Abstract
Objective
To explore the DPP4 expression changes and functions in ovarian cancer (OV), as well as the regulation mechanism for DDP4.
Methods
GEPIA2, GSE18520, GSE26712 and UALCAN were used to analyze differences in DPP4 expression between OV tumors and control tissues. Serum DPP4 levels were measured by ELISA. The prognostic values of DPP4 were evaluated using a Kaplan–Meier (KM) plotter. Small interfering RNA was used for DPP4 knockdown in OVCAR-3 and SKOV-3 cells. CCK-8 and scratch healing assays were used to determine the cells’ proliferation and migration abilities. Flow cytometry (FCM) was used to detect the cell cycle and apoptosis. A dual-luciferase assay was designed to confirm the regulatory effect of miR-29a-3p on DPP4.
Results
The expressions of DPP4 mRNA and protein were decreased in OV tumor tissues. Serum DPP4 levels decreased in OV patients. KM plotter analysis showed correlation between high DPP4 expression and a poor prognosis in OV patients. By targeting knockdown of DPP4, we found that OVCAR-3 and SKOV-3 cells’ proliferation was inhibited, while cell’s migration ability was significantly promoted. FCM analysis showed that DPP4 knockdown induced a decrease in the S phase. Furthermore, DPP4 was shown to be downregulated by miR-29a-3p and TGFβ1 in OVCAR-3 cells, and miR-29a-3p expression was upregulated by TGFβ1. The effects of miR-29a-3p and TGFβ1 on OVCAR-3 cells’ biological behaviors were consistent with DPP4 knockdown.
Conclusion
DPP4 was downregulated in OV patients. DPP4 knockdown significantly inhibited OVCAR-3 and SKOV-3 cell proliferation and promoted cell migration. DDP4 can be downregulated by TGFβ1 through the upregulation of miR-29a-3p in OV cells.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Ovarian cancer (OV) is a common malignant tumor of the female reproductive system. Globally, the incidence and mortality rates of OV were 3.4% and 4.7% respectively [1]. The 5-year survival rate has remained around 40% for the past 30 years [2]. The mechanism of OV occurrence and development remains unclear. Additionally, issues such as drug resistance, recurrence and metastasis remain problems for clinical OV therapy. Thus, there is an urgent need to identify novel molecules that are involved in OV development revealing the mechanism and improving the clinical therapeutic effects.
Dipeptidyl peptidase 4 (DPP4, also known as CD26) is an intrinsic type II transmembrane glycoprotein and a member of the serine protease family. DPP4 exists in both cell membrane-anchored and soluble forms, occurring in the serum [3]. DPP4 cleaves X-proline dipeptides from the N-terminus of polypeptides and has a diverse range of substrates, including incretins, chemokines, growth factors and neuropeptides [4]. DPP4 has been associated with multiple biological progresses [5,6,7,8]. Previous studies showed that DPP4 expression levels were significantly altered in tumors and might play a vital role in cancer development [9, 10]. In this study, we analyzed the expression changes and potential prognostic value of DPP4 in OV. Furthermore, we investigated the effect of DPP4 knockdown on the biological behavior OV cancer cells. Moreover, we explore potential mechanisms involved in DDP4 expression regulation. This study may present evidence for DPP4 involvement in OV progression.
2 Materials and methods
2.1 Differential expression analysis of DPP4
Based on TCGA and GTEx database, GEPIA2 (http://gepia2.cancer-pku.cn) bioinformatic tool was used to analyze the change of DPP4 mRNA expression in 426 OV tumor tissues compared with 88 control non-tumor tissues. Select Cutoff as |Log2FC|= 0.5. GSE18520 and GSE26712 datasets were used to verify the results from GEPIA2. UALCAN (http://ualcan.path.uab.edu/) was used to analyze the DDP4 protein expression in OV based on the CPTAC (Clinical Proteomic Tumor Analysis Consortium) database. The basic information of GSE18520 and GSE26712 were list in Table 1.
