Abstract
Melanoma remains one of the most therapy-resistant forms of human cancer despite recent introductions of highly efficacious targeted therapies. The intrinsic therapy resistance of human melanoma is largely due to abundant expression of a repertoire of xenobiotic efflux pumps of the ATP-binding cassette (ABC) transporter family. Here, we report that GH action is a key mediator of chemotherapeutic resistance in human melanoma cells. We investigated multiple ABC efflux pumps (ABCB1, ABCB5, ABCB8, ABCC1, ABCC2, ABCG1, and ABCG2) reportedly associated with melanoma drug resistance in different human melanoma cells and tested the efficacy of five different anti-cancer compounds (cisplatin, doxorubicin, oridonin, paclitaxel, vemurafenib) with decreased GH action. We found that GH treatment of human melanoma cells upregulates expression of multiple ABC transporters and increases the EC50 of melanoma drug vemurafenib. Also, vemurafenib-resistant melanoma cells had upregulated levels of GH receptor (GHR) expression as well as ABC efflux pumps. GHR knockdown (KD) using siRNA in human melanoma cells treated with sub-EC50 doses of anti-tumor compounds resulted in significantly increased drug retention, decreased cell proliferation and increased drug efficacy, compared to mock-transfected controls. Our set of findings identify an unknown mechanism of GH regulation in mediating melanoma drug resistance and validates GHR as a unique therapeutic target for sensitizing highly therapy-resistant human melanoma cells to lower doses of anti-cancer drugs.
Similar content being viewed by others
Introduction
Human melanoma has remained the most aggressive form of skin cancer and one of the most therapy-resistant forms of cancer worldwide. In some countries, recent reports have claimed it to be of epidemic proportions [1–6]. Studies indicate increased UV exposure, use of tanning-beds, hormone replacement therapies, as well as improved and increased diagnostic screening as the underlying causes of heightened melanoma incidence [3, 7–9]. Developing a melanoma vaccine have had limited success [10] while, in the last 5 years, a number of highly efficacious immunotherapies (CTLA-4 antibody, ipilimumab; PD-1/PD-L1 antibody, pembrolizumab) and targeted therapies (V600E BRAF inhibitor, vemurafenib) had been approved by the FDA [11–16] while several are in various stages of development [17]. Despite the success, chemotherapeutic interventions for melanoma often result in drug resistance that may occur by several cellular mechanisms leaving persistent concerns for clinicians and patients [18–24]. Effective therapy with one of the most successful drugs, pembrolizumab, has been reported to necessitate pre-existence of active cytotoxic T cells [25] while resistance to most other known chemotherapies, including ipilimumab and vemurafenib, have been reported [26].
Melanoma is unique among other types of cancers in possessing multiple mechanisms of chemotherapeutic resistance, including a repertoire of drug efflux pumps [27–29], as well as upregulation of epithelial-mesenchymal transition (EMT) markers [30–32]. Molecular mechanisms defining melanoma drug resistance are lacking. Studies with MCF-7 breast cancer cells indicated an upregulated doxorubicin resistance in presence of supra-physiological doses (1000 ng/mL) of GH [33] while over the last decade Lobie and colleagues have reported GH-induced chemotherapy resistance in acromegaly patients [34], autocrine GH-induced resistance to radiation therapy in breast and endometrial cancers [35, 36], and increased propensity to EMT in breast cancer [37]. A number of clinical reports have pointed towards a distinct association of human GH (hGH) with melanoma [9, 38, 39]. Significantly elevated levels of the hGH receptor (GHR) were observed by immune-staining of different stages of melanoma tumors, where 34 of 37 tested cases were moderate to strongly positive for GHR expression [40]. We previously showed that the GHR RNA level was highest specifically in human melanoma cells in the entire NCI60 panel of 9 different cancer types [41]. Recently, we reported a comprehensive mechanism of GH-GHR action in human melanoma cells regulating key intracellular signaling pathways like the JAK, STAT (1, 3, and 5), SRC, ERK1/2, AKT, and mTOR [42] which are known to be critical mediators of early gene activation and drug resistance in several forms of cancer, especially melanoma [43–48]. We also observed robust expression of endogenous GH and GH-dependent modulation of HGF, MET, and ERBB3 RNAs in human melanoma cells which indicate a GH driven mechanism of therapy refractoriness [49–52]. Most importantly, the GH-GHR interaction was found to positively drive EMT in human melanoma cells [42], a mechanism deemed increasingly important in the incidence of drug refractoriness [53–56]
Here, we specifically investigated the effect of siRNA mediated GHR knockdown (KD) on the drug resistance property of four human melanoma cells (SK-MEL-28, MALME-3M, MDA-MB-435, and SK-MEL-5) which express both GH and GHR. We compared changes in RNA expression of seven different ATP-binding cassette (ABC) transporter family efflux pumps, following treatment with sub-EC50 doses (EC50 = effective concentration for 50% of maximum observed effect to occur) of the following selected anti-tumor drugs: cisplatin, doxorubicin, oridonin, paclitaxel, and vemurafenib under basal and excess GH (50 ng/mL) conditions as well as in the absence of GH action (GHRKD). We report that prolonged exposure to GH alone leads to increased resistance to anti-cancer drugs whereas GHRKD results in significant suppression of the effects. The incidence of drug resistance coincided with increased GHR levels while increased drug sensitivity followed GHRKD. Collectively, we present a critical mechanism of abetting drug resistance by GH in human melanoma cells. Our results indicate that targeting GHR is an effective mechanism to curb drug efflux and markedly sensitize highly therapy-resistant melanoma cells to different classes of established and developing anti-cancer compounds.
Methods
Cell Culture
Human melanoma cells SK-MEL-5 (#HTB-70), SK-MEL-28 (#HTB-72), MALME-3M (#HTB-64), MDA-MB-435S (#HTB-129), and normal melanocyte ST-MEL (ATCC # 30-2001) were purchased from American Type Culture Collection (ATCC; Manassas, Virginia) and grown in recommended media with 10% fetal bovine serum (FBS; ATCC # 30-2020) and 1X antibiotic-antimycotic (Thermo Fisher Scientific #15240) at 37 °C/5% CO2 in a humidified incubator. Recombinant human (h) GH (Antibodies Online # ABIN2017921) was added to media at 50 ng/mL as an acute dose or at 20 ng/mL for prolonged exposure. Vemurafenib-resistance cell line was developed in vitro as described by McDermott et al. [57] by continuous exposure of SK-MEL-28 and SK-MEL-5 cells to 10 nM vemurafenib for eight cycles of 3-day exposure to vemurafenib followed by drug free growth to confluency (4–5 days) with or without 20 ng/mL hGH (replaced every feeding cycle 3 days) in the media. Following eight cycles, the different subtypes of cells were frozen separately until use. On subsequent thawing and re-growth for 4 cycles (media containing 10% FBS and 1X antibiotic-antimycotic), the cells were used for evaluation of EC-50 and RNA analysis.
