Abstract
Purpose
Papillary thyroid carcinoma (PTC) is characterized by epithelial malignancy and is the most prevalent thyroid neoplasm with the best overall prognosis. Notably, recently published studies have indicated remarkably high expression of dipeptidyl peptidase 4 (DPP4) in PTC. However, the underlying molecular mechanism and regulatory factors of PTC progression remain unknown. Therefore, the current study aimed to elucidate the effects of DPP4 gene silencing on PTC and further investigated whether the mechanism of PTC progression is related to the mitogen-activated protein kinase (MAPK) pathway.
Methods
Herein, microarray-based gene expression profiling of PTC was conducted to identify the differentially expressed genes between tumor thyroid tissue and normal thyroid tissue as well as the underlying signaling pathway involved in PTC pathogenesis. Moreover, protein quantification was performed to assess the protein expression of DPP4 in PTC tissues collected from 65 patients. In addition, DPP4 was silenced in PTC cell lines (GLAG-66 and TPC-1) through siRNA-mediated DPP4 knockdown or sitagliptin (inhibitor of DPP4)-mediated inhibition to assess the effects of DPP4 on the MAPK pathway and cellular processes, including proliferation, apoptosis, and epithelial-to-mesenchymal transition (EMT).
Results
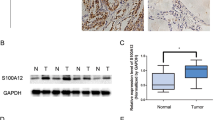
Intriguingly, our data revealed markedly high expression of DPP4 in PTC tissues. However, in GLAG-66 and TPC-1 cells, the silencing of DPP4 resulted in significantly reduced expression of ERK1/2, JNK1, P38 MAPK, VEGF, FGFR-1, TGF-β1, Snail, HIF-1α, N-cadherin, and Bcl-2 along with reduced phosphorylation of ERK1/2, JNK1, and P38 MAPK, whereas the expression of E-cadherin and Bax was increased. Furthermore, DPP4 silencing was found to hinder cell proliferation and potentiate cell apoptosis.
Conclusion
Collectively, the present study demonstrated that DPP4 gene silencing inhibits PTC cell proliferation and EMT and promotes cell apoptosis via suppression of the MAPK pathway, thus highlighting a possible regulatory pathway in PTC progression.









Similar content being viewed by others
References
LiVolsi VA (2011) Papillary thyroid carcinoma: an update. Mod Pathol 24(Suppl 2):S1-9
Chen W, Zheng R, Baade PD et al (2016) Cancer statistics in China, 2015. CA Cancer J Clin 66:115–132
Toniato A, Boschin I, Casara D et al (2008) Papillary thyroid carcinoma: factors influencing recurrence and survival. Ann Surg Oncol 15:1518–1522
Stern S, Dagan E, Alon EE et al (2016) Papillary thyroid carcinoma treatment—is the pendulum shifting? Harefuah 155:511–515
Liu FC, Lin HT, Lin SF et al (2017) Nationwide cohort study on the epidemiology and survival outcomes of thyroid cancer. Oncotarget 8:78429–78451
McLeod DS, Sawka AM, Cooper DS (2013) Controversies in primary treatment of low-risk papillary thyroid cancer. Lancet 381:1046–1057
Abdullah MI, Junit SM, Ng KL et al (2019) Papillary thyroid cancer: genetic alterations and molecular biomarker investigations. Int J Med Sci 16:450–460
Glorie LL, Verhulst A, Matheeussen V et al (2012) DPP4 inhibition improves functional outcome after renal ischemia-reperfusion injury. Am J Physiol Renal Physiol 303:F681-688
Sato Y, Kamada T, Yamauchi A (2014) The role of dipeptidyl peptidase 4 (DPP4) in the preservation of renal function: DPP4 involvement in hemoglobin expression. J Endocrinol 223:133–142
Ozog J, Jarzab M, Pawlaczek A et al (2006) Expression of DPP4 gene in papillary thyroid carcinoma. Endokrynol Pol 57(Suppl A):12–17