2.2 Serum DPP4 levels detection by ELISA assay
Ethical approval was obtained from the Ethics Committee of Tangdu Hospital, Fourth Military Medical University. Informed consent was exempted from this study. Serum from 24 OV patients including16 with high-grade serous ovarian cancer (HGSOC, age range: 47–76 years) and 8 with other forms of OV (age range: 45–68 years). Additionally, serum from 23 healthy subjects (age range: 42–75 years) was collected. Peripheral blood was collected and centrifuged at 4000 rpm and 4 °C for 5 min to obtain serum. The serum samples were stored at − 80 °C. Serum DPP4 levels were detected using an ELISA kit according to the operation manual (R&D Systems, DY1180). In short, the capture antibody was coated in a 96-well plate and incubated overnight at room temperature. After blocking for 2 h, serum samples were added to the plate and incubated for 2 h. Then, streptavidin-conjugated detection antibody was added and incubated for 2 h. Then streptavidin-HRP antibody was added and incubated for 20 min. Finally, the TMB substrate was added, and the optical density (OD450nm) was measured. Serum DPP4 levels were calculated according to the standard curves.
2.3 Kaplan–Meier (KM) plotter analysis
The KM plotter bioinformatic tool (http://www.kmplot.com/analysis/index.php?p=service) [11] was used to evaluate the potential prognostic value of DPP4 expression in OV patients. The best performing cutoff value was computed by using “auto select best cutoff” model. The source data was based on TCGA and GEO datasets (GSE18520, GSE26712).
2.4 Cell culture
Human ovarian cancer cell lines, OVCAR-3 and SKOV-3, were purchased from Procell Life Science &Technology Co., Ltd (Wuhan, China). OVCAR-3 and SKOV-3 were used to investigate the effect of DPP4 on the cells’ biological behavior. OVCAR-3 was cultured with RPMI-1640 (Gibco, Carlsbad, NY, USA) supplemented with 20% Fetal Bovine Serum fetal bovine serum (ExCell Bio, Shanghai, China). SKOV-3 was cultured with Dulbecco’s Modified Eagle Medium (Gibco) plus 10% Fetal Bovine Serum (ExCell Bio). The cells were cultured in a 5% CO2 incubator at 37 °C.
2.5 Small-interfering RNA (siRNA) and miR-29a-3p transfection
siRNA (Sangon Biotech, Shanghai, China) was used to knock down the expression of DPP4. miR-29a-3p (Sangon Biotech) was used to investigate the effect of miR-29a-3p on DPP4 expression. Approximately 1 × 105 cells were seeded in 6 well plates and cultured for,
24 h Subsequently siRNA was transfected into OVCAR-3 and SKOV3 cells, and miR-29a-3p mimics were transfected into OVCAR-3 cells. The siRNA and miR-29a-3p transfection were carried out using Lipofectamine 2000 (Invitrogen, Carlsbad, CA, USA) according to the manufacturer's instructions. The sequences are listed in Table 2.
2.6 Quantitative RT-PCR and western blot
After 48 h of siRNA or miR-29a-3p mimic transfection, RNA and protein were extracted from the cells. Total RNA was extracted from cells using a TRIzol Reagent Kit (GLPBIO, Cat: GK20008) For mRNA, cDNA was prepared using the Evo M-MLV Mix Kit (Accurate Biology AG) and cDNA for micro-RNA was prepared by using the Mir-X™ miRNA First-strand Synthesis Kit (Takara Bio, Japan). By using the Blastaq™ MasterMix kit (ABM, Canada), the reference genes for mRNA and miRNA were GAPDH and U6, respectively Quantitative real-time RT-PCR was used to detect the DPP4 mRNA and miR-29a-3p expression levels. Real-time PCR was performed on a QIAGEN Rotor-gene Q Real-Time PCR system (QIAGEN, Germany). Relative expression was calculated using the ΔΔCt method. U6 primers and miR29a-3p reverse primers were provided by the Mir-X™ miRNA First-Strand Synthesis Kit mentioned above. The primers for RT-PCR were list in Table 3.
For Western blotting, cell proteins were separated using SDS-PAGE and transferred onto a nitrocellulose membrane. The membranes were blocked for 2–3 h at room temperature, and then incubated overnight at 4 °C with an anti-DPP4 antibody (Cell Signaling Technology, Danvers, MA, USA). After washing with TBST three times, the membranes were incubated with an HRP labeled secondary antibody (ZSGB-BIO, Beijing, China) for 2 h. Proteins were detected using a Western-blotting luminol reagent (4A Biotech Co., Ltd, Beijing, China), GAPDH was used as the internal standard. Protein bands were visualized using a chemiluminescence detection system (Bio-Rad). The relative density of specific protein expression was determined using Image Lab software.
