more than 10 years by a household and none of the members showed symptoms of CKDu, it was selected as a non-patient water sample source.
According to CKDu patient definition if a person has persistent albuminuria, i.e., Albumin-Creatinine Ratio (ACR) ≥ 30 mg/g in an initial urine sample and at a repeat with no past history of glomerulonephritis, pyelonephritis, renal calculi or snake bites and if not on treatment for diabetes is considered to be decisive of CKDu (Jayatilake et al. 2013). Accordingly, in this research, non-patient households were confirmed of absence of CKDu conditions by carrying out a repeated persistent albuminuria test for negative results (urine Albumin–Creatinine Ratio less than 30 mg/g).
Sample collection period was from December 2010 to August 2011. The sampling team consisted of the first author, a research partner and a medical doctor. Heavy rains were prevailing in December 2010 in the research area. Therefore, data collection was extended up to August 2011 so that samples were not flawed by rainwater. More than 80% of the primary data were collected from May to September 2011.
Samples from Anuradhapura patients and non-patients were grouped as ANU-P and ANU-NP, respectively. Samples from Polonnaruwa patients and non-patients were grouped as POL-P and POL-NP, respectively. Control area samples from Gampaha were termed as GAM.
Temperature and pH values of each sample were tested in the field using thermometer and pH meter (model ATPH-6). Chemical parameters analysed in water samples were chloride (Cl−), fluoride (F−), nitrate (NO3−), phosphate (PO43−), calcium (Ca2+), magnesium (Mg2+), sodium (Na+), cadmium (Cd2+) and arsenic (As3−). Water samples were collected in PVC bottles, and preservatives of HNO3 and H2SO4 were added according to the standard procedures before they were transported to a commercially accredited laboratory named SGS Laboratories Pvt. Ltd in Colombo. Cd2+, As3−, Na+, Mg2+ and Ca2+ were tested using 3120 APHA methods, and F−, Cl−, PO43− and NO3− were tested using 4500 APHA 21st Ed methods.
All tested parameters were compared with WHO standards. ANOVA and Kruskal–Wallis (KW) tests were used to differentiate water parameters between groups (group significance), of ANU-P, ANU-NP, POL-P and POL-NP based on mean and median values, respectively. ANOVA and Kruskal–Wallis (KW) tests measure significant group difference by comparing the group mean or median values with the mean or median of total group, respectively. The null hypotheses (H0) for ANOVA and KW tests were that mean and median values of a variable were not significantly different from group mean and median values, respectively. Dunnett’s T3 post hoc test was used to identify which two groups were significantly different based on mean values, and Mann–Whitney’s post hoc test was used to identify which two groups were significantly different based on median values. Significant differences were determined at p < 0.05 at 95% confidence level. Discriminant analysis (DA) was used to determine whether sampling categories of ANU-P, ANU-NP, POL-P and POL-NP were appropriately classified.
Factorial analysis (FA) was applied to identify any chemical associations which are shown as factor combinations. KMO and Bartlett (Kaiser–Meyer–Olin and Bartlett) test of sphericity was the first step of FA. It is a measure of sampling adequacy on the hypothesis that the observed data are a sample of a multivariate normal population. Sample adequacy was tested at KMO and Bartlett test values higher than 0.5 at p < 0.05 as specified by Colman and Pulford (2006). Initial eigenvalues and percentage variances were used to isolate the significant factors which represent the strength of cross-correlations among the observed variables. Eigenvalues indicate how much of variance is explained by a factor. If a factor has an eigenvalue less than 1 (percentage variance below 10%), it shows the cross-correlations among the variables are insignificant. On the other hand, if a factor has an initial eigenvalue above 1 (percentage variance above 10%), it shows variables have significant cross-correlations within the factor (Green and Salkind 2005). Those factors having an initial eigenvalue above 1 (percentage variance above 10%) were extracted in this analysis.
Factor rotations are carried out in FA to improve the interpretability of associations among variables. Factor rotations generate a simple structure of high loadings of absolute values, which helps to differentiate one factor from the other. An orthogonal rotation technique, namely Varimax, was used in the analysis which rotates the axes at right angles assuming that underlying factors are not correlated with each other (Gaur and Gaur 2009).
Cronbach’s Alpha values gave a measure of reliability of derived factors. Cronbach’s alpha value of 0.7 and above is considered to be acceptable, and a value of 0.8 and above gives a higher reliability of the variables in a factor (Kaiser 1974) which was used in this study. Component plots in rotated space were used to explain the factor results graphically.