Transfection
Transfection was performed using siLentFect lipid reagent (Biorad #170-3360, Hercules, CA) following the manufacturer’s protocol. Pre-designed siRNA duplex against human GHR (Origene #SR301794, Rockville, MD) at 20 nM was used (siRNA-B: AGCUAGAAUUGAGUGUUUAAAGUTC) that resulted in a >80% decrease in GHR transcript in all four melanoma cells while a universal scrambled siRNA duplex (Origene #SR30004) was used as control. Cells were seeded at 25,000–30,000 cells/cm2, incubated overnight and siRNA duplex (scramble or GHR specific) and siLentFect reagent at 1:1 M ratio was added to the cells and incubated at 37 °C/5% CO2. Media was changed after 24 h. RNA levels were analyzed 48 h post-transfection while protein levels were analyzed at 60 h post-transfection. For drug treatment, drugs at the specified concentrations noted below were added to the cells 48 h post-transfection and treated for 24 h prior to quantitation of RNA levels.
Drug Treatments
For treatment of melanoma cells, the following five anti-tumor compounds were obtained from the sources mentioned: cisplatin (Calbiochem #232120, Darmstadt, Germany), doxorubicin (Sigma-Aldrich #D-1515, St. Louis, MO), oridonin (Sigma-Aldrich #O-9639, St. Louis, MO), Paclitaxel (Sigma-Aldrich #C-7191), and vemurafenib/PLX4032 (ApexBio #A-3004, Houston, TX). We performed evaluation of EC50 values for each drug in each cell line and observed the following EC-50 ranges for the four melanoma cell lines; cisplatin (3–15 uM), doxorubicin (25–100 nM), oridonin (2–8 uM), paclitaxel (2–8 nM), and vemurafenib (5–15 nM). In the subsequent experiments, the following drug concentrations were used unless specified otherwise: cisplatin (0.5 uM), doxorubicin (10 nM), oridonin (0.5 uM), paclitaxel (1 nM), and vemurafenib (2 nM). Treatments were performed for 24 h starting 48 h post-transfection with siRNA for GHRKD cells, and for 24 h for 50 ng/ml GH treated cells, prior to RNA extraction and analysis.
RNA Extraction and RT-qPCR
IBI-Trizol based total RNA purification kit (MidSci #IB47632, St. Louis, Missouri) was used for RNA extraction from the cells, and reverse transcription was performed using the Maxima First Strand cDNA synthesis kit (Thermo Fisher Scientific #K1642, Waltham, MA) following the manufacturers’ protocols. Real-time-quantitative PCR and melt curve analysis were performed using Maxima SYBR-Green qPCR master mix (Thermo Fisher Scientific #K0241) and a T100 thermal cycler (Biorad #1861096, Hercules, CA). RNA and DNA concentrations were estimated using Nanodrop2000 (Thermo Fisher Scientific, Waltham, MA) spectrophotometer.
Primers were obtained from Sigma-Aldrich for the following human genes and primer efficiencies were confirmed: GAPDH, β-Actin, GH1, GHR, E-Cadherin (CDH1), N-Cadherin(CDH2), Vimentin, ABCB1, ABCB5, ABCB8, ABCG1, ABCG2, ABCC1, and ABCC2. Each sample was a pool of two replicates per experiment; RNA expression analysis by qPCR was done in triplicates from three different experiments.
Protein Extraction
Total cellular protein was collected 60 h post-transfection using RIPA buffer (Sigma-Aldrich #R-0278, St. Louis, Missouri) containing 1X Halt protease and phosphatase inhibitor cocktail (Thermo Fisher #78442, Waltham, MA) following the manufacturer’s protocol. Briefly, cells were washed twice with 1X phosphate-buffered saline (PBS) and RIPA buffer at 1 mL per million cells was added and incubated for 5 min/4 °C. Cell lysate was clarified by centrifuging at 8000×g/10 min/4 °C and the supernatant was collected and stored at −80 °C for subsequent analysis. Each sample was a pool of three replicates per experiment; each experiment was done three times. Protein concentration was estimated using the Bradford reagent (Sigma-Aldrich #B6916), Spectramax250 spectrophotometer (Molecular Devices, Sunnyvale, California) and SoftmaxPro software (ver4.7.1).
Western Blotting
Western blotting was performed following standard laboratory protocol with a few modifications [58]. Briefly, intracellular proteins were separated by SDS-PAGE and transferred onto a PVDF membrane and blocked with 5% bovine serum albumin (BSA) in 1X TBS-T (Tris-buffered saline, pH 7.2 with 0.1% Triton-X100) for 12–16 h/4 °C. Membranes were incubated with primary antibody (at specific dilutions mentioned below) for 12–16 h at 4 °C followed by three washes with 1X TBS-T and then incubated with the corresponding secondary antibodies (at specific dilutions mentioned below) for 2 h/25 °C. Membranes were then washed four times with 1X TBS-T and treated with WestFemto Chemilumiscence detection reagents (Thermo Fisher Scientific) and the chemiluminiscent signal was captured using a GelDoc (Biorad) fluorescence reader. Densitometry analysis of the blots was done using ImageJ software.
Primary antibodies were used to detect the following human proteins: GHR (Mouse, 1:300, SCBT #137185; Goat, 1:100, R&D Systems #AF1210; Rabbit, 1:200, Abcam #ab134078), Actin (Goat, 1:3000, SCBT #sc1616), GAPDH (Goat, 1:3000, SCBT #sc20357), Vimentin (Rabbit, 1:3000, CST #5741), E-cadherin (Rabbit, 1:1000, CST #3195), N-cadherin (Rabbit, 1:500, CST #13116), Vimentin (Rabbit, 1:3000, CST #5741), ABCG1 (Rabbit, 1:100, Abiocode #R0254), ABCB8 (Rabbit, 1:100, SAB #31025), and ABCB1/MDR1 (Mouse, 1:100, SCBT #sc55510). The secondary antibodies were anti-rabbit HRP-linked IgG (Donkey, 1:2000, CST #7074P2), anti-goat HRP-linked IgG (Donkey, 1:1000, SCBT #sc2020), anti-rabbit HRP-linked IgG (Donkey, 1:2000, GE #NA934), and anti-mouse HRP-linked IgG (Rat, 1:1000, Antibodies Online #ABIN1589975).
Immunofluorescence
Cells were seeded at 10,000 cells/cm2 in 8-well chamber slides and transfection was performed as described above. The cells were treated 48 h post-transfection with 10 nM doxorubicin or 1 nM paclitaxel for 24 h. Subsequently, cells were fixed with 100% methanol, permeabilized with 0.2% Triton-X100 in 1X PBS for 15 min at 25 °C, and blocked with 1% BSA for 4 h at 25 °C. Incubation time was 12 h at 4 °C for the primary antibody and 2 h at 25 °C for the secondary antibody. Finally, the slides were washed four times with 1X PBS and the sample was mounted with Fluoroshield mounting medium containing DAPI (Abcam #ab104139, Cambridge, UK), covered with a 60-mm coverslip and stored at 4 °C for microscopy. Microscopic imaging was done using a Nikon Eclipse E600 compound fluorescent microscope fitted with a Nikon DS-Fi1CC camera (Nikon, Tokyo, Japan) and NIS-Elements BR3.2 imaging software. The antibodies used were Rabbit anti-human-Ki67 monoclonal antibody with an AlexaFluor488 tag (Abcam #ab154201, 1:300 dilutions), and Goat anti-rabbit secondary antibody with AlexaFluor488 tag (Life Technologies #R37116, 1:500 dilution).
Quantification of immunofluorescence expression was performed from images of three separate experiments. Images of six different areas from each coverslip were counted using ImageJ, per experiment to have a minimum of 4000 cells per treatment group. Ki67-positive (Ki67+) cell number was normalized against DAPI+ cell numbers per image and the Ki67+/DAPI+ ratio was used to quantitate cell proliferation.