2.7 CCK8 assay
The CCK8 assay was performed to detect cell proliferation according to the manufacturer's instructions (GLPBIO, Cat: GSK10001). First, 2 × 103 cells were seeded in a 96-well plate. second, at different time points during culture (0, 24, 48, 72 h), 10μL of CCK8 reagent was added into each well and the cells were incubated for 2 h at 37 °C. Then, the OD450nm was measured, and the relative cell viability was calculated. as a percentage using the formula: (mean OD450 of treated cells/mean OD450 of control cells) × 100%.
2.8 Flow cytometry (FCM) analysis
Cells were collected 48 h after siRNA transfection for cell cycle and apoptosis detection. FCM was used to examine the cell cycle and apoptosis. For cell cycle analysis, cells were treated by using a cell cycle detection kit (KeyGENBiotech, Nanjing, China) according to the manufacturer's instructions. In brief, cells were fixed for 2 h with ethanol and stained with propidium iodide (PI) in buffer containing 10 μg RNase A. The cells were then kept in the dark at room temperature for 30 min. Cell cycle distribution was assessed using a FACS Calibur flow cytometer (BD Biosciences, San Jose, USA). Cell apoptosis was detected using the annexin V–FITC apoptosis detection kit (4A Biotech Co, Ltd, Beijing, China, Cat: FXP022). Cells were collected, washed twice with PBS and stained with annexin V-FITC and PI in the dark at room temperature for 15 min. The percentage of apoptotic cells was calculated using CellQuest 6.0 (BD Biosciences).
2.9 Cell migration assay
As previously described [12], a scratch assay was used to investigate the cell’s migration ability. In brief, cells were seeded and cultured in a 6-well plate. The scratch was performed when the cell density reached 80–90%. A monolayer of cells was then scratched with a new 200μL pipette tip across the center of the well, and washed gently twice with PBS, Then, cells were cultured for 24 h without serum. The scratch images were captured using a Cytation1 (BioTek, Vermont, USA). The cell migration ratio = (start distant—end distant)/start distant.
2.10 Luciferase reporter assay
To predict the binding sites between DPP4 and miR-29a-3p, TargetScan Human (https://www.targetscan.org/vert_71/) was used. The pGL3-DPP4-wild-type (WT) and pGL3-DPP4-mutant (MUT) were synthesized by Tsingke Biotechnology Co., Ltd (Beijing, China). Then, HEK293 cells were seeded into a 24-well plate and transiently co-transfected with synthesized plasmids (WT [1 µg] or MUT[1 µg]), pRL-TK-Renilla(20 ng), miR-29a-3p mimics(20 pM) or mimics-NC(20 pM) by using Lipofectamine™ 2000 (Invitrogen). After 48 h, the luciferase activities in cells were detected by using the Dual-Luciferase Reporter Assay Kit (Cat No. E1910, Promega, USA) and the Glomax luminescence detector (Promega). The results were expressed as the relative firefly luciferase activity, which is obtained after normalization to Renilla luciferase activity.
2.11 Statistical analysis
Serum DPP4 levels were expressed as the mean ± standard error. Student’s t-test was used to analyze the difference of serum DPP4 levels between OV patients and controls. The data from cell experiments are expressed as the mean ± standard error. Student’s t-test was used to analyze the difference of the cell proliferation rate, cell migration, cell cycle and apoptosis between different groups. All experiments were confirmed in three biological replicates. p < 0.05 was considered to be statistically significant. Statistical analysis was performed using GraphPad Prism 5.01.
3 Results
3.1 DPP4 expression was decreased in OV patients
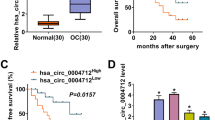
Based on the TCGA and GTEx databases, GEPIA2 analysis showed a decrease in DPP4 mRNA expression in OV tumor tissues (Fig. 1A). The results from the GSE26712 and GSE18520 datasets also showed downregulation of DPP4 expression in OV tumor tissues (Fig. 1B, C). Furthermore, based on the CPTAC database, UCLUC analysis showed a decrease in DPP4 protein expression in OV tumor tissues (Fig. 1D). We further investigated the DPP4 levels in the serum of OV patients. As shown in Fig. 1E, serum DPP4 levels were significantly lower in HGSOC patients than in healthy controls (HCs) (OV: 295 ± 118 ng/ml; HC: 399 ± 141 ng/mL, p = 0.023). There was no significant difference in serum DPP4 levels between HGSOC and other subtypes of OV (Fig. 1F).