Statistical analysis of drinking water data
Comparisons between sampling groups and WHO standards
The mean, median, maximum and minimum values of variables of chloride (Cl−), nitrate (NO3), fluoride (F−), phosphate (PO43−), calcium (Ca2+), magnesium (Mg2+) and sodium (Na+) in each sampling group are given in Table 3 along with WHO recommended maximum permissible limits. The average and median value of each variable did not exceed the WHO recommended values in any of the sampling groups. However, some individual samples have exceeded the WHO limits (e.g. in Anuradhapura patients: Cl− 11.75%, F− 3.92%, NO3− 2.94%, Ca2+ 1.96% and Na+ 5.88%; in Anuradhapura non-patients: Cl− 5% and NO3− 20%; in Polonnaruwa patients: NO3− 5.43%). PO43− and Mg2+ values have not exceeded WHO limits in both Anuradhapura and Polonnaruwa patient and non-patient samples as well as in Gampaha control area samples.
In Anuradhapura patient group, 3.92% of the samples have exceeded the fluoride levels above WHO maximum permissible limit of 1.5 mg/L, but Anuradhapura non-patient, Polonnaruwa patient and Polonnaruwa non-patient groups have not exceeded this limit. Out of Anuradhapura patients and non-patients, 29.41% and 21.67% samples, respectively, have exceeded 0.6 mg/L fluoride of MPL for tropical countries. In Polonnaruwa patients and non-patients, 35.56% and 25% samples, respectively, have exceeded this limit. Fluoride levels in Anuradhapura patient samples were in a wider range than Polonnaruwa patients; however, median value of fluoride in Anuradhapura patients was lower than Polonnaruwa patients and non-patients. The mean value of F− as shown in Table 3 was highest in Anuradhapura patients out of all the groups. The median value of F− was highest in Polonnaruwa patients. Both mean and median values of Cl−, Mg2+ and Na+ were found highest in Anuradhapura non-patient samples. Mean and median Ca2+ ions in both Anuradhapura and Polonnaruwa patient and non-patient water samples were higher than Gampaha control area samples but they did not exceed the WHO standard of 200 mg/L except in 1.96% Anuradhapura patient samples.
Statistical significance tests for different sampling groups
Univariate analysis of variance (ANOVA) and Dunnett’s T3 post hoc test results on mean values of different sampling groups are presented in Table 4. ANOVA results showed group differences to be significant at p < 0.05 for all the ions except F− and Ca2+. Dunnett’s T3 post hoc test results showed Cl− and PO43− to be significantly different (p < 0.05) between all the groups except ANU-P and ANU-NP. Even though the group differences were not significant for F−, it was significantly different between ANU-P and ANU-NP. NO3− was significantly different between all the groups except ANU-P and POL-P and ANU-NP and POL-P. Mg2+ was significantly different between ANU-P and POL-NP, POL-P and POL-NP and ANU-NP and POL-NP. Ca2+ was found to be not significantly different among any of the groups. Na+ was significantly different between all the groups except ANU-P and ANU-NP and POL-P and POL-NP.
Nonparametric results of Kruskal–Wallis (KW) and Mann–Whitney’s tests on median values of different sampling groups are presented in Table 5. Kruskal–Wallis results showed significant group differences (p < 0.05) in all the ions except F−. Mann–Whitney’s post hoc test results showed Cl− was significantly different between all the groups except ANU-P and POL-P. NO3− was significantly different between all the groups except ANU-P and POL-NP. PO43− was significantly different between the groups except ANU-P and ANU-NP and ANU-NP and POL-P. Ca2+ was significantly different between ANU-P and POL-P, ANU-P and POL-NP and POL-P and POL-NP. Na+ was significantly different between all the groups except ANU-P and ANU-NP and POL-P and POL-NP. Mg2+ showed similar grouping results as ANOVA and Dunnett’s T3 post hoc tests.
In order to compare these two sets of results easily, superimposed table (Table 6) was prepared based on mean and median test results. Table 6 showed that most groups were significantly different to each other both in mean and median value (Dif) compared to groups with no significant difference (nDif). Groups were significantly different more in median values (nDif-dif) than in mean values (Dif-nDif). This is due to the reason that means are flawed by extreme values but the medians are not. As such, both ANOVA with Dunnett’s T3 post hoc test and Kruskal–Wallis with Mann–Whitney’s post hoc test gave different traits of explanation in relation to mean and median values.
Factorial analysis (FA) for identifying chemical combinations
Factorial analysis (FA) results are summarized in Table 7. All sampling groups from CKDu endemic areas had KMO and Bartlett’s test values above 0.5, indicating sample adequacy. Control area Gampaha gave a value of 0.482 which was marginally lower than 0.5 but the sample adequacy was significant at p < 0.05. Therefore, this group was also deemed adequate.
FA results after factor rotations showed that two factors could be extracted from Anuradhapura patient samples with the first factor having an initial eigenvalue of 3.78 and a percentage variance of 54%. Factors extracted from other groups were: three factors from Polonnaruwa patients, three factors from Anuradhapura non-patients, two factors from Polonnaruwa non-patients and three factors from Gampaha controls. In Anuradhapura patient, Polonnaruwa patient and Polonnaruwa non-patient groups, F−, Na+ and Mg2+ combined in a single factor having significantly high initial eigenvalues and percentage variances. This situation could be explained by their natural occurrence in water as ion combinations. In all the four groups from CKDu endemic area, NO3− and PO43− combined in the same factor but not in control of Gampaha. Anuradhapura non-patient samples were different from other three groups from CKDu endemic area for the fact that fluoride did not combine with any other variables to form a factor in this group.