Cell Proliferation Assay
We performed a resazurin-based absorption assay measuring cell proliferation, as a measure of drug effect. There are several commonly practiced assays using tetrazolium (MTT, MTS, XTT), or resazurin which give a quantitative reflection of cell viability. Although ATP detection assay is the most sensitive of the available options, resazurin-based assay is considered adequate and is routinely used to measure compound EC50s or cell viability following cytotoxic treatments [59, 60]. A stock solution of 1% (w/v) resazurin (Sigma-Aldrich #R7017) in 1X PBS was made and filter-sterilized. The final concentration of resazurin in the assay was 0.004%. Proliferating cells can be quantified by spectrophotometric measurement of a bright pink fluorescent product called resorufin (stable for 4 h) formed when mildly fluorescent blue resazurin enters a reducing intracellular environment characteristic of proliferating cells [61]. Cells were seeded at 10,000 cells/cm2 into 96-well plates and transfected as described above. The resazurin assay was performed 60 h after transfection (unless specified otherwise) and resorufin absorbance was measured at 570 nm (reference wavelength = 600 nm) using Spectramax250 (Molecular Devices, Sunnyvale, CA) and SoftmaxPro software. In all cases, cells were incubated at 37 °C/5% CO2 for 45–60 min following resazurin addition for adequate sensitivity of detection. The resazurin assay for cell proliferation was used to measure EC-50 of relevant drugs and Graph Pad Prism was used to calculate EC-50 shifts (EC50 shift = EC50 of treated cell line/EC50 of parental cell line as described [57].
Drug Retention Assay
The presence of multiple drug resistance pumps along the cellular membrane is key to the resistance against chemotherapy in some cells like melanoma. ABC-transporter pumps in the MDR and MRP family are involved in exclusion of xenobiotics from inside the cells to outside. This reduces the retention time of drugs inside a cell and confers decreased sensitivity to the drug effects. For our purpose, we chose the Vybrant multidrug resistance assay kit (Molecular Probes #V13180, Eugene, OR) which was developed initially by Tiberghein and Loor [62]. The assay uses the non-fluorescent calcein acetoxymethylester (calcein-AM) as a drug-mimic and a substrate for the melanoma cell efflux pumps. Calcein-AM is highly lipid soluble and permeates the cell membrane where it is converted to a fluorescent calcein by the intracellular esterases. In the absence or decreased activity of the efflux pumps, the intensely fluorescent calcein is retained and can be measured as an indication of drug retention inside the cell. The assay was performed as per the manufacturer’s protocol with some necessary optimizations. Briefly, the siRNA treated cells were trypsinized 48 h after transfection, counted and seeded at 50,000 cells/well in a black, clear bottom Costar 96-well plate (Corning #3603, Corning, NY) and then calcein-AM was added at a final concentration of 2 uM, and incubated at 37 °C for 2 h. After thorough washing, fluorescence was measured at 494 (excS) / 517 nm (emi) in a spectramax M2 fluorescence plate reader (Molecular Devices, Sunnyvale, CA) and SoftMax Pro v6.2.1 software. Experiments were done in quadruplicate.
Statistical Analyses
Parametric and non-parametric statistical analyses were performed using R software (ver3.0.2). For RT-qPCR analysis of RNA, the levels were normalized first against two reference genes (GAPDH and beta-actin) and the relative values (ΔΔCt) were compared by Wilcoxon signed rank test for significance. One-way ANOVA and Bonferroni post-hoc tests were done for comparing Ki67+/DAPI+ ratios. A p value less than 0.05 was considered as significant. The densitometry analyses and resazurin-based assays were compared by a paired Student’s t test and ANOVA was performed (using GraphPad Prism software) to compare for significance (p < 0.05 is considered significant).
Results
GHRKD Exacerbates Drug Effects on Melanoma Cell Proliferation
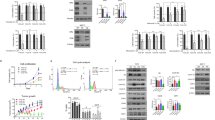
We and others had previously shown that human melanoma cells have robust expression of GH and GHR and GH action regulates critical oncogenic pathways in this type of cancer including driving epithelial-to-mesenchymal transition (EMT), cell migration, invasion, colony formation and proliferation [42]. In the four cell lines tested, we observed GHR RNA and protein levels in the order of SK-MEL-28 > MALME-3 M > MDA-MB-435 > SK-MEL-5. A reduction in cell proliferation was assayed by quantifying the levels of Ki67, a commonly used marker for cellular proliferation [63]. To evaluate the effect of combining anti-cancer compounds in GHRKD-induced suppression of cell proliferation levels [post-transfection RT-qPCR analyses showed 85–90% reduction in GHR RNA levels for all melanoma cell lines except SK-MEL-5 (50% reduction in GHR was observed); Fig S1(a-d)], we performed immunofluorescence studies on scramble (scr) or GHR-siRNA-transfected samples, following 24 h treatment with sub-EC50 doses of the anti-cancer compounds; doxorubicin (10 nM), cisplatin (0.5 uM), oridonin (0.5 uM), paclitaxel (1 nM), or vemurafenib (2 nM). From the results of cell proliferation assay using resazurin, we observed that GHRKD in combination with anti-cancer drugs exerted a marked suppression of cell proliferation in SK-MEL-28 human melanoma cells when exposed to cisplatin, doxorubicin and vemurafenib (Figs 1a, b). Similar effects of significantly decreased cell proliferation following GHRKD was observed in MALME-3M cells in response to cisplatin, doxorubicin, paclitaxel, and vemurafenib (Figs 1c, S2). The effects were less pronounced in MDA-MB-435 (cisplatin, vemurafenib) and SK-MEL-5 (paclitaxel, vemurafenib) melanoma cells, which were previously observed to have a lower expression of GHR compared to SK-MEL-28 and MALME-3M (Figs 1d, e, S3, S4). Quantification and normalization of Ki67-positive (Ki67+) cells with DAPI-positive (DAPI+) cells show that in the melanoma cells, GHRKD alone significantly decreased cell proliferation (ranging from 30.7% reduction in MALME-3M to 17.2% reduction in MDA-MB-435 cells) except SK-MEL-5; whereas the anti-cancer drugs alone, administered at sub-EC50 doses did not cause more than 10% reduction in cell proliferation (Fig. 1). However, combining GHRKD with the drugs at the same doses resulted in a significantly higher reduction in cell proliferation than either treatment alone, and was observed differently in the four human melanoma cell lines in response to specific drugs only, as described above. This variation in the extent of GHRKD effects can be due to several additional intrinsic genetic differences influencing drug efficacy between the samples. The results suggest that GHRKD appears to have an effect of either increasing the anti-cancer potency of the drugs or in sensitizing the melanoma cells to low doses of the drugs. Melanoma cells are unique in having a robust expression of ABC transporters for drug efflux, which serves as one of the most critical mechanisms of developing chemotherapy refractoriness. Therefore, we chose to assess the changes in the levels of selected ABC drug efflux pumps that are abundantly expressed in human melanoma cancers [28, 64, 65].