Expression difference analysis of DPP4 between OV and controls. A DPP4 mRNA expression was reduced in OV tumor tissues. TPM Transcripts per kilobase of exon model per million mapped reads. B, C Differential DPP4 expression between OV tumor tissues and controls in the GSE26712 and GSE18520 datasets. D The difference in DPP4 protein expression between OV tumor tissues and controls is based on the CPTAC database. E The serum DPP4 levels in OV patients. F The serum DPP4 levels in HGSOC and the other subtypes of OV
3.2 Prognostic value of DPP4 in OV patients
The KM plotter was used to evaluate the relationship between DPP4 expression and prognosis. Based on the TCGA database (Fig. 2A), high DPP4 expression was correlated with poor overall survival (OS) of OV patients (hazards ratio [HR] 1.36; logrank p = 0.018). Based on the GEO database, the GSE18520 datasets showed a significant correlation between high DPP4 expression and poor OS (Fig. 2B), while in the GSE26712 datasets, there was no significant correlation between DPP4 expression and OS (Fig. 2C).
3.3 DPP4 knockdown inhibited proliferation and promoted migration of OV cancer cells
DPP4 mRNA and protein expression were significantly downregulated by siRNA transfection (Fig. 3A, B). By using the CCK8 assay, we found that OVCAR-3 and SKOV-3 cell proliferation was significantly inhibited by DPP4 knockdown (Fig. 3C). FCM analysis showed that there was a significant decrease in DPP4 knockdown cells in the S phase (Fig. 3D). There was no significant effect of DPP4 knockdown on OVCAR-3 and SKOV-3 cell apoptosis (Fig. 3E). Furthermore, a scratch assay was performed to investigate the effect of DPP4 knockdown on the migration of OVCAR-3 and SKOV-3 cells. As show in Fig. 3F, DPP4 knockdown significantly promoted the cell’s migration ability.
The effect of DPP4 knockdown on cell biological behaviors. A, B The inhibitory effect of siDPP4 transfection on DPP4 mRNA and protein expression levels. C DPP4 knockdown inhibited cell proliferation. D DPP4 knockdown resulted in a decrease of the cells in the S phase. E There was no significant effect of DPP4 knockdown on cell apoptosis. F DPP4 knockdown promoted cell migration
3.4 DPP4 was downregulated by TGFβ1/miR-29a-3p
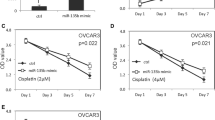
To explore the potential mechanism behind DPP4 downregulated in OV, TargetScan was employed to predict the regulators of DPP4 and miR-29a-3p was predicted to be a potential regulator of DPP4. In OVCAR-3 cells, DDP4 expression was significantly inhibited by miR-29a-3p mimics transfection (Fig. 4A, B). Then, adual-luciferase assay was designed to confirm the regulatory effect of miR-29a-3p on DPP4. The cells cotransfected with miR-29a-3p mimics and DPP4-WT showed significantly lower luciferase activity than those cotransfected with mimics-NC and DPP4-WT (p < 0.01), while cells transfected with DPP4-MUT exhibited no obvious changes in luciferase activity (Fig. 4C). Furthermore, miR-29a-3p mimics inhibited OVCAR-3 cell proliferation and promoted cell migration, which was consistent effects with si-DPP4 (Fig. 4D, E). Notably, miR-29a-3p is known to be a downstream target of TGF-β1[13]. Thus, we investigated the changes in miR-29a-3p and DPP4 expression in response to TGFβ1 stimulation. We found that TGFβ1 treatment significantly promoted miR-29a-3p expression and inhibited DPP4 expression (Fig. 4F, G). Furthermore, TGFβ1 treatment inhibited OVCAR-3 cell proliferation and promoted cell migration (Fig. 4H, I). Taken together, these results indicated that the TGFβ1/miR-29a-3p might be involved in DPP4 downregulation and OV development.
