Abstract
Mineral dissolution reactions actively participate in controlling the composition of mineral water. In this study, water soluble, acidic–alkaline and carbonated solution experiments were designed, and mineral reaction mechanisms were researched using chemical kinetics and the minimum free-energy method. The results showed that the release of metasilicate was controlled by pH, CO2, and rock characteristics. In the water soluble experiment, the release process of metasilicate in powdered rocks reached equilibrium after 40 days, while metasilicate in solid rocks took 170 days. The release process of metasilicate in solid rocks satisfied an asymptotic model, while in powdered rocks it accorded with the Stanford reaction kinetic model. In the acidic–alkaline experiment, metasilicate was released earlier under acidic conditions (2.46 < pH < 7) than under alkaline conditions (7 < pH < 10.61). The release process of metasilicate under acidic conditions reached equilibrium in 40 days, compared with 60 days for alkaline conditions. The addition of CO2 to the water solution was beneficial to the formation of metasilicate. Under neutral pH conditions, the reaction barely occurred. Under alkaline conditions, metasilicate was produced by the hydrolysis of metasilicate minerals. Under acidic and additional CO2 conditions, metasilicate formation was mainly via the reaction of H+, CO2, and metasilicate minerals. From these results, we concluded that the metasilicate mineral water from the Changbai Mountains, Jingyu County, is generated by a combination of the hydrolysis of metasilicate minerals and the reaction of H+, CO2, and metasilicate minerals. These results can contribute to a better development and protection of the mineral water resources in the Changbai Mountains.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Formation and distribution of mineral waters is under the control of active faulted structure and lava rocks commonly, and the characteristic elements mainly come from water–rock interaction (Zhang 2001; Bahri and Saibi 2012). Many studies have been conducted on chemical composition of mineral water in relation with rock materials. Most of these studies focused on mica minerals and carbonate rocks; little is known about the mechanism of metasilicate mineral dissolution in mineral water in basalt area.
Key factors influencing water–rock interaction include temperature, pH, pressure, catalysis, and rock characteristics (Gérard et al. 2002; Oelkers et al. 2008; Gysi and Stefánsson 2011; Pachana et al. 2012). Gérard et al. (2002) investigated the relationships between Si concentration, soil temperature, and H+ concentration, and concluded that silica concentration was controlled by soil temperature and H+ concentration. Oelkers et al. (2008) gave an overview on the steady-state muscovite dissolution rates at temperatures from 60 to 201 °C and 1 ≤ pH ≤ 10.3 as a function of reactive solution K, Si, and Al concentration. The findings of Pachana et al. (2012) in analyzing the dissolution generates etch in the surface of biotite and muscovite basal at different pH values (pH 1.1, 3.3, and 5.7) and different temperatures (T = 2.5, 120, and 200 °C) were very important, as he concluded that dissolution regime changes with pH values and temperatures. Skopljak and Vlahović (2012) wrote about the genesis of mineral waters in Kiseljak surroundings, and pointed out that the water origin from atmospheric and water descends in the primary aquifer where it becomes enriched with CO2 and minerals. The source of elements in mineral water mainly generated in the reaction with groundwater and surrounding rocks (Shen and Zhou 1987; Zhou 1992; Ai et al. 1998; Zhang et al. 1999). Shen and Zhou (1987) studied that the hydrogeochemical mechanism of mineral water was formed containing silicon by the chemical thermodynamic in Huaying mountain, and came up with the reason of soluble silica in the groundwater. Zhou (1992) studied the chemical causes of natural drinking mineral water containing silicon of Wolong, because of feldspar quartz sandstone, microcline, and albite dissolutions, containing silicon and calcium, which produced silicon and Strontium. Dang and Hou (1995) researched the dissolution mechanism of basalt in acidic and alkaline solution at closed system with normal temperature and pressure, and acknowledged that the pH value of solution eventually became neutral. Ai et al. (1998) analyzed the trend of mineral dissolution and precipitation of the interaction process of groundwater and basalt in Xuyi using the saturation index of the groundwater components by establishing water chemical equilibrium reaction model. Zhang et al. (1999) discussed the sources of soluble silicon, strontium, lithium, zinc, selenium which were mainly produced by water–rock reaction in the groundwater.