Huang Y, Prasad M, Lemon WJ et al (2001) Gene expression in papillary thyroid carcinoma reveals highly consistent profiles. Proc Natl Acad Sci USA 98:15044–15049
Gubala E, Wiench M, Oczko-Wojciechowska M et al (2005) Gene expression analysis by DNA microarray in papillary thyroid cancer. Endokrynol Pol 56:752–757
Javidroozi M, Zucker S, Chen WT (2012) Plasma seprase and DPP4 levels as markers of disease and prognosis in cancer. Dis Markers 32:309–320
Zeng Y, Li C, Guan M et al (2014) The DPP-4 inhibitor sitagliptin attenuates the progress of atherosclerosis in apolipoprotein-E-knockout mice via AMPK- and MAPK-dependent mechanisms. Cardiovasc Diabetol 13:32
Lee S, Rauch J, Kolch W (2020) Targeting MAPK signaling in cancer: mechanisms of drug resistance and sensitivity. Int J Mol Sci 21:1102
Wang DP, Tang XZ, Liang QK et al (2020) Overexpression of long noncoding RNA SLC26A4-AS1 inhibits the epithelial-mesenchymal transition via the MAPK pathway in papillary thyroid carcinoma. J Cell Physiol 235:2403–2413
Gautier L, Cope L, Bolstad BM et al (2004) affy—analysis of Affymetrix GeneChip data at the probe level. Bioinformatics 20:307–315
Smyth GK (2004) Linear models and empirical Bayes methods for assessing differential expression in microarray experiments. Stat Appl Genet Mol Biol 3:1–25
Kim J, So S, Lee HJ et al (2013) DigSee: disease gene search engine with evidence sentences (version cancer). Nucleic Acids Res 41:W510-517
Shannon P, Markiel A, Ozier O et al (2003) Cytoscape: a software environment for integrated models of biomolecular interaction networks. Genome Res 13:2498–2504
Kim K, Kim JH, Park IS et al (2018) The updated AJCC/TNM staging system for papillary thyroid cancer (8th edition): from the perspective of genomic analysis. World J Surg 42:3624–3631
Song HM, Luo Y, Li DF et al (2015) MicroRNA-96 plays an oncogenic role by targeting FOXO1 and regulating AKT/FOXO1/Bim pathway in papillary thyroid carcinoma cells. Int J Clin Exp Pathol 8:9889–9900
Ayuk SM, Abrahamse H, Houreld NN (2016) The role of photobiomodulation on gene expression of cell adhesion molecules in diabetic wounded fibroblasts in vitro. J Photochem Photobiol B 161:368–374
Occhi G, Barollo S, Regazzo D et al (2015) A constitutive active MAPK/ERK pathway due to BRAFV600E positively regulates AHR pathway in PTC. Oncotarget 6:32104–32114
Romitti M, Wajner SM, Ceolin L et al (2016) MAPK and SHH pathways modulate type 3 deiodinase expression in papillary thyroid carcinoma. Endocr Relat Cancer 23:135–146
Wronkowitz N, Gorgens SW, Romacho T et al (2014) Soluble DPP4 induces inflammation and proliferation of human smooth muscle cells via protease-activated receptor 2. Biochim Biophys Acta 1842:1613–1621
Cancer Genome Atlas Research N (2014) Integrated genomic characterization of papillary thyroid carcinoma. Cell 159:676–690
Hong S, Yu S, Li J et al (2016) MiR-20b displays tumor-suppressor functions in papillary thyroid carcinoma by regulating the MAPK/ERK signaling pathway. Thyroid 26:1733–1743
Lee JJ, Wang TY, Liu CL et al (2017) Dipeptidyl peptidase IV as a prognostic marker and therapeutic target in papillary thyroid carcinoma. J Clin Endocrinol Metab 102:2930–2940
Wagner EF, Nebreda AR (2009) Signal integration by JNK and p38 MAPK pathways in cancer development. Nat Rev Cancer 9:537–549
Ta NN, Li Y, Schuyler CA et al (2010) DPP-4 (CD26) inhibitor alogliptin inhibits TLR4-mediated ERK activation and ERK-dependent MMP-1 expression by U937 histiocytes. Atherosclerosis 213:429–435