The effect and mechanism of TGFβ1 on DPP4 expression and on cell biologic behaviors. A, B The effect of miR-29a-3p on DPP4 mRNA and protein expression. C Dual-luciferase assay verified the target binding of miR-29a-3p and DPP4. D miR-29a-3p inhibited cell proliferation. E miR-29a-3p promotes cell migration. F miR-29a-3p expression was upregulated by TGFβ1 treatment. G TGFβ1 treatment inhibited DPP4 expression. H TGFβ1 treatment inhibited cell proliferation. I TGFβ1 treatment promoted cell migration
4 Discussion
Ovarian cancer is one of the major causes of cancer-related deaths among women worldwide. Targeted therapies have shown a good clinical effect for certain OV patients, such as PARP inhibitors [14]. However, due to the complexity and heterogeneity of tumors, there is still an urgent need to find new therapeutic targets for OV. In this study, we analyzed the changes in DPP4 levels in OV patients and the effect of DPP4 on OVCAR-3 and SKOV-3 cells’ behavior. The major findings are summarized as follows: First, DPP4 levels were reduced in OV patients Second, DPP4 knockdown significantly affected the proliferation and migration of OVCAR-3 and SKOV-3 cells. Third, the TGFβ1-DPP4 might play an important role in OV development.
Based on the TCGA, GEO and CPTAC databases, we found a significant decrease in DPP4 expression in OV tumor tissues compared to control tissues. Additionally, serum DPP4 levels were significantly lower in OV patients than in healthy subjects. Previous studies have reported a decrease in serum DDP4 levels in several cancer types, such as gastric cancer, and colorectal cancer [3, 15]. Boccardi and colleagues showed that serum DPP4 levels were decreased in gastric cancer and may serve as an early detection marker [3]. Elzefzafy et al.’s study showed that serum DPP4 levels were lower in colorectal cancer compared to colorectal diseases and healthy controls [15]. Notably, Shao et al.’s study showed that serum CD26 levels were decreased in high-grade serous ovarian carcinoma patients, and may serve as an independent diagnostic marker [16]. For prognosis, based on the TCGA database, OV patients with high DPP4 mRNA expression levels showed a poor prognosis. Base on the GEO database, high DPP4 expression in the GSE18520 dataset was associated with a poor prognosis of ovarian cancer, while DPP4 expression in the GSE26712 was not associated with a prognosis. Taken together, these data demonstrated that DPP4 levels were decreased in OV patients (both in tumor tissues and serum). However, the prognostic value of DPP4 requires further verification, as there may be more confounding factors influencing the prognosis of OV patients, including CD26 expression.
To explore the roles of DPP4 in OV, we further investigated the effects of DPP4 knockdown on OVCAR3 and SKOV-3 cells’ proliferation and migration. By using si-DPP4 transfection, we observed that DPP4 knockdown significantly inhibited cell’s proliferation, which might be due to a decrease in cell numbers in the S phase. Consistently, previous studies have shown the inhibitory effects of DPP4 knockdown on the proliferation of other cancer cells [9, 10, 17]. For example, Okamoto T and colleagues found that DPP4 knockdown significantly inhibited the proliferation of malignant pleural mesothelioma [17]. Hu X’s study showed an inhibitory effect of DPP4 knockdown on papillary thyroid carcinoma cell’s proliferation [9].Furthermore, Yang et al.’s study showed that DPP4 overexpression promotes endometrial carcinoma cell proliferation [18]. These results suggest that DPP4 overexpression may promote OV cell proliferation, which could contribute to the poor prognosis in OV patients.
The effect of DPP4 on cell migration appears different in different cancer types. Our results in this study and Yang’s report [19] showed the promoted effect of DPP4 knockdown on OV cell lines and 4 T-1 (breast cancer cell line) cell migration respectively. However, Ng et al. found that DPP4 knockdown significantly inhibited the migration of colorectal carcinoma cells (HT29) [20]. Epithelial-mesenchymal transition (EMT) plays an important role in facilitating cancer cell migration. Yang et al. found that DPP4 knockdown induces EMT in breast cancer cells [19]. However, Hu X’s study showed the inhibited effect of DPP4 knockdown on the EMT of papillary thyroid carcinoma cells [9]. Taken together, there were significant differences in the effect of DPP4 on the migration and EMT progress among different types of cancer cells, which demonstrated CD26’s complex roles involved in cancer development. The correlation between DPP4 and EMT in OV cancer needs further study.