Effect of drug treatment on cell proliferation in growth hormone receptor knockdown (GHRKD) SK-MEL-28 cells. a SK-MEL-28 cells were exposed to either DMSO (a), or 0.5 um cisplatin (b), or 10 nM doxorubicin (c), or 0.5 uM oridonin (d), or 1 nM paclitaxel (e), or 15 nM vemurafenib (f) for 24 h. Treatments were done 48 h post-transfection with either scr-siRNA (1 and 2) or GHR-siRNA (3 and 4). Panels 1 and 3 show cellular DNA stained with DAPI while panels 2 and 4 show fluorescence signals from AF488-tagged anti-Ki67 antibody. Picture was taken at ×40 magnification; scale bar represents 500 um. Similar profiles for MALME-3M (Fig S2), MDA-MB-435 (Fig S3), and SK-MEL-5 (Fig S4) melanoma cells are in supplementary. b–e Quantitation of Ki67+ and DAPI+ cells in drug-treated GHRKD and control cells in SK-MEL-28 (b), MALME-3M (c), MDA-MB-435 (d), and SK-MEL-5 (e) using ImageJ. The Ki67+/DAPI+ ratio represents proliferating cells normalized to cell number. GHRKD significantly reduced cell proliferation in combination with drug treatment. [*p < 0.05, one-way ANOVA, n = 3]
GHRKD significantly Suppresses Expression of ABC-Transporter Pumps in Human Melanoma Cells
Melanoma cell express a subset of the 47 different human ABC efflux pumps of which we specifically investigated the RNA levels of ABCB1 [66], ABCB5 [67, 68], ABCB8 [27], ABCC1 [69], ABCC2 [19], ABCG1 [70], and ABCG2 [71], based on reports of their presence and drug resistance activity in human melanoma. The RNA levels of these seven different ABC drug efflux pumps were evaluated following a 24 h exposure to sub-EC50 doses of cisplatin, doxorubicin, oridonin, paclitaxel, and vemurafenib, and compared between GHRKD or mock-transfected cells. GHRKD resulted in a significant suppression of ABC efflux pump RNA levels, but in varying levels to different drugs, in different cell lines (Figs. 2 and S5-S8). The results for the significant variations off all 35 combinations (five drugs × seven transporters) for each of the four-different human melanoma cell lines are depicted in Fig. 2b and tabulated in Table 1. Following addition of the five different drugs, the GHRKD SK-MEL-28 cells, in comparison to corresponding scr-siRNA treated samples, showed reduced RNA levels of different sets of ABC transporters against cisplatin (ABCB1, ABCB5, ABCB8, ABCG1) doxorubicin (ABCB8, ABCG1, ABCG2), oridonin (ABCB5, ABCC1, ABCG1), paclitaxel (ABCB5, ABCC1, ABCG1, ABCG2), or vemurafenib (ABCB8, ABCC2, ABCG1, ABCG2) (Fig. 2a, b). Figure 2b summarizes similar sets of transporter expression changes for each drug in each cell line. We further checked the levels of protein expression of these efflux pumps and could consistently detect markedly reduced levels of ABCB8 and ABCC1 proteins, following GHRKD, in all four cell lines, compared to mock-treated controls (Fig. 2c). These results indicate of a unique role of GH in cancer cells, namely the regulation of drug efflux pumps and subsequent drug resistance. This prompted us to investigate the role of excess GH exposure to the drug responsiveness of human melanoma cells.
Effect of GHRKD on ABC efflux pump expression following drug treatment in human melanoma cells. a Significant downregulation of ABC-transporter expressions in GHRKD SK-MEL-28 cells following treatment with anti-cancer drugs. b Heat-map showing the statistically significant variations in RNA expressions of ABC transporters following GHRKD, in all four human melanoma cell lines, following treatment with all five drugs (35 combinations per cell line, or 28 combinations per drug). Detailed comparison for SK-MEL-28 (Fig S5), MALME-3M (Fig S6), SK-MEL-5 (Fig S7), and MDA-MB-435 (Fig S8) melanoma cells are in supplementary. Experiments were conducted in presence of 50 ng/mL hGH and 0.5% final concentration of DMSO was used as control. In all cases, drug treatment was for 24 h starting 48 h post-transfection. RNA expressions were quantified by RT-qPCR and normalized against expression of ACTB and GAPDH as reference genes [*p < 0.05, Wilcoxon sign rank test, n = 3]. c Changes in protein expressions of ABCC1 and ABCB8 were analyzed. Western blot comparison was done for protein extracted from all four melanoma cells, 60 h post-transfection with GHR-or scr-siRNA. Blots were quantified using ImageJ software and mean of three blots per sample was taken. Expressions were normalized against expression of ACTB (β-actin) [*p < 0.05, Student’s t test, n = 3]
Extended GH Treatment significantly Increases Expression of GHR, ABC-Transporter Pumps and Chemotherapeutic Drug-EC50s in Human Melanoma Cells
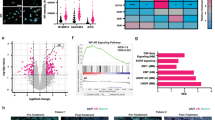
We and others have had previously observed GH production by human melanoma and other forms of human cancer [34, 42, 72–74]. In order to determine whether sustained exposure or an acute dose to exogenously added GH affects our observations following GHRKD, we cultured SK-MEL-28 and SK-MEL-5 human melanoma cell lines for 8 weeks with 20 ng/mL of recombinant hGH (changed every 3 days) in the culture media. The same type of melanoma cells (SK-MEL-28 and SK-MEL-5) were also grown with vemurafenib, for developing vemurafenib resistance, in either presence or absence of 20 ng/mL hGH as described in the “Methods” section. We found that hGH treatment alone resulted in an ~5-fold increase in vemurafenib EC-50 in SK-MEL-28 and a 2-fold increase in SK-MEL-5 human melanoma cell lines (Figs 3a, b). In SK-MEL-28 cells, the vemurafenib-EC50 shifted from 9 to 38 nM (Fig. 3a) while in SK-MEL-5 cells, the EC-50 value increased from 10.2 to 24.6 nM (Fig. 3b). Vemurafenib-resistant (VemR) cells that were grown in absence of GH in media showed an EC50 shift for vemurafenib to 139 nM (>11-fold shift) for SK-MEL-28 and to 75.3 nM (> 6-fold shift) for SK-MEL-5 cells. The presence of GH in the media during culture to develop VemR cells, lead to an even greater increase in vemurafenib resistance in both melanoma cell types. SK-MEL-28 had an EC50 of 278 nM (>25-fold shift), and SK-MEL-5 cells had an EC50 of 98.8 nM (>8-fold shift) for vemurafenib, when grown in presence of GH (Fig. 3a, b).