Cronbach’s Alpha value is a measure of the reliability of the factor in factor analysis where a higher Cronbach’s Alpha value (close to 1.000) gives a higher reliability of a factor combination. Table 8 shows the change of reliability when a variable was eliminated from a factor. If Cronbach’s Alpha value decreased after a variable was eliminated from a factor, that meant a better reliability of the factor with the variable. In oppose, if Cronbach’s Alpha value increased after a variable was eliminated from a factor, the given variable is supposed to make the factor less reliable (Green and Salkind 2005).
In Anuradhapura patients, the first factor is comprised of variables Cl−, F−, Ca2+, Mg2+ and Na2+, which was reliable with a Cronbach’s Alpha value of 0.903. In Anuradhapura patient samples, Cl− was more reliable than F− in factor combinations. When Ca2+, Mg2+ and Na2+ were eliminated from the factor in Anuradhapura patient samples, Cronbach’s Alpha value dropped (from 0.903) to 0.674, 0.742 and 0.594, respectively, indicating that Na+ was the most reliable variable to form associations with Cl− and F− ions in this factor than Ca2+ and Mg2+.
In Polonnaruwa patients, the first factor was made of F−, Mg2+ and Na+ with a Cronbach’s Alpha value of 0.871. When Mg2+ and Na+ were eliminated from this factor, Cronbach’s Alpha value dropped to 0.035 and 0.037, respectively, explaining both the variables were reliable in explaining the given factor. As such, Na+ and F− as well as Mg2+ and F− were both reliable chemical combinations in Polonnaruwa patient samples.
Polonnaruwa non-patient samples consisted of variables Cl−, F−, Ca2+, Mg2+ and Na2+ with a Cronbach’s Alpha value of 0.892. When Ca2+, Mg2+ and Na+, respectively, were deleted from Polonnaruwa non-patients, Cronbach’s Alpha value dropped to 0.748, 0.677 and 0.705, respectively, indicating Mg2+ was more reliable to combine with F− and Cl− than Ca2+ and Na+. Cronbach’s Alpha if item deleted was 0.708 and 0.836, respectively, for Cl− and F−, respectively, indicating Cl− was more reliable to combine with Mg2+ than F−. Therefore, it was concluded that Na2+ and F− combination was secondary to Mg2+ and Cl− combination in Polonnaruwa non-patient sample.
Anuradhapura non-patient samples were different from other three groups in which F− itself formed a separate factor, which did not combine with any other variables. As a result, reliability test for F− was not plausible for this factor. Furthermore, Mg2+ and Cl− combined in the same factor, whereas Na+ did not group with Cl− or F− to form combinations in this group.
Gampaha control area samples showed different factor results to CKDu endemic area samples, where F− was not detected and Na+ and or F− combining in a factor was not identified. Furthermore, NO3− and PO43− did not go in a single factor in Gampaha control samples.
Discriminant analysis (DA) for appropriate classification
Discriminant analysis (DA) was used to analyse whether Anuradhapura and Polonnaruwa patient and non-patient groups were appropriately classified. The results in Table 9 showed that 54.9% of Anuradhapura patient samples were correctly classified under their own group, yet 16.6, 14.7 and 13.7% samples were classified under Polonnaruwa patient, Anuradhapura non-patient and Polonnaruwa non-patient groups, respectively, having similarities to those groups. When Polonnaruwa patient samples were considered, around 43% samples were under their own classification, while around 41% had similarity to Polonnaruwa non-patient classification. This indicated that Polonnaruwa patients had nearly equal affinities to Polonnaruwa non-patient samples. Anuradhapura and Polonnaruwa non-patient groups showed 60 and 85% similarities to their own groups, respectively, indicating a higher percentage of these samples having affinity of their own groups. Out of all samples, 58.3% were correctly classified within their own groups.
When FA results were compared with DA results, approximately 55% of data in Anuradhapura patients could be classified under their own group, having Na+ and F− and Na+ and Cl− as the more probable combinations and Mg2+ and F− and Mg and Cl− as the less probable combinations. In Anuradhapura non-patients, 60% of the samples falling under their own classification give this percentage of samples having Mg2+ and Cl− as a reliable combination. Polonnaruwa patient samples had almost equal distributions under their own classification (43.3%) and non-patient classification (41.1%), having equally reliable combinations of Na+ and F− and Mg2+ and F−. In Polonnaruwa non-patients, 85% of samples falling under their own classification explain this % of data showing a more reliable combination of Mg2+ and Cl + which was secondary to Na2+ and F− combination.