Although there are many researches about the origin of the mineral water elements, the mechanism of metasilicate mineral water in the basalt area of JingYu County and the effects of pH and CO2 on water–rock interaction are not clear. The formation of metasilicate mineral requires about 40 years by tritium isotope (Yan et al. 2015), which is a long time for human, and the flow discharges of the springs were reducing. So detecting the formation mechanism of mineral water and making appropriate measures to protect it are crucial. Rocks of JingYu County were sampled as experimental materials. Water soluble, acidic–alkaline, carbonated solution experiments were designed, and studied the influences of pH and CO2 on the formation mechanism of metasilicate mineral water through the simulation test in door. This paper was also completed on the basis of field investigated and the results of experiments in door.
Study area
Jingyu County, covering a total land area of 3094.4 km2, is located in the southeast of Jilin Province, northeast China. The area is characterized by a temperate continental monsoon climate, with an annual average temperature of 3.7 °C and an annual average precipitation of 749.7 mm, much of which occurs between June and September. Mineral water is produced by 18 springs located in a 15306-ha protection zone in the Changbai Mountain (Fig. 1), southwest Jingyu. Among the 18 springs, Feilong, Jiulong, and Wulong have maximum flow discharge.
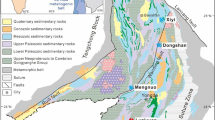
The study area is within Changbai Mountain. It is located on the basalt platform of the Quaternary Pleistocene, surrounded by the volcanic cluster of the Longgang Region, which had an olivine basalt lithology (Fig. 2). The study area is at the point where the latitudinal tectonic system and Neocathaysian structural system joint, existing east–west break (XingHua-Mount Paektu break), northwest break, and northeast break (JiAn-Songjiang lithosphere break) meet. The neotectonic movement in the region has strong drape faults that are well developed, and there are extensive basalt outcrops. The basalt varies from 120 to 15 m thick, and the degree of the fracture also declines, from the middle of the platform to the edge of the protected area, which has resulted in a reduction in the groundwater recharge capacity and the quantity of water in the aquifer. Based on the groundwater storage conditions, physical properties of water and the hydrological and hydraulic characteristics, the groundwater in the study area can be divided into pore water in loose beds, basalt pore-fissured water, and granite fissure water.
Experiments and methods
Rock materials
The test rock samples were mainly collected from Jingyu County, including Baijiang, Sihailongwan, Feilong, and Wangdashan springs as well as experimental well. The mineral compositions of rock consisted of forsterite and plagioclase, and matrix was composed of plagioclase, magnetite, and pyroxene. Table 1 shows the chemical compositions of the rocks. There are no specific permissions required for the study area (Jingyu County, Changbai Mountain), because it is just a scientific research and would help the local people. Meanwhile, the field studies did not involve endangered or protected species.
Water soluble experiment
The materials of water soluble experiment contain powdered rocks and solid rocks. Powdered rocks with particle size of 200 meshes were sampled from four springs above and experimental well, while solid rocks with 1.50 × 0.35 × 1.40 cm3 were sampled from experimental well. All of the experimental rock samples were placed into deionized water with the water–rock ratio of 5:1 in a sealed container with initial pH value of 7.1. Measurements were made daily during the first week and weekly from 2 to 30 weeks. After reaction, the solution from each container was collected using a syringe and filtered through a 0.2-μm pore-size minisart filter. Sampled solutions were divided into two aliquots: one for SiO2 analysis and the other for pH analysis.
Acidic–alkaline experiment
The materials of acidic–alkaline experiment were powdered rocks with particle size of 200 meshes from four springs above and experimental well. All of the experimental rock samples were placed into deionized water with the water–rock ratio of 5:1 in a sealed container at varied initial pH values of 2.46 and 10.61. Measurements were made daily during the first week and weekly from 2 to 30 weeks. After reaction, the solution from each container was collected using a syringe and filtered through a 0.2-μm pore-size Minisart filter. Sampled solutions were divided into two aliquots: one for SiO2 analysis and the other for pH analysis.