Effect of GH treatment in vemurafenib resistance in human melanoma cells. SK-MEL-28 and SK-MEL-5 cells grown in normal media (C), or in presence of 20 ng/mL GH (CGH), or with 3-day cycles of 20 nM vemurafenib (V) or with 3-day cycles of 20 nM vemurafenib in presence of 20 ng/mL GH (VGH). EC-50 of all four groups per cell line was measured and the ratio to untreated control (C) was expressed as EC50 ratio for SK-MEL-28 (a) and SK-MEL-5 (b). Relative RNA expression in SK-MEL-28 (c) and SK-MEL-5 (d) cells of ABCB1, ABCB5, ABCB8, ABCC1, ABCC2, ABCG1, ABCG2 and GH, GHR, CDH1, CDH2, and vimentin. e Heat-map showing the statistically significant variations in RNA expressions of ABC transporters in three human melanoma cell lines, following treatment with all five drugs, in presence of 50 ng/mL GH for 24 h (e1) or 20 ng/mL GH treatment for 8-weeks (e2). Detailed comparison for SK-MEL-28 (Fig S9), MALME-3M (Fig S10), and SK-MEL-5 (Fig S11) melanoma cells are in supplementary. In all cases, drug treatment was for 24 hr with 0.5% final DMSO concentration. RNA expressions were quantified by RT-qPCR and normalized against expression of ACTB and GAPDH as reference genes [*p < 0.05, Wilcoxon sign rank test, n = 3]
We further determined RNA levels of GH and GHR in these VemR cells, and found that while GH levels were mostly unchanged, the GHR levels were significantly upregulated in SK-MEL-28 (>8-fold increase in GH alone and >12-fold in vemurafenib treated) and SK-MEL-5 (<2-fold increase) cells (Fig. 3c, d). In the light of our previous findings that GH-GHR in human melanoma drives epithelial-to-mesenchymal transition (EMT), we inquired into the RNA levels of E-cadherin (CDH1), N-cadherin (CDH2), and vimentin, in these VemR cell lines. GH in presence or absence of vemurafenib treatments caused robust downregulation of CDH1 levels in both SK-MEL-28 (3-fold) and SK-MEL-5 (2-fold) cells (Fig. 3c, d). On the other hand, SK-MEL-28 VemR cells grown with GH, were found to have a >10-fold increase in CDH2 and >3-fold increase in vimentin levels (Fig. 3c).
Subsequently, we analyzed the changes in RNA levels of ABC transporters in the VemR cell lines. Consistent with the drug resistant phenotypes induced by GH-GHR as shown above, hGH treatment alone resulted in a drastic upregulated expression of ABCB1 (>25-fold in GH alone, >13-fold in vemurafenib treated), ABCB8 (>2-fold in GH treated and >3-fold in GH + vemurafenib treated), ABCC2 (>5-fold in GH alone, >6-fold in vemurafenib treated), and ABCG1 (>15-fold in both GH and vemurafenib treated) in SK-MEL-28 cells (Fig. 4b). Additionally, we saw similar results in SK-MEL-5 cells where RNA levels of ABCB8 (>2-fold in both vemurafenib and GH + vemurafenib treated) and ABCG1 (>3-fold in GH alone, >2-fold in vemurafenib treated) was particularly elevated with hGH treatment and drug resistance (Fig. 3d). The results for ABC efflux pumps whose expressions significantly increased with GH and/or vemurafenib treatment are listed in Table 2. This consistent change in ABC-transporter levels observed following a more chronic exposure or pretreatment with GH (Fig 3e2) were not observed for a 24-h acute treatment with 50 ng/mL GH given along with drug exposure (Fig 3e1). The acute treatment with GH caused upregulation as well as downregulation of several ABC transporters (listed in Table 3). Although some of those variations were consistent with GHRKD results, the results were not conclusive. The observations indicate that a rather sustained GH exposure might lead to a more aggressive chemo-refractory form of melanoma.
GHRKD resulted in increased drug retention and drastically reduced proliferation of melanoma cells. Changes in amounts of calcein retained inside cells following treatment with calcein-AM ester was analyzed by the fluorescence readout from intracellular calcein. Increased abundance of transporter pumps is reflected by decreased levels of intracellular calcein. a Significantly lower calcein retention in human melanoma cells compared to human melanocyte ST-MEL. b Human melanoma cells exhibit significantly higher levels of intracellular calcein following GHRKD. Assays were performed 48 h post-transfection with either scr-siRNA or GHR-siRNA. Effect of GHRKD on cell proliferation following 24 h exposure to EC50 levels of cisplatin and paclitaxel was tested. c SK-MEL-28 and d MALME-3M cells were exposed to DMSO (vehicle), or 10 um cisplatin (Cis), or 5 nM paclitaxel (Pac) for 24 h. Treatments were done 48 h post-transfection with either scr-siRNA (scr) or GHR-siRNA (GHR). Mean of three independent experiments performed in triplicate was taken [*p < 0.05, Student’s t test, n = 3]
GHR Knockdown Leads to significantly Higher Drug Retention and Sensitizes Human Melanoma Cells to Low Doses of Chemotherapy
In view of the above findings, it was reasonable to expect that targeting GHR by siRNA mediated KD might cripple important mechanisms of drug efflux in melanoma cells. In that case, GHRKD should also result in increased intracellular drug retention, leading to cytotoxicity and cell-death for the tumor cells. In the fluorometric calcein retention assay, which quantifies ABC efflux pump mediated drug efflux activity, we indeed observed a significantly lower concentration of retained calcein in the human melanoma cells compared to melanocyte (Fig. 4a). Following GHRKD, significantly higher concentration of calcein was retained inside GHR-siRNA-transfected melanoma cells compared to the scramble siRNA-transfected controls (Fig. 4b). The results corroborated our above observations demonstrating the critical role of the GH action in multidrug resistance in melanoma.
A significant suppression of expression of several efflux pumps leading to a longer retention of xenobiotic compounds inside the GHRKD melanoma cells compared to the scr-siRNA-treated controls should cause an increased growth inhibition and lower the required amount of drug required for required anti-tumor effects. Therefore, we quantified the cell proliferation levels of SK-MEL-28 and MALME-3M cells following exposure to EC-50 levels of cisplatin (10 uM) and paclitaxel (5 nM) with and without siRNA-mediated KD of GHR. We observed drastic inhibition (>90%) in all cases (Fig. 4c, d). The results underscore the effect of targeting GHR in sensitization of the human melanoma cells to low doses of anti-cancer drugs.
Discussion
The ATP-binding cassette (ABC) transporters are ATP-dependent xenobiotic efflux pumps which are employed by various cancer cells as an important mechanism of lowering the intracellular accumulation of cytotoxic anti-cancer drugs [64, 65, 75–77]. ABCB1 is expressed in of several tissues and has been extensively reported to function in conferring resistance to a number of anti-tumor drugs in different forms of human cancers including melanoma in vitro [65, 77] and in mice [78]. In our study, we observed robust downregulation of ABCB1 following GHRKD especially with cisplatin treatment across all four melanoma cell lines. Further ABCB1 expression was increased to >25-fold by GH treatment alone and also >13-fold in vemurafenib-resistant SK-MEL-28 cells, which indicates GH conferred drug resistance to melanoma tumors and subsequent sensitization following GHR blockade. ABCB8 was one of the most abundantly expressed ABC drug efflux pumps in human melanoma cells. Although the physiological role of ABCB8, located at the inner mitochondrial membrane is not well-defined [65], its distinct role in protecting the mitochondrial genome against doxorubicin in melanoma has been identified [27]. In this study, we observed that ABCB8 levels in response to doxorubicin treatment were significantly downregulated by GHRKD across all four melanoma cell lines. GHRKD also successfully suppressed ABCB8 levels in response to paclitaxel and vemurafenib in three out of four cell lines tested. ABCC1 and ABCC2 together with ABCG1 and ABCG2 have also been extensively observed to be critical mediators of cancer drug resistance in several forms of human cancer [71, 78, 79] and more prominently in melanoma [28, 69]. They have overlapping substrates in drug efflux [65] and have been reported to be involved with melanoma resistance to multiple drugs including vemurafenib [69, 77] and cisplatin [19]. We observed that following GHRKD, ABCC1 levels in response to paclitaxel was consistently suppressed across all four melanoma cell lines whereas ABCC2 levels in response to vemurafenib was suppressed in three out of four melanoma cell lines. ABCG1 was especially important in SK-MEL-28, MALME-3M, and MDA-MB-435 cells where GHRKD significantly lowered its expression compared to the scr-siRNA-transfected controls for all five drugs tested. In addition, GH treatment especially upregulated ABCG1 levels by >10-fold in SK-MEL-28 cells, wherein we also observed a >5-fold shift in the vemurafenib EC50. Lastly, GHRKD markedly suppressed ABCG2 levels in response to doxorubicin and vemurafenib treatments compared to corresponding controls in all melanoma cell lines except MDA-MB-435. It was particularly interesting to find significantly reduced cell proliferation rates of human melanoma cells, even up to >50% in some cases, when GHRKD was combined with sub-EC50 doses of the drugs. This was corroborated with complete inhibition of proliferation of the same melanoma cells when GHRKD was combined with EC50 doses of the drugs. We had a further confirmation of the GH-mediated drug resistance in melanoma cells from the 5–25-fold increase in vemurafenib EC50 when the cells were grown in presence of GH. Therefore, our results show a sensitization to chemotherapy of the human melanoma cells due to the GHR blockade, and indicate that a lower dose of anti-tumor drugs could be more efficacious when combined with GHR inhibition. These results could act as a springboard for subsequent detailed analysis of this unique aspect of GH-regulated drug efflux in melanoma cells in a drug type or ABC-transporter class specific manner.