Analysis of secondary data
Secondary data have been categorized as CKD endemic area and non-endemic area depending on CKD prevalence by Chandrajith et al. (2011) given in Table 2. Accordingly, Medawachchiya and Padaviya have been identified as CKDu endemic samples from NCP and Girandurukotte and Nikawewa as CKDu endemic samples from Uva and North Western Provinces, respectively. Huruluwewa has been identified as a CKDu non-endemic sample from NCP and Wellawaya as a non-endemic sample from Uva Province.
The average and median values of magnesium and sodium in Huruluwewa have exceeded the maximum permissible limits (MPL) recommended by WHO but the average and median values of all other chemical ions have not exceeded these limits except for some individual samples (Chandrajith et al. 2011).
When ANOVA and Kruskal–Wallis tests were applied to secondary data, ANOVA did not give interpretable results for majority of the ions as total degrees of freedom for the variables were too large. The data from Medawachchiya (endemic area) and Wellawaya (non-endemic area) could not be analysed due to inadequate sample sizes. However, ANOVA and Kruskal–Wallis tests for secondary data showed F− values were significantly different among all the groups at p < 0.05. Dunnett’s T3 and Mann–Whitney’s post hoc tests showed significantly different F− levels between CKDu endemic areas samples of Girandurukotte and Nikawewa and Nikawewa and Padaviya. Fluoride was not significantly different between Girandurukotte and Padaviya, Girandurukotte and Huruluwewa as well as Padaviya and Huruluwewa. Mann–Whitney’s post hoc test results showed that Na+ was significantly different in all sampling locations.
The secondary data were analysed using FA to identify chemical combinations among ions, and the results are shown in Table 10. Factor results of Girandurukotte endemic samples were comparable to Anuradhapura and Polonnaruwa patient results with first factor consisting of F−, Mg2+ and Na+. The reliability results (Table 11) of Girandurukotte samples also showed that Na+ was more significant than Mg2+ in making a factor with F−. Samples obtained from other CKDu areas of Nikawewa and Padaviya also showed Na+ and F− combining in one factor. However, in Nikawewa, it was grouped in the third factor with a lower Cronbach’s Alpha value. Reliability by deleting variable could not be tested for Nikawewa and Padaviya as these factors were made with only two variables.
Huruluwewa also showed Na+ and F− combining in one factor; however, reliability results showed that Na+ and Mg2+ were highly and equally significant in making a factor with F− which was similar to the factor results of Polonnaruwa patient samples. Although Huruluwewa has been identified as a non-patient location by Chandrajith et al. (2011), it is situated close to CKDu areas in NCP of Sri Lanka. KMO and Bartlett’s test of sampling adequacy did not give results for Wellawaya control samples as the sample size was not adequate. Furthermore, it was noted that Na+ and F− did not combine to form a factor in Wellawaya control samples.
Discriminant analysis results for secondary data (Table 12) showed a total of 68.1% samples falling under the right classification with 93.5% of Girandurukotte samples, 58.8% of Nikawewa samples, 50% of Padaviya samples and 65.5% of Huruluwewa samples classified under their own classification.
Sodium to calcium ratios for primary data
Sodium to calcium ratios in the Anuradhapura and Polonnaruwa water samples are shown in Table 13. Na+/Ca2+ ratios for patient and non-patient samples of Anuradhapura and Polonnaruwa were in a much lower range than the values estimated by Chandrajith et al. (2011). In Gampaha control area, Na+/Ca2+ ratios were several times higher compared to Anuradhapura and Polonnaruwa samples; however, those values were also in a much lower range compared to secondary data by Chandrajith et al. (2011), which are shown in Table 13.
Heavy metal analysis for primary data in CKD endemic areas
Heavy metals analysed using primary data for CKDu endemic samples are shown in Table 14 along with some results from the literature. According to primary data, Fe, Cu, Zn, As, Pb and Cd were below WHO limits in average, median, maximum and minimum counts for all the samples. However, average (mean) and maximum values of Mn in CKDu endemic samples have exceeded WHO limit. Cadmium was not detected in Gampaha control samples.
Discussions
CKDu is an epidemic affecting agricultural communities of NCP in Sri Lanka. CKDu patients in Sri Lanka as well as in El Salvador, Nicaragua and Costa Rica have been found similar renal pathological and epidemiological characteristics where this condition has been named as Chronic Interstitial Nephritis in Agricultural Communities more recently. Existing hypotheses suggest exposure to occupational and environmental toxins in agriculture as a primary trigger and dehydration caused by inadequate fluid intake in a hot environment as a contributory factor of this disease. Previous research suggests cadmium, aluminium, fluoride, Na+/Ca2+ ratio, algal toxins and glyphosate complexation with hardness of water as possible CKDu-causing factors in NCP. Agricultural communities in CKDu endemic area obtain drinking water mainly from shallow wells. This research was designed to examine the possible CKDu risk factors in drinking water from shallow wells in NCP of Sri Lanka. Drinking water from CKDu patients and non-patients was drawn from Anuradhapura and Polonnaruwa districts. Water samples were examined for chloride (Cl−), fluoride (F−), nitrate (NO3−), phosphate (PO43−), calcium (Ca2+), magnesium (Mg2+), sodium (Na+), cadmium (Cd2+) and arsenic (As3−). Control samples were drawn from Gampaha which was free from this disease.