The mechanism of DPP4 expression regulation remains unclear. Chen Z’s study has shown the negative regulation of DPP4 expression by TGFβ1 in keloid-derived fibroblasts [21]. In the present study, we found that TGFβ1 treatment reduced DPP4 expression in OVCAR-3 cells. Furthermore, the effects of TGFβ1 on OVCAR-3 cell behavior were consistent with the outcomes observed DDP4 knockdown by siRNA. These data indicated the important role of TGFβ1-DPP4 signaling in OV development. Notably, miR-29a-3p might serve as an intermediary molecule between TGFβ1 and DPP4. In OVCAR-3 cells, TGFβ1 upregulated miR-29a-3p and miR-29a-3p inhibited DPP4 expression. The regulatory effect of miR-29a-3p on DPP4 was confirmed by the dual-luciferase assay in this study. Thus, these results indicated that DPP4 is downregulated by TGFβ1/miR-29a-3p in OV cells. Furthermore, the effects of miR-29a-3p and TGFβ1 on OVCAR-3 cells’ biological behaviors were consistent with DPP4 knockdown using si-DPP4. In contrast to this study, previous studies have shown downregulation of miR-29a-3p in response to TGFβ1 in human fetal lung fibroblast IMR-90 cells and hepatic stellate cell line LX-2 [13, 22]. Thus, the relationship between TGFβ1 and miR-29a-3p exhibits variability variation in different cell types.
There are some limitations to this study. First, the function of CD26 on OV progression was not evaluated in animal models. Thus, the efficacy of targeting CD26 therapy is also unknown for OV. Second, the effects of siDPP4 on downstream molecules were also unclear in this study, and these data could be further studied by using omics screening.
In conclusion, our study showed reduced levels of DPP4 levels in OV. Furthermore, DPP4 knockdown could inhibit OVCAR-3 cell’s proliferation and promote cell’s migration. DDP4 could be downregulated by TGFβ1 through the upregulation of miR-29a-3p in OV cells. Taken together, our study presents the potential roles of DPP4 in OV development.
Data availability
All data generated or analysed during this study are included in this published article.
References
Sung H, Ferlay J, Siegel RL, et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2021;71(3):209–49.
Jayson GC, Kohn EC, Kitchener HC, Ledermann JA. Ovarian cancer. Lancet. 2014;384(9951):1376–88.
Boccardi V, Marano L, Rossetti RR, et al. Serum CD26 levels in patients with gastric cancer: a novel potential diagnostic marker. BMC Cancer. 2015;15:703.
Torrecillas-Baena B, Gálvez-Moreno MÁ, Quesada-Gómez JM, et al. Influence of dipeptidyl peptidase-4 (DPP4) on mesenchymal stem-cell (MSC) biology: implications for regenerative medicine—review. Stem Cell Rev Rep. 2022;18(1):56–76.
Ye C, Tian X, Yue G, et al. Suppression of CD26 inhibits growth and metastasis of pancreatic cancer. Tumour Biol. 2016;37:15677.
Tan CW, Lee YH, Tan HH, et al. CD26/DPPIV down-regulation in endometrial stromal cell migration in endometriosis. Fertil Steril. 2014;102(1):167-177.e9.
Beckenkamp A, Willig JB, Santana DB, et al. Differential expression and enzymatic activity of DPPIV/CD26 Affects migration ability of cervical carcinoma cells. PLoS One. 2015;10(7):e0134305.
Komiya E, Ohnuma K, Yamazaki H, et al. CD26-mediated regulation of periostin expression contributes to migration and invasion of malignant pleural mesothelioma cells. Biochem Biophys Res Commun. 2014;447(4):609–15.
Hu X, Chen S, Xie C, et al. DPP4 gene silencing inhibits proliferation and epithelial-mesenchymal transition of papillary thyroid carcinoma cells through suppression of the MAPK pathway. J Endocrinol Invest. 2021;44(8):1609–23.