The results identify that GHRKD attenuates expression of ABC transporters mediating multidrug resistance in human melanoma and reveal a cell-specific and multiple drug-specific variations of seven different ABC transporters. The pattern of response between different melanoma cell lines to anti-cancer drugs following GHRKD is variable, and allows us to arrive at the following important inferences: (i) ABCG1, an important protein for conferring drug resistance in melanoma, is consistently downregulated by GHRKD in SK-MEL-28, MALME-3M, and SK-MEL-5 following all five drug treatments; (ii) ABCC1, found to confer paclitaxel resistance in melanoma side-population cells [80], was downregulated in all four melanoma cell lines following GHRKD in response to paclitaxel treatment; (iii) ABCB1 levels in response to cisplatin treatment and ABCB8 levels in response to doxorubicin were significantly suppressed by GHRKD in all melanoma cells tested; and (iv) in SK-MEL-28 and SK-MEL-5 cells, GHRKD suppressed levels of ABCC2, and ABCG2 in response to vemurafenib treatment. Thus, we hypothesize that targeting GHR can be an effective modality for specific classes of chemotherapeutic treatments in sensitizing melanoma to the drug action. For example, GHR antagonism coupled with vemurafenib or paclitaxel for GH-responsive (GHR expressing) tumors using in vivo models can readily verify our hypothesis.
Melanomas are one of the most drug resistant forms of human cancer and were also found to express one of the highest levels of GHR among all human cancers in the NCI’s panel of human cancer cell lines [41]. Significant reduction in expression of multiple different ABC-transporter pumps following a decrease in GHR indicates a GH action dependent mechanism regulating drug efflux from melanoma cells. In fact, we recently reported the existence of GH-GHR-mediated regulation of the mTOR pathway in melanoma cells [42] and GH-induced activation of the pathway is known to be necessary for rapid activation of protein synthesis [17, 81] as might be expected to be required in the case of expression of transporter pumps in response to exposure to drugs. Further, the ubiquitous transcription factor NF-Y, known to be involved in several processes including upregulating transcription of ABC drug transporters [82–84], has been found to work in conjunction with the GH-regulated transcription factor STAT5 in female hamster liver cells [85]. We are currently verifying our observations in vivo using appropriately designed mouse models of differential growth hormone action: transgenic (bGH), GH antagonist transgenic mice (GHRA) or GHR deficient (GHR−/−) mice [86].
In conclusion, this study presents a novel GH action in mediating multidrug resistance in a human melanoma cells. This study is in alignment with our observation of a significant GH-dependent variation in RNA and protein levels of several intracellular mediators of oncogenic signaling pathways in melanoma and adds important and unknown information of the downstream effects of our earlier findings. Decreased drug efflux machinery, increased drug retention, a reversal in EMT markers, and a markedly reduced cell proliferation at low doses of chemotherapy following GHRKD together support the idea of approaching GH-GHR interaction as a suitable chemotherapeutic target of intervention as a combination therapy for several classes of anti-tumor compounds. If effective in vivo, this approach can have several important downstream effects in cancer therapy: (i) a significantly lower drug dose and possibly shorter duration of chemotherapy can be applied in combination or following pretreatment with GHR antagonists. This in effect might significantly reduce the harsh side effects associated with chemotherapy. (ii) GHR inhibition as a means of sensitizing the tumor cells should be a valuable approach in drug development. Combination of GHR inhibition and chemotherapy can not only markedly improve the efficacy of available anti-melanoma drugs but may also assist the development of novel chemotherapeutic compounds. Decreased drug retention in tumors is a major hurdle in establishing efficacy of numerous drug candidates in pharmaceutical research and development. Our study identifies a possible breakthrough in this problem by identifying that GH-GHR interaction is a critical mediator of melanoma drug resistance and targeting this interaction may successfully lead to improved drug action and might greatly benefit millions of melanoma and cancer patients worldwide.
References
Welch HG (2005) Skin biopsy rates and incidence of melanoma: population based ecological study. BMJ 331:481. doi:10.1136/bmj.38516.649537.E0
Linos E, Swetter SM, Cockburn MG, Colditz GA, Clarke CA (2009) Increasing burden of melanoma in the United States. J. Invest. Dermatol. 129:1666–1674. doi:10.1038/jid.2008.423
Hery C, Tryggvadottir L, Sigurdsson T, Olafsdottir E, Sigurgeirsson B, Jonasson JG, Olafsson JH, Boniol M, Byrnes GB, Dore J-F, Autier P (2010) A melanoma epidemic in Iceland: possible influence of sunbed use. Am J Epidemiol 172:762–767. doi:10.1093/aje/kwq238
Geller AC, Clapp RW, Sober AJ, Gonsalves L, Mueller L, Christiansen CL, Shaikh W, Miller DR (2013) Melanoma epidemic: an analysis of six decades of data from the Connecticut Tumor Registry. J Clin Oncol 31:4172–4178. doi:10.1200/JCO.2012.47.3728
Hallberg Ö, Johansson O (2013) Increasing melanoma—too many skin cell damages or too few repairs? Cancers (Basel) 5:184–204. doi:10.3390/cancers5010184
Lowe GC, Saavedra A, Reed KB, Velazquez AI, Dronca RS, Markovic SN, Lohse CM, Brewer JD (2014) Increasing incidence of melanoma among middle-aged adults: an epidemiologic study in Olmsted County, Minnesota. Mayo Clin Proc 89:52–59. doi:10.1016/j.mayocp.2013.09.014
Berwick M (2010) Invited commentary: a sunbed epidemic? Am J Epidemiol 172:768–770. doi:10.1093/aje/kwq232
Boniol M, Autier P, Boyle P, Gandini S (2012) Cutaneous melanoma attributable to sunbed use: systematic review and meta-analysis. BMJ 345:e4757–e4757. doi:10.1136/bmj.e4757
Handler MZ, Ross AL, Shiman MI, Elgart GW, Grichnik JM (2012) Potential role of human growth hormone in melanoma growth promotion. Arch Dermatol 148:1179. doi:10.1001/archdermatol.2012.2149
Ozao-Choy J, Lee DJ, Faries MB (2014) Melanoma vaccines. Surg Clin North Am 94:1017–1030. doi:10.1016/j.suc.2014.07.005
Zitvogel L, Kroemer G (2012) Targeting PD-1/PD-L1 interactions for cancer immunotherapy. Oncoimmunology 1:1223–1225. doi:10.4161/onci.21335
Khattak M, Fisher R, Turajlic S, Larkin J (2013) Targeted therapy and immunotherapy in advanced melanoma: an evolving paradigm. Ther Adv Med Oncol 5:105–118. doi:10.1177/1758834012466280
N. Comfere, Chakraborty, Wieland, Molecular targeted therapies in metastatic melanoma, Pharmgenomics Pers Med (2013) 6:49. doi:10.2147/PGPM.S44800.