The average and median values of all the above ions did not exceed the WHO recommended values in any of the sampling groups, but some individual samples exceeded these limits. In Anuradhapura patients, 3.92% of the samples have exceeded the F− levels above WHO maximum permissible limit of 1.5 mg/L, but Anuradhapura non-patient, Polonnaruwa patient and Polonnaruwa non-patient samples have not exceeded this limit. The mean value of F− was highest in Anuradhapura patients, and the median value was highest in Polonnaruwa patients. The mean and median Ca2+ ions in both Anuradhapura and Polonnaruwa patient and non-patient samples were higher than Gampaha control area samples but they did not exceed the WHO limit (200 mg/L).
ANOVA results showed F− and Ca2+ to be not significantly different at p < 0.05 in Anuradhapura and Polonnaruwa patient and non-patient groups. Nonparametric results of Kruskal–Wallis test showed F− to be not significantly different at p < 0.05 in Anuradhapura and Polonnaruwa patient and non-patient groups. Median values given by Mann–Whitney’s post hoc test results showed significant differences among groups more than mean values given by Dunnett’s T3 post hoc test.
Chemical constituents do not exist singly in water and may form associations. Factorial analysis (FA) was applied to identify those chemical associations which are shown as factor combinations in sampling groups. KMO and Bartlett’s test values above 0.5 indicated sample adequacy, and Cronbach’s Alpha value gave a measure of the reliability in FA. Those factors which were significant at p < 0.05 and reliable with a Cronbach’s α > 0.7 were extracted in the analysis. In Anuradhapura patient, Polonnaruwa patient and Polonnaruwa non-patient groups F− combined with Na+ and Mg2+ to form reliable factors (Cronbach’s α > 0.7). In Anuradhapura patient samples, Na+ and F− and Na+ and Cl− combinations were more reliable than Mg2+ and F− and Mg2+ and Cl− combinations. In Polonnaruwa patient samples, Na+ and F− combination was equally reliable to Mg2+ and F− combination. In Polonnaruwa non-patient samples, Mg2+ and Cl− and Ca2+ and Cl− combinations were more reliable than Na+ and F− and Mg2+ and F− combinations. Anuradhapura non-patient samples were different from other groups from NCP for the fact that F− did not combine with any other variables to form a factor in this group. F− was not detected in Gampaha control area samples. DA results showed approximately 55% of data in Anuradhapura patient samples, 43% of data in Polonnaruwa patient samples, 60% of data in Anuradhapura non-patient samples and 85% of data in Polonnaruwa non-patient samples falling under their own group classification having above reliable factor combinations.
According to secondary data analysis results, the average and median values of magnesium and sodium in Huruluwewa have exceeded the maximum permissible limits (MPL) recommended by WHO but the average and median values of all other chemical ions have not exceeded these limits. ANOVA and Kruskal–Wallis tests for secondary data showed F− values to be significantly different among all the groups at p < 0.05. Dunnett’s T3 and Mann–Whitney’s post hoc tests showed significantly different F− levels between CKDu endemic areas samples of Girandurukotte and Nikawewa and Nikawewa and Padaviya.
Factor analysis results for secondary data showed Girandurukotte endemic samples were comparable to Anuradhapura and Polonnaruwa patient results with first factor consisting of F−, Mg2+ and Na+. The reliability results of Girandurukotte samples showed that Na+ was more reliable than Mg2+ in making a factor with F−.
In primary data from CKDu area, Fe, Cu, Zn, As, Pb and Cd were below WHO limits in average, median, maximum and minimum counts. There is a possibility of variation of the heavy metal concentrations depending on seasonal factors such as the monsoonal rainfall and droughts. Also according to the hypotheses on heat stress and dehydration, the agricultural workers consuming large quantities of water containing heavy metals below the allowable limits in the long run may also have harmful effects of aetiological significance for CKDu.
The average (mean) and maximum values of Mn have exceeded the WHO limits in primary data. Mn intake is known to cause hypertension in patients older than 40 (Emhemmed et al. 2014); however, a direct relationship between Mn consumption and CKDu cannot be found in literature.
Na+/Ca2+ ratios were several times higher in Gampaha control area compared to CKDu endemic area samples; however, they were not in the range identified by Chandrajith et al. (2011) in earlier research.