Wang WJ, Chang CH, Sun MF et al (2014) DPP-4 inhibitor attenuates toxic effects of indoxyl sulfate on kidney tubular cells. PLoS ONE 9:e93447
Beckers PAJ, Gielis JF, Van Schil PE et al (2017) Lung ischemia reperfusion injury: the therapeutic role of dipeptidyl peptidase 4 inhibition. Ann Transl Med 5:129
Wang X, Zhang J, Fan M et al (2009) The expression of E-cadherin at the invasive tumor front of oral squamous cell carcinoma: immunohistochemical and RT-PCR analysis with clinicopathological correlation. Oral Surg Oral Med Oral Pathol Oral Radiol Endod 107:547–554
Lowy AM, Knight J, Groden J (2002) Restoration of E-cadherin/beta-catenin expression in pancreatic cancer cells inhibits growth by induction of apoptosis. Surgery 132:141–148
Huang CN, Wang CJ, Yang YS et al (2016) Hibiscus sabdariffa polyphenols prevent palmitate-induced renal epithelial mesenchymal transition by alleviating dipeptidyl peptidase-4-mediated insulin resistance. Food Funct 7:475–482
Sun J, Chu S, Lu M et al (2020) The roles of dipeptidyl peptidase-4 and its inhibitors in the regulation of airway epithelial-mesenchymal transition. Exp Lung Res 46:163–173
Li S, Fan Y, Kumagai A et al (2020) Deficiency in dipeptidyl peptidase-4 promotes chemoresistance through the CXCL12/CXCR4/mTOR/TGFbeta signaling pathway in breast cancer cells. Int J Mol Sci 21:805
Abdelzaher WY, Rofaeil RR, Ali DME et al (2020) Protective effect of dipeptidyl peptidase-4 inhibitors in testicular torsion/detorsion in rats: a possible role of HIF-1alpha and nitric oxide. Naunyn Schmiedebergs Arch Pharmacol 393:603–614
Shi S, Kanasaki K, Koya D (2016) Linagliptin but not Sitagliptin inhibited transforming growth factor-beta2-induced endothelial DPP-4 activity and the endothelial-mesenchymal transition. Biochem Biophys Res Commun 471:184–190
El-Sherbeeny NA, Nader MA (2016) The protective effect of vildagliptin in chronic experimental cyclosporine A-induced hepatotoxicity. Can J Physiol Pharmacol 94:251–256
Wang Y, Han J, Lv Y et al (2019) miR-29a inhibits proliferation, invasion, and migration of papillary thyroid cancer by targeting DPP4. OncoTargets Ther 12:4225–4233
Acknowledgements
We would like to give our sincere appreciation to the reviewers for their helpful comments on this article.
Funding
None.
Author information
Authors and Affiliations
Contributions
XH, SC, CX, ZL, ZW, and ZY designed the study. XH, SC, and CX collected the data, carried out the data analyses and produced the initial draft of the manuscript. ZL, ZW, and ZY contributed to drafting the manuscript. All the authors have read and approved the final submitted manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no conflicts of interest.
Ethical approval
The study was approved by the ethics committee of the Institutional Review Board of Affiliated Dongguan People's Hospital, Southern Medical University (Dongguan People's Hospital) and performed in strict accordance with the Declaration of Helsinki.
Informed consent
All the participants, or their parents or guardians, provided informed consent before enrollment.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Hu, X., Chen, S., Xie, C. et al. DPP4 gene silencing inhibits proliferation and epithelial-mesenchymal transition of papillary thyroid carcinoma cells through suppression of the MAPK pathway. J Endocrinol Invest 44, 1609–1623 (2021). https://doi.org/10.1007/s40618-020-01455-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40618-020-01455-7