McDermott D, Srivastava N (2014) Update on benefit of immunotherapy and targeted therapy in melanoma: the changing landscape. Cancer Manag Res 279. doi:10.2147/CMAR.S64979
Luke JJ, Ott PA (2015) PD-1 pathway inhibitors: the next generation of immunotherapy for advanced melanoma. Oncotarget 6:3479–3492. doi:10.18632/oncotarget.2980
Adam D, Rajakulendran T (2015) Spotlight on pembrolizumab in the treatment of advanced melanoma. Drug Des Devel Ther 2883. doi:10.2147/DDDT.S78036
Russo A, Ficili B, Candido S, Pezzino F, Guarneri C, Biondi A, Travali S, McCubrey J, Spandidos D, Libra M (2014) Emerging targeted therapies for melanoma treatment (review). Int. J, Oncol. doi:10.3892/ijo.2014.2481
Drake WM, Grossman AB, Hutson RK (2005) Effect of treatment with pegvisomant on meningioma growth in vivo. Eur J Endocrinol 152:161–162. doi:10.1530/eje.1.01825
Liedert B, Materna V, Schadendorf D, Thomale J, Lage H (2003) Overexpression of cMOAT (MRP2/ABCC2) is associated with decreased formation of platinum-DNA adducts and decreased G2-arrest in melanoma cells resistant to cisplatin. J Invest Dermatol 121:172–176. doi:10.1046/j.1523-1747.2003.12313.x
Heerboth S, Housman G, Leary M, Longacre M, Byler S, Lapinska K, Willbanks A, Sarkar S (2015) EMT and tumor metastasis. Clin Transl Med 4:6. doi:10.1186/s40169-015-0048-3
Hernandez-Davies JE, Tran TQ, Reid MA, Rosales KR, Lowman XH, Pan M, Moriceau G, Yang Y, Wu J, Lo RS, Kong M (2015) Vemurafenib resistance reprograms melanoma cells towards glutamine dependence. J Transl Med 13:210. doi:10.1186/s12967-015-0581-2
Thang ND, Nghia PT, Kumasaka MY, Yajima I, Kato M (2015) Treatment of vemurafenib-resistant SKMEL-28 melanoma cells with paclitaxel. Asian Pac J Cancer Prev 16:699–705 http://www.ncbi.nlm.nih.gov/pubmed/25684511 (accessed September 26, 2016)
Bu X, Mahoney KM, Freeman GJ (2016) Learning from PD-1 resistance: new combination strategies. Trends Mol Med 22:448–451. doi:10.1016/j.molmed.2016.04.008
Zaretsky JM, Garcia-Diaz A, Shin DS, Escuin-Ordinas H, Hugo W, Hu-Lieskovan S, Torrejon DY, Abril-Rodriguez G, Sandoval S, Barthly L, Saco J, Homet Moreno B, Mezzadra R, Chmielowski B, Ruchalski K, Shintaku IP, Sanchez PJ, Puig-Saus C, Cherry G, Seja E, Kong X, Pang J, Berent-Maoz B, Comin-Anduix B, Graeber TG, Tumeh PC, Schumacher TNM, Lo RS, Ribas A (2016) Mutations associated with acquired resistance to PD-1 blockade in melanoma. N Engl J Med 375:819–829. doi:10.1056/NEJMoa1604958
Tumeh PC, Harview CL, Yearley JH, Shintaku IP, Taylor EJM, Robert L, Chmielowski B, Spasic M, Henry G, Ciobanu V, West AN, Carmona M, Kivork C, Seja E, Cherry G, Gutierrez AJ, Grogan TR, Mateus C, Tomasic G, Glaspy JA, Emerson RO, Robins H, Pierce RH, Elashoff DA, Robert C, Ribas A (2014) PD-1 blockade induces responses by inhibiting adaptive immune resistance. Nature 515:568–571. doi:10.1038/nature13954
Luke JJ, Hodi FS (2013) Ipilimumab, vemurafenib, dabrafenib, and trametinib: synergistic competitors in the clinical management of BRAF mutant malignant melanoma. Oncologist 18:717–725. doi:10.1634/theoncologist.2012-0391
Elliott AM, Al-Hajj MA (2009) ABCB8 mediates doxorubicin resistance in melanoma cells by protecting the mitochondrial genome. Mol Cancer Res 7:79–87. doi:10.1158/1541-7786.MCR-08-0235
Chen KG, Valencia JC, Gillet J-P, Hearing VJ, Gottesman MM (2009) Involvement of ABC transporters in melanogenesis and the development of multidrug resistance of melanoma. Pigment Cell Melanoma Res. 22:740–749. doi:10.1111/j.1755-148X.2009.00630.x
Walsh N, Kennedy S, Larkin AM, Tryfonopoulos D, Eustace AJ, Mahgoub T, Conway C, Oglesby I, Collins D, Ballot J, Ooi WS, Gullo G, Clynes M, Crown J, O’Driscoll L (2010) Membrane transport proteins in human melanoma: associations with tumour aggressiveness and metastasis. Br J Cancer 102:1157–1162. doi:10.1038/sj.bjc.6605590
Lackner MR, Wilson TR, Settleman J (2012) Mechanisms of acquired resistance to targeted cancer therapies. Future Oncol 8:999–1014. doi:10.2217/fon.12.86
Fischer KR, Durrans A, Lee S, Sheng J, Li F, Wong STC, Choi H, El Rayes T, Ryu S, Troeger J, Schwabe RF, Vahdat LT, Altorki NK, Mittal V, Gao D (2015) Epithelial-to-mesenchymal transition is not required for lung metastasis but contributes to chemoresistance. Nature 527:472–476. doi:10.1038/nature15748
Mitra A, Mishra L, Li S (2015) EMT, CTCs and CSCs in tumor relapse and drug-resistance. Oncotarget 6:10697–10711. doi:10.18632/oncotarget.4037
Zatelli MC, Minoia M, Molè D, Cason V, Tagliati F, Margutti A, Bondanelli M, Ambrosio MR, degli Uberti E (2009) Growth hormone excess promotes breast cancer chemoresistance. J Clin Endocrinol Metab 94:3931–3938. doi:10.1210/jc.2009-1026
Perry JK, Mohankumar KM, Emerald BS, Mertani HC, Lobie PE (2008) The contribution of growth hormone to mammary neoplasia. J Mammary Gland Biol Neoplasia 13:131–145. doi:10.1007/s10911-008-9070-z
Pandey V, Perry JK, Mohankumar KM, Kong X-J, Liu S-M, Wu Z-S, Mitchell MD, Zhu T, Lobie PE (2008) Autocrine human growth hormone stimulates oncogenicity of endometrial carcinoma cells. Endocrinology 149:3909–3919. doi:10.1210/en.2008-0286