Conclusions
None of the analysed ions exceeded WHO limits in their mean or median values in any of CKDu endemic area drinking water samples. Therefore, drinking water quality could not be concluded on average and median values of water parameters analysed. ANOVA and Kruskal–Wallis (KW) results showed F− to be not significantly different between the groups at p < 0.05. Therefore, ANOVA and Kruskal–Wallis tests followed by their post hoc tests were inadequate to identify significant differences between the investigated drinking water samples among Anuradhapura and Polonnaruwa patients and non-patients.
Factor analysis (FA) reflects the combinations of variables in drinking water samples. According to FA, Na+ and F− was the common reliable combination that could be identified in both Anuradhapura and Polonnaruwa patient samples. In Polonnaruwa non-patient samples, Mg2+ and Cl− and Ca2+ and Cl− combinations were more reliable than Na+ and F−. Anuradhapura non-patient samples were different from other three groups for the fact that fluoride did not combine with any other variables to form a factor in this group. The secondary data analysis also showed that CKDu endemic area samples were associated with Na+ and F− combination, whereas in CKDu non-endemic samples, Na+ and Mg2+ equally combined with F−.
The above results give a comparative account of significant ion combinations in drinking water samples of CKDu patients and non-patients in NCP. These combinations reflect the multiple interactions among commonly found ions in natural drinking water but they do not give conclusive evidence as to the cause of CKDu. Most significant common combination of Na+ and F− in patient samples and the secondary combination of Mg2+ and F− found in patient and non-patient drinking water samples need further investigation for any influence on CKDu in the endemic area of NCP.
Abbreviations
- ANOVA:
-
Univariate analysis of variance
- APHA:
-
American Public Health Association
- CKD:
-
Chronic Kidney Disease
- CKDu:
-
Chronic Kidney Disease of unknown aetiology
- CINAC:
-
Chronic Interstitial Nephritis in Agricultural Communities
- CYN:
-
Cylindrospermopsin
- DA:
-
Discriminant analysis
- DCYN:
-
Deoxy-cylindrospermopsin
- FA:
-
Factorial analysis
- KMO:
-
Kaiser–Meyer–Olin
- KW:
-
Kruskal–Wallis
- MAL:
-
Maximum allowed levels
- MPL:
-
Maximum permissible limits
- NCP:
-
North Central Province
- TSP:
-
Triple super phosphate
- WHO:
-
World Health Organization
References
Agalakova NI, Gusev GP (2012) Molecular mechanisms of cytotoxicity and apoptosis induced by inorganic fluoride. ISRN Cell Biol 2012, Article ID 403835. https://doi.org/10.5402/2012/403835
Bandara JMRS, Senevirathna DMAN, Dasanayake DMRSB, Herath V, Bandara JMRP, Abeysekara T, Rajapaksha KH (2008) Chronic renal failure among farm families in cascade irrigation systems in Sri Lanka associated with elevated dietary cadmium levels in rice and freshwater fish (Tilapia). Environ Geochem Health 30:465–478
Bandara JMRS, Wijewardena HVP, Liyanege J, Upul MA, Bandara JMUA (2010) Chronic renal failure in Sri Lanka caused by elevated dietary cadmium: Trojan horse of the green revolution. Toxicol Lett 198:33–39
Central Bank of SRI LANKA (2010) Annual Report 2010
Chandrajith R, Seneviratna S, Wickramaarachchi K, Attanayake T, Aturaliya TNC, Dissanayake CB (2010) Natural radionuclides and trace elements in rice field soils in relation to fertilizer application: study of a Chronic Kidney Disease of unknown etiology area in Sri Lanka. Environ Earth Sci 60:193–201
Chandrajith R, Dissanayake CB, Ariyarathna T, Herath HMJMK, Padmasiri JP (2011) Dose-dependent Na and Ca in fluoride-rich drinking water—another major cause of chronic renal failure in tropical arid regions. Sci Total Environ 409:671–675
Cittanova ML, Lelongt B, Verpont MC, Geniteau-Legendre M, Wahbe F, Prie D, Coriat P, Ronco PM (1996) Fluoride ion toxicity in human kidney collecting duct cells. Anesthesiology 84:428–435
Colman A, Pulford B (2006) A crash course in SPSS for windows: updated for versions 10, 11, 12 and 13, Blackwell Publishers, Inc
Department of Meteorology of Sri Lanka (2012) Climate in Sri Lanka, https://www.google.com.au/search?q=Climate+in+Sri+Lanka&oq=Climate+in+Sri+Lanka&aqs=chrome.69i57j0l5.488j0j7&sourceid=chrome&es_sm=93&ie=UTF-8. Accessed 16 July 2014
Dharma-wardana MWC (2018) Chronic kidney disease of unknown etiology and the effect of multiple-ion interactions. Environ Geochem Health 40(2):705–719