Carbonated solution experiment
The materials of acidic–alkaline experiment were powdered rocks with particle size of 200 meshes from Wangdashan spring. Rock samples were placed into deionized water with the water–rock ratio of 5:1 in a container at varied initial pH values of 3.20, 7.64, and 11.48. CO2 was injected into the container at a rate of 0.05–0.1 m3/min for 5 min every other day. Measurements were taken daily during the first week and weekly from 2 to 10 weeks. After reaction, the solution from each container was collected using a syringe and filtered through a 0.2-μm pore-size minisart filter. Sampled solutions were divided into two aliquots: one for SiO2 analysis and the other for pH analysis.
Chemical kinetic analysis
Using Stanford reaction kinetic model (Stanford and Smith 1972; Zhang et al. 2012) to simulate the release process of metasilicate with powder samples of the water soluble experiment and carbonated solution experiment (initial pH acidic), cumulative release was estimated by Eq. 1.
where M t (mg/kg) is the metasilicate cumulative release; M 0 (mg/kg) is the potential release of metasilicate; k m (days−1) is release rate constant; t (days) is the reaction time.
Using asymptotic model (Knauss and Wolery 1986) to simulate the process of metasilicate with solid samples of the water soluble experiment, cumulative release was estimated by Eq. 2.
where M t (mg/kg) is the metasilicate cumulative release; k m (days−1) and k d (days−1) are release rate constants; t (days) is the reaction time.
Using Boltzmann reaction kinetic model (Yan and Zhang 2008; Liu and Zhou 2009) to simulate the release process of metasilicate of the carbonated solution experiment (initial pH neutral and alkaline), cumulative release was estimated by Eq. 3.
where M t (mg/kg) is the metasilicate cumulative release; A 0, A 1, and A 2 (mg/kg) are release rate constants; t (d) is the reaction time.
Minimum free-energy method
Chemical reaction follows mass conservation principle, charge conservation principle, and electronic balance principle. The smaller the free-energy consumption, the chemical reaction is more prone. The mineral reaction linear programming model was established by the lowest energy principle and chemical thermodynamics principle using water samples information balanced of wangdashan spring of the water soluble experiment, pH alkaline experiment, and carbonated experiment (initial pH acidic). The objective function (4) is the lowest free-energy change ∆G of the chemical reactions (He et al. 2008; Liu et al. 2010; Hareeparsad et al. 2011).
where X (J/L) is the free energy consumed of chemical reaction; ∆G ri is the free-energy change of i chemical reaction (J/mol); X i is the free energy consumed of i chemical reaction (mol/L); n is the total number of reactions.
Results
pH, metasilicate, and chemical kenetic process in water solution
All sample solutions showed a similar trend of pH with time (Fig. 3). In the powdered rocks, it was found that pH rose during the first 25 days, reduced afterward to day 52, and then increased again afterward and remained weak alkaline after 71 days. The pH of the solid rocks changed lag than in powdered rocks, pH increased in the first 40 days, and reduced between 40 and 63 days, and later pH tended to neutral.
All sample solutions showed a similar trend of metasilicate with an initial rise within the first 25–40 days and steady-state afterward, but at different magnitudes (Fig. 4). In the powdered rocks, it was found that the metasilicate cumulative releases increased quickly in the first 25 days of the investigation. On the reaction of 40th day, the reaction of Baijiang spring and Feilong spring reached equilibrium while others at the reaction on the 60th day. The maximum of the cumulative releases of metasilicate were different on the equilibrium solution as the samples rock varied. In the solid rocks, the cumulative release of metasilicate increased slowly, and the reaction reached equilibrium after the 160th day. The maximum of the cumulative release of metasilicate at the equilibrium reaction with solid sample was less than powdered sample.
The release process of metasilicate of solid rocks satisfied asymptotic model while that of the powdered rocks accorded with Stanford reaction kinetic model. Equation 5 shows the asymptotic model and Table 2 shows the parameters of Stanford model.
pH and metasilicate in acidic and alkaline solutions
All sample solutions showed a similar trend of pH with time (Fig. 5). In the acidic solution, the initial pH value was 2.46, and then increased rapidly from 2.46 to above 7.00 in the first 20 days in the reaction of Baijiang spring and Feilong spring, and declined between 20 and 30 days, then increased again and tended to neutral finally (Figs. 6, 7). In the alkaline solution, the initial pH value was 10.61, and then reduced rapidly from 10.61 to about 8.00, and tended to neutral finally.