Bougen NM, Steiner M, Pertziger M, Banerjee A, Brunet-Dunand SE, Zhu T, Lobie PE, Perry JK (2012) Autocrine human GH promotes radioresistance in mammary and endometrial carcinoma cells. Endocr Relat Cancer 19:625–644. doi:10.1530/ERC-12-0042
Zhang W, Qian P, Zhang X, Zhang M, Wang H, Wu M, Kong X, Tan S, Ding K, Perry JK, Wu Z, Cao Y, Lobie PE, Zhu T (2015) Autocrine/paracrine human growth hormone-stimulated MicroRNA 96-182-183 cluster promotes epithelial-mesenchymal transition and invasion in breast cancer. J Biol Chem 290:13812–13829. doi:10.1074/jbc.M115.653261
Wyatt D (1999) Melanocytic nevi in children treated with growth hormone. Pediatrics 104:1045–1050
Caldarola G, Battista C, Pellicano R (2010) Melanoma onset after estrogen, thyroid, and growth hormone replacement therapy. Clin Ther 32:57–59. doi:10.1016/j.clinthera.2010.01.011
Lincoln DT, Sinowatz F, Kolle S, Takahashi H, Parsons P, Waters M (1999) Up-regulation of growth hormone receptor immunoreactivity in human melanoma. Anticancer Res 19:1919–1931
Sustarsic EG, Junnila RK, Kopchick JJ (2013) Human metastatic melanoma cell lines express high levels of growth hormone receptor and respond to GH treatment. Biochem Biophys Res Commun 441:144–150. doi:10.1016/j.bbrc.2013.10.023
Basu R, Wu S, Kopchick J (2017) Targeting growth hormone receptor in human melanoma cells attenuates tumor progression and epithelial mesenchymal transition via suppression of multiple oncogenic pathways. Oncotarget 5. doi:10.18632/oncotarget.15375
Vergani E, Vallacchi V, Frigerio S, Deho P, Mondellini P, Perego P, Cassinelli G, Lanzi C, Testi MA, Rivoltini L, Bongarzone I, Rodolfo M (2011) Identification of MET and SRC activation in melanoma cell lines showing primary resistance to PLX4032. Neoplasia 13:1132–IN17. doi:10.1593/neo.111102
Boone B, Jacobs K, Ferdinande L, Taildeman J, Lambert J, Peeters M, Bracke M, Pauwels P, Brochez L (2011) EGFR in melanoma: clinical significance and potential therapeutic target. J Cutan Pathol 38:492–502. doi:10.1111/j.1600-0560.2011.01673.x
Girotti MR, Pedersen M, Sanchez-Laorden B, Viros A, Turajlic S, Niculescu-Duvaz D, Zambon A, Sinclair J, Hayes A, Gore M, Lorigan P, Springer C, Larkin J, Jorgensen C, Marais R (2013) Inhibiting EGF receptor or SRC family kinase signaling overcomes BRAF inhibitor resistance in melanoma. Cancer Discov 3:158–167. doi:10.1158/2159-8290.CD-12-0386
Goetz EM, Ghandi M, Treacy DJ, Wagle N, Garraway LA (2014) ERK mutations confer resistance to mitogen-activated protein kinase pathway inhibitors. Cancer Res 74:7079–7089. doi:10.1158/0008-5472.CAN-14-2073
Kim H, Frederick DT, Levesque MP, Cooper ZA, Feng Y, Krepler C, Brill L, Samuels Y, Hayward NK, Perlina A, Piris A, Zhang T, Halaban R, Herlyn MM, Brown KM, Wargo JA, Dummer R, Flaherty KT, Ronai ZA (2015) Downregulation of the ubiquitin ligase RNF125 underlies resistance of melanoma cells to BRAF inhibitors via JAK1 deregulation. Cell Rep 11:1458–1473. doi:10.1016/j.celrep.2015.04.049
Zhao C, Li H, Lin H-J, Yang S, Lin J, Liang G (2016) Feedback activation of STAT3 as a cancer drug-resistance mechanism. Trends Pharmacol Sci 37:47–61. doi:10.1016/j.tips.2015.10.001
Heynen GJ, Fonfara A, Bernards R (2014) Resistance to targeted cancer drugs through hepatocyte growth factor signaling. Cell Cycle 13:3808–3817. doi:10.4161/15384101.2014.988033
Ma J, Lyu H, Huang J, Liu B (2014) Targeting of erbB3 receptor to overcome resistance in cancer treatment. Mol Cancer 13:105. doi:10.1186/1476-4598-13-105
Fattore L, Malpicci D, Marra E, Camerlingo R, Roscilli G, Belleudi F, Ribas A, Mancini R, Torrisi M, Aurisicchio L, Ascierto P, Ciliberto G (2015) ErbB3 plays a key role in the early phase of establishment of resistance to BRAF and/or MEK inhibitors. J Transl Med 13:K3. doi:10.1186/1479-5876-13-S1-K3
Cao H-H, Cheng C-Y, Su T, Fu X-Q, Guo H, Li T, Tse AK-W, Kwan H-Y, Yu H, Yu Z-L (2015) Quercetin inhibits HGF/c-Met signaling and HGF-stimulated melanoma cell migration and invasion. Mol Cancer 14:103. doi:10.1186/s12943-015-0367-4
Kim HR, Kim WS, Choi YJ, Choi CM, Rho JK, Lee JC (2013) Epithelial-mesenchymal transition leads to crizotinib resistance in H2228 lung cancer cells with EML4-ALK translocation. Mol Oncol 7:1093–1102. doi:10.1016/j.molonc.2013.08.001
Du B, Shim J (2016) Targeting epithelial–mesenchymal transition (EMT) to overcome drug resistance in cancer. Molecules 21:965. doi:10.3390/molecules21070965
Mallini P, Lennard T, Kirby J, Meeson A (2014) Epithelial-to-mesenchymal transition: what is the impact on breast cancer stem cells and drug resistance. Cancer Treat Rev 40:341–348. doi:10.1016/j.ctrv.2013.09.008
Liang S-Q, Marti TM, Dorn P, Froment L, Hall SRR, Berezowska S, Kocher G, Schmid RA, Peng R-W (2015) Blocking the epithelial-to-mesenchymal transition pathway abrogates resistance to anti-folate chemotherapy in lung cancer. Cell Death Dis 6:e1824. doi:10.1038/cddis.2015.195
McDermott M, Eustace AJ, Busschots S, Breen L, Crown J, Clynes M, O’Donovan N, Stordal B (2014) In vitro development of chemotherapy and targeted therapy drug-resistant cancer cell lines: a practical guide with case studies. Front Oncol 4. doi:10.3389/fonc.2014.00040
Aksamitiene E, Hoek JB, Kholodenko B, Kiyatkin A (2007) Multistrip western blotting to increase quantitative data output. Electrophoresis 28:3163–3173. doi:10