Dissanayake CB (1991) The fluoride problem in the groundwater of Sri Lanka -environmental management and health. Int J Environ Stud 38:137–155
Dissanayake CB (2005) Of stones and health: medical geology in Sri Lanka. Science 309:883–885
Dissanayake CB, Chandrajith R (2007) Medical geology in Sri Lanka. Environ Geochem Health 29:155–162
Dissanayake CB, Weerasooriya S (1985) The hydrogeochemical atlas of Sri Lanka. Natural Resources Energy and Science Authority, Sri Lanka
Dissanayake CB, Senaratne A, Weerasooriya S (1982) Geochemistry of well water and cardiovascular diseases in Sri Lanka. Int J Environ Stud 19:195–203
Dissanayake DM, Jayasekera JMKB, Ratnayake KB, Wickramasinghe W, Radella YA, Palugaswewa WB (2012) Effect of concentrated water from reservoirs of high prevalence area for Chronic Kidney Disease of unknown etiology (CKDUu) of unknown origin in Sri Lanka on mice In: Water quality and human health: challenges ahead. University of Peradeniya, Sri Lanka. Postgraduate Institute of Science
Edmunds WM, Smedley P (2005) Fluoride in natural waters. In: Selinus O (ed) Essentials of medical geology. Springer, Dordrecht
Emhemmed A, Albriky K, Bushita A (2014) Concentration of heavy metals in underground water wells in Gharian district, Libya. In: International conference on agricultural, ecological and medical sciences (AEMS-2014), Bali, Indonesia
Fawell J, Bailey K, Chilton J, Dahi E, Fewtrell L, Magara Y (2006) Fluoride in drinking-water. WHO. ISBN 92-4-156319-2. Human Health Effects, pp 29–36
Gaur AS, Gaur SS (2009) Statistical methods for practice and research: a guide to data analysis using SPSS. SAGE Publications India Pvt. Ltd, New Delhi
Gooneratne I, Ranaweera A, Liyanarachchi N, Gunawardane N, Lanerolle R (2008) Epidemiology of chronic kidney disease in a Sri Lankan population. Int J Diabetes Dev Ctries 28:60–64
Green S, Salkind N (2005) Using SPSS for Windows and Macintosh: understanding and analysing data, 4th edn. Prentice-Hall, Upper Saddle River
Gunatilake S, Illangasekera T (2015) Hydro-epidemiology of chronic kidney disease (CKD) in Sri Lanka and its similarities to the CKD epidemic in Meso-America. In: American Geophysical Union Fall Meeting, 14–18 December, San Francisco, CA
Herath K, Ileperuma O, Dharmagunawardhane H, Haller KJ (2005) Environmental health risk for the chronic renal failure in Sri Lanka. In: 31st congress on science and technology, Suranree University of Technology, Thailand
Kawakami T, Weragoda SKT, Serikawa, Motoyama AY (2012) Contamination of arsenic in well water and rice in Sri Lanka. In: Ileperuma OA, Priyantha N, Navaratne A, Yatigammana SK, Weragoda SKT (eds) International symposium on water quality and human health: challenges ahead. PGIS, University of Peradeniya, Sri Lanka
Ileperuma OA (2012) Water quality and disease: chemical amplification of pollutants. In: Ileperuma OA, Priyantha N, Navaratne A, Yatigammana SK, Weragoda SK (eds) Water quality and human health: challenges ahead. University of Peradeniya, Sri Lanka. Postgraduate Institute of Science
Jayasekara KB, Dissanayake DM, Sivakanesan R, Ranasinghe A, Karunarathna RH, Priyantha Kumara GWG (2015) Epidemiology of chronic kidney disease, with special emphasis on chronic kidney disease of uncertain etiology, in the North Central Region of Sri Lanka. J Epidemiol 25(4):275–280
Jayasumana C, Gunatilake S, Senanayake P (2014) Glyphosate, hard water and nephrotoxic metals: are they the culprits behind the epidemic of chronic kidney disease of unknown etiology in Sri Lanka? Int J Environ Res Public Health 11(2):2125–2147
Jayasumana C, Orantes C, Herrera R, Almaguer M, Lopez L, Silva LC, Ordunez P, Siribaddana S, Gunatilake S, De Broe ME (2017) Chronic interstitial nephritis in agricultural communities: a worldwide epidemic with social, occupational and environmental determinants. Nephrol Dial Transplant 32(2):234–241
Jayatilake N, Mendis S, Maheepala P, Mehta FR (2013) Chronic kidney disease of uncertain aetiology: prevalence and causative factors in a developing country. BMC Nephrol 14:180
Jayawardana DT, Pitawala HMTGA, Ishiga H (2010) Geological evidences from soil and water leading to chronic renal failure of unknown etiology in dry zone Sri Lanka. In: Water quality and human health: challenges ahead. University of Peradeniya, Sri Lanka. Postgraduate Institute of Science
Jayawardana DT, Pitawala HMTGA, Ishiga H (2012) Groundwater quality in different climatic zones of Sri Lanka: focus on the occurrence of fluoride. Int J of Environ Sci Dev 1(3):244