All sample solutions showed a similar trend of metasilicate with an initial rise within the first 40–60 days and steady-state afterward, but at different magnitudes (Figs. 7, 8). For Sihailongwan spring, the metasilicate cumulative releases reached maximum value (133.18 mg/kg) at 40th days in acidic experiment, while the time of maximum value (90.75 mg/kg) at 60th days in alkaline experiment.
The release process of metasilicate of the acidic–alkaline experiment complies with Stanford reaction kinetic mode. Tables 3 and 4 show the kinetic model parameters.
pH and metasilicate in carbonated solution
In carbonated solution, pH value tended to faintly acid (Fig. 9). Initial pH alkaline and neutral solutions showed a similar trend of metasilicate. Metasilicate of solution was slowly increased within 7 days, and then increased quickly between 7 and 42 days. Metasilicate was increased quickly at beginning of reaction (Fig. 10).
The release process of metasilicate of the carbonated solution (initial pH neutral or alkaline) complied with Boltzmann reaction kinetic model (6, 7), while the initial pH acidic complied with Stanford reaction kinetic model (8).
Mineral reaction mechanism
Table 5 shows the chemical reactions that may occur in the water soluble experiment and ∆G of the reactions with the constraints was (9). Table 6 shows the ion concentration of the initial solution and balanced solution of the water soluble experiment. Table 7 shows the optimal feasible solution.
Table 8 shows the physical and chemical reactions that may occur in the alkaline pH experiment and ∆G of the reactions with the constraints was (10). Table 9 shows the ion concentration of the initial solution and balanced solution of the alkaline pH experiment. Table 10 shows the optimal feasible solution.
Table 11 shows the physical and chemical reactions that may occur in the carbonated solution experiment (initial pH acidic) and ∆G of the reactions with the constraints was (11). Table 12 shows the ion concentration of the initial solution and balanced solution of the carbonated solution experiment (initial pH acidic). Table 13 shows the optimal feasible solution.
Discussions
The pH of the solution, catalyst, samples state generally have the great impact on chemical reaction (Christoskova and Stoyanova 2009), and when varying these factors our experimental results showed different magnitudes for metasilicate release. In the water soluble experiment, the pH of the reaction solution increased to weakly alkaline because the basalt rock samples were alkaline (Chen and Zhang 1994). In the alkaline solution, H2O and OH− provide a strong base, while strongly acidic mineral elements are also present in basalt, such as Al3+, based on Pearson’s hard and soft acid–base theory (HSAB) (Pearson 1963). H2O and OH− combined with metal cation with basalt sample forming hydrolyzate or hydroxide, and SiO2 combined with alkali forming silicate and metasilicate according to the “hard pro hard” law (Wisniewski 2006). Plagioclase, olivine, and pyroxene reacted with H2O-generated silicate and metasilicate, but also produced precipitates that hinder the reaction’s progress by covering the mineral surface. In the acidic solution, the dissolution reaction of silicate minerals can be summarized by Eq. 12, and the rate of the dissolution reaction of silicate minerals increases with the concentration of H+ (Helgeson 1970; Yu 1994).
In deionized water with neutral pH, the solubility of the silicate mineral is extremely low, but with the addition of the catalyst (CO2), a large amount of H+ is produced by CO2 and H2O, making the reaction solution acidic. Thus, in the carbonated solution experiments with an initially neutral or alkaline pH, a change was found after 7 days when the pH of reaction solution reduced to acidic.