Kawaguchi T, Kodama T, Hikita H, et al. Synthetic lethal interaction of combined CD26 and Bcl-xL inhibition is a powerful anticancer therapy against hepatocellular carcinoma. Hepatol Res. 2015;45(9):1023–33.
Gyorffy B, Lánczky A, Szállási Z. Implementing an online tool for genome-wide validation of survival-associated biomarkers in ovarian-cancer using microarray data from 1287 patients. Endocr Relat Cancer. 2012;19(2):197–208.
Gao ZW, Liu C, Yang L, et al. CD73 severed as a potential prognostic marker and promote lung cancer cells migration via enhancing EMT progression. Front Genet. 2021;12: 728200.
Xu XY, Du Y, Liu X, et al. Targeting Follistatin like 1 ameliorates liver fibrosis induced by carbon tetrachloride through TGF-β1-miR29a in mice. Cell Commun Signal. 2020;18(1):151.
Klotz DM, Wimberger P. Overcoming PARP inhibitor resistance in ovarian cancer: what are the most promising strategies? Arch Gynecol Obstet. 2020;302(5):1087–102.
Elzefzafy WM, Abd Elrahman S, Mohmmed ZA, et al. Diagnostic utility of serum dipeptidyl peptidase (DPP- IV) /CD26 as a serum marker in Egyptian patients with colorectal cancer. J Immunoassay Immunochem. 2020;41(4):729–44.
Shao S, Wang C, Tian J, et al. Diagnostic and prognostic significance of serum CD26 level in Asian women with high-grade serous ovarian carcinoma. Future Oncol. 2019;15(16):1863–71.
Okamoto T, Yamazaki H, Hatano R, et al. Targeting CD26 suppresses proliferation of malignant mesothelioma cell via downmodulation of ubiquitin-specific protease 22. Biochem Biophys Res Commun. 2018;504(2):491–8.
Yang X, Zhang X, Wu R, et al. DPPIV promotes endometrial carcinoma cell proliferation, invasion and tumorigenesis. Oncotarget. 2017;8(5):8679–92.
Yang F, Takagaki Y, Yoshitomi Y, et al. Inhibition of dipeptidyl peptidase-4 accelerates epithelial-mesenchymal transition and breast cancer metastasis via the CXCL12/CXCR4/mTOR Axis. Cancer Res. 2019;79(4):735–46.
Ng L, Wong SK, Huang Z, et al. CD26 induces colorectal cancer angiogenesis and metastasis through CAV1/MMP1 signaling. Int J Mol Sci. 2022;23(3):1181.
Chen Z, Gao Z, Xia L, et al. Dysregulation of DPP4-CXCL12 balance by TGF-β1/SMAD pathway promotes CXCR4+ inflammatory cell infiltration in Keloid scars. J Inflamm Res. 2021;14:4169–80.
Cushing L, Kuang PP, Qian J, et al. miR-29 is a major regulator of genes associated with pulmonary fibrosis. Am J Respir Cell Mol Biol. 2011;45(2):287–94.
Acknowledgements
This study was supported by the key RD projects of shanxi province (NO.2021SF-005), the Scientist Fund of Air Force Medical University (NO.2022XC054) and the Scientist Fund of Tangdu Hospital (NO.2021SHRC031).
Author information
Authors and Affiliations
Contributions
KD and XMZ directed the study; CL and ZWG conceived and designed this study. CL, YQL and LY conducted all experiments and analyzed the data. XNW provided statistical and technical guidance. YQL conducted reference search and paper format modification. CL drafted the manuscript. ZWG, KD and XMZ revised the manuscript All authors read and approved the final manuscript.
Corresponding authors
Ethics declarations
Ethics approval and consent to participate
Ethical approval was obtained from the Ethics Committee of Tangdu Hospital, Fourth Military Medical University (No.202106-03), Informed consent was exempted for this study, which is approved by Ethics Committee of Tangdu Hospital. The study conformed to the principles outlined in the World Medical Association Declaration of Helsinki.
Competing interests
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Liu, C., Gao, ZW., Liu, YQ. et al. Down-regulation of DPP4 by TGFβ1/miR29a-3p inhibited proliferation and promoted migration of ovarian cancer cells. Discov Onc 14, 195 (2023). https://doi.org/10.1007/s12672-023-00815-y
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s12672-023-00815-y