Kaiser HF (1974) An index of factorial simplicity. Psychometrika 39(1):31–36
Karunaratne N (1983) Some Problems encountered in groundwater exploration in Anuradhapura district. Hydrogeological Workshop on Groundwater Resources. Institute of Geological Sciences and the Water Resources Board, Sri Lanka, 14–23 March, Sri Lanka
Lantz O, Jouvin M, De Vernejoul M, Druet P (1987) Fluoride-induced chronic renal failure. Am J Kidney Dis 10(2):136–138
Liyanage HM, Magana-Arachchi DN (2012) Molecular identification of cylindrospermopsin producing cylindrospermopsis raciborskii from anuradhapura water reservoirs. In: Water quality and human health: challenges ahead. University of Peradeniya, Sri Lanka. Postgraduate Institute of Science
Navas-Acien A, Tellez-Plaza M, Guallar E, Muntner P, Silbergeld E, Jaar B, Weaver V (2009) Blood cadmium and lead and Chronic Kidney Disease of unknown etiology in US adults: a joint analysis. Am J Epidemiol 170(9):1156–1164
Orantes-Navarro C, Herrera-Valdés R, Almaguer-López M, López-Marín L, Vela Parada X, Hernandez-Cuchillas M, Barba LM (2017) Toward a comprehensive hypothesis of chronic interstitial nephritis in agricultural communities. Adv Chronic Kidney Dis 24(2):101
Ozsvath DL (2006) Fluoride concentrations in a crystalline bedrock aquifer Marathon County Wisconsin. Environ Geol 50:132–138
Padmasiri JP, Jayawardhene WM, Dissanayake CB (2012) Water quality improvements in the dry zone areas in Sri Lanka. In: Proceedings of the international symposium on challenges ahead: water quality and human health. University of Peradeniya, Sri Lanka. Postgraduate Institute of Science
Panabokke CR, Ariyaratne BR (2008) A diagnostic study of the micro-variability in groundwater fluoride content in the hard rock landscape of the dry zone. In: Proceedings of Workshop on Groundwater in Sri Lanka: a most precious but highly threatened resource. National Academy of Sciences, Sri Lanka
Perera APGRL, Gonawela JKL, Wijekoon D (2008) Groundwater quality in Anuradhapura district with special reference to fluoride. In: Proceedings of workshop on groundwater in Sri Lanka: a most precious but highly threatened resource. National Academy of Sciences, Sri Lanka
Pethiyagoda U (2011) NCP kidney disease—a call for urgent action, The Island, Sri Lanka, 5 Jan 2011
Poulter NR, Mendis S (2009) Finalized multidisciplinary research proposal on Chronic Kidney Disease of unknown etiology of uncertain aetiology (CKDUu) in Sri Lanka. WHO Country office, Colombo
Ramseyer WF, Smith C, McCay C (1957) Effect of sodium fluoride administration on body changes in old rats. J Gerontol 12:14–19
Schiffl H (2018) Fluoridation of drinking water and chronic kidney disease: absence of evidence is not evidence of absence. Nephrol Dial Transplant 23(1):411
Wasana HMS, Aluthpatabendi D, Bandara J (2012) Drinking water quality assessment towards “Chronic Kidney Disease of unknown etiology of unknown aetiology (CKDUu)” in North Central province (NCP) of Sri Lanka. In: Water quality and human health: challenges ahead, University of Peradeniya, Sri Lanka. Postgraduate Institute of Science
WHO, World Health Organization (1992) Environmental Health Criteria 134: Cadmium Environmental Health Criteria, Geneva
WHO, World Health Organization (2006) Guidelines for drinking-water quality, 2nd edn. WHO, Geneva
WHO, World Health Organization (2009) Guidelines for drinking-water quality policies and procedures. Public Health and the Environment, Geneva
WHO, World Health Organization (2012) Investigation and evaluation of Chronic Kidney Disease of unknown etiology of uncertain aetiology in Sri Lanka, Final Report. Ministry of Health (MoH)
Young SM, Pitawala A, Ishiga H (2011) Factors controlling fluoride contents of groundwater in north-central and northwestern Sri Lanka. Environ Earth Sci 63:1333–1342
Acknowledgements
I would like to acknowledge Prof C.B.Dissanayaka, Prof. Rohana Chandrajith, Prof. W.A. Abeywickrema and Mr. V.A. Waduge for the immense support given in this research by providing data, assisting with data collection and research advice.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Paranagama, D.G.A., Bhuiyan, M.A. & Jayasuriya, N. Factors associated with Chronic Kidney Disease of unknown aetiology (CKDu) in North Central Province of Sri Lanka: a comparative analysis of drinking water samples. Appl Water Sci 8, 151 (2018). https://doi.org/10.1007/s13201-018-0792-9
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s13201-018-0792-9