In the water soluble experiment, the release process of metasilicate for the powdered sample matched the Stanford reaction kinetic model, while the process of metasilicate release for the solid sample followed an asymptotic model. In the powdered sample experiments, rock particles and the reaction solution have full contact, leading to an increased reaction rate and to the reaction reaching equilibrium faster. In the acidic–alkaline experiment, the release process of metasilicate for the powdered samples all matched the Stanford reaction kinetic model, but the reaction mechanisms differed. For alkaline experiments (7.00 < pH < 10.61), metasilicate formation is produced by the hydrolysis of SiO2, plagioclase, olivine, and pyroxene, but the secondary minerals also inhibit the reaction by covering the surface of the rock samples. For acidic (2.46 < pH < 7.00) experiments, metasilicate formation is predominantly from the reaction of H+ and SiO2, plagioclase, olivine, and pyroxene. The addition of CO2 can adjust the pH of the solution (Aiman and Al 2010). In the carbonated solution experiment, the release process of metasilicate with the initially neutral or alkaline solutions complied with the Boltzmann reaction kinetic model, while initially acidic solutions complied with the Stanford reaction kinetic model. For the carbonated experiment, metasilicate formation is mainly from the reaction of CO2 and H2O, plagioclase, olivine, and pyroxene. Since CO2 reacted as an aqueous solution, its products were H2CO3 with a neutral or alkaline pH, meaning the rate of metasilicate release is relatively small initially, until the pH of the reaction solution becomes acidic.
The pH value of precipitation of Jingyu was faintly acid. Rainfall infiltrated into groundwater through humus layer, causing pH of groundwater to reduce. In the exposed bedrock area, the pH of groundwater was found to be alkaline. So the metasilicate mineral water of Jingyu was generated by the hydrolysis of metasilicate mineral and the reaction of H+, CO2, and metasilicate mineral together.
Conclusions
The study presents the impact of the pH and CO2 on the formation of Metasilicate mineral water in Changbai Mountain, Northeast China by experimental methods, chemical kinetic analysis, and minimum free-energy method. The general conclusions can be drawn from this study.
-
(1)
The laws of the metasilicate release process all increased firstly and then reached equilibrium state. pH values of the reaction solutions become essentially neutral.
-
(2)
The release process of metasilicate of solid rocks was slower than that of powdered rocks in the same experimental conditions; and the release process of metasilicate of solid rocks satisfied asymptotic model while that of powdered rocks fit in with Stanford reaction kinetic model.
-
(3)
It was earlier to forming metasilicate in the condition of acidic pH (2.46 < pH < 7.00) than in the condition of alkaline pH (7.00 < pH < 10.61), as the release process of metasilicate fits in with Stanford reaction kinetic model. Addition of CO2 was beneficial to the formation of metasilicate, and the release process of metasilciate accorded with Boltzmann equation in the condition of neutral and alkaline pH.
-
(4)
In the condition of neutral pH, metasilicate formation was mainly from the hydrolysis of SiO2, but the reaction was difficult. In the condition of alkaline pH (7.00 < pH < 10.61), metasilicate formation was produced from the hydrolysis of SiO2, Plagioclase, Olivine, and Pyroxene; but the secondary mineral also inhibit the reaction covering the surface of rock samples. In the condition of acidic pH (2.46 < pH < 7.00), metasilicate formation was mainly from the reaction of H+ and SiO2, Plagioclase, Olivine, and Pyroxene. In the condition of CO2 catalytic, metasilicate formation was mainly from the reaction of CO2, H2O, Plagioclase, Olivine, and Pyroxene.
-
(5)
The metasilicate mineral water of Jingyu was generated by the hydrolysis of metasilicate mineral and the reaction of H+, CO2, and metasilicate mineral together.
References
Ai Y, Gao M, Dai SH (1998) The trend analysis of groundwater and basalt reaction. Geol J China Univ 4:220–222
Aiman E, Al R (2010) CaCO3-CO2-H2O system in falm on a bank of horizontal tubes: model verification. J Ind Eng Chem 16:1050–1058
Bahri F, Saibi H (2012) Characterization, classification, bacteriological and evaluation of groundwater from 24 wells in six departments of Algeria. Arab J Geosci 5:1449–1458
Chen ZD, Zhang BZ (1994) Basic characteristics of groundwater in Basalt in the Changbai Mountain area. J Fuxin Min Inst (Nat Sci) 13:26–29
Christoskova ST, Stoyanova M (2009) Catalytic oxidation of cyanides in an aqueous phase over individual and manganese-modified cobalt oxide systems. J Hazard Mater 165:690–695
Dang Z, Hou Y (1995) Experimental study on the dissolution kinetics of basalt-water interaction. Acta petrological sinica 11:10–12
Gérard F, François M, Ranger J (2002) Processes controlling silica concentration in leaching and capillary soil solutions of an acidic brown forest soil. Geoderma 107:197–226
Gysi AP, Stefánsson A (2011) CO2-water-basalt interaction. Numerical simulation of low temperature CO2 sequestration into basalts. Geochim Cosmochim Acta 75:4728–4751
Hareeparsad S, Barna LT, Brouckaert CJ, Buckley CA (2011) Quantitative geochemical modelling using leaching tests: application for coal ashes produced by two South African thermal processes. J Hazard Mater 186:1163–1173
He L, Zhou CZ, Xie YK, Gao YF (2008) Water and Rock reaction model solution by the excel programming. Chin J Undergr Space Eng 4:265–268
Helgeson (1970) Reaction rates in hydrothermal systems. Geochim.et Cosmochim Acta 34:299–303
Knauss KG, Wolery TJ (1986) Dependence of albite dissolution kinetics of pH and time at 25°C and 70°C. Geochim Comochim Acta 50:2481–2497
Liu HF, Zhou GJ (2009) Lattice Boltzmann model for shallow water flows in curved and meandering channels. Int J Comput Fluid Dyn 23:209–220
Liu F, Mou WJ, Sun XS, Zhang GP (2010) Application of MATLAB to inverse Geochemical Simulation. J Water Res Water Eng 21:151–153
Oelkers EH, Schott J, Gauthier JM, Herero-Roncal T (2008) An experimental study of the dissolution mechanism and rates of muscovite. Geochim Cosmochim Acta 72:4948–4961
Pachana K, Zuddas P, Censi P (2012) Influence of pH and temperature on the early stage of mica alteration. Appl Geochem 27:1738–1744
Pearson RGJ (1963). Am Chem Soc 85:3533–3539
Shen ZA, Zhou JM (1987) The hydrogeochemical mechanism of mineral water formed in Huayingshan nine holes. Hydrogeol Eng Geol 4:13–17
Skopljak F, Vlahović T (2012) The origin of mineral waters in Kiseljak near Sarajevo, Bosnia and Herzegovina. Environ Earth Sci 66:809–822
Stanford G, Smith SJ (1972) Nitrogen Mineralization Potentials of Soils. Soil Sci Soc Am J 36:465–472
Wisniewski M (2006) Pearson’s hard-soft acid-base principle as a means of interpreting the reactivity of caron materials. Adsorpt Sci Technol 24:389–402
Yan GW, Zhang JY (2008) A multi-entropy-level lattice Boltzmann model for the one-dimensional compressible Euler equations. Int J Comput Fluid Dyn 22:383–392
Yan BZ, Xiao CL, Liang XJ, Wei RC, Wu SL (2015) Characteristics and genesis of mineral water from Changbai Mountain, Northeast China. Environ Earth Sci 73:4819–4829
Yu CW (1994) Mineralization dynamics. Earth Sci Front (China University of Geosciences) 1:54–73
Zhang BF (2001) The formation distribution characteristics and development strategy recommendations of mineral water orefiled in JingYu Changbai mountain. Jilin Geol 20:59–61
Zhang NL, Li JP, Li SZ (1999) The analysis of beneficial elements of mineral water in ShanXi. Res Dev Mark 15:177–178
Zhang XF, Li H, Hong M (2012) Effects of Saline-Alkali Soil on Nitrogen Transformations. J Jilin Univ (Earth Sci Ed) 42:1145–1150
Zhou JM (1992) The chemical genesis and development of natural silicon mineral water. Res Dev Conserv 8:135–136
Acknowledgments
This work was financially supported by the “11th Five-Year Plan” science and technology project of China (2006BAB04A09-02, 2007BAB28B04-03) and by the Science and Technology Key Research of Jilin Province (20100452).
Conflict of interest
The authors declare no competing financial interests.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Yan, B., Xiao, C., Liang, X. et al. Influences of pH and CO2 on the formation of Metasilicate mineral water in Changbai Mountain, Northeast China. Appl Water Sci 7, 1657–1667 (2017). https://doi.org/10.1007/s13201-015-0317-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13201-015-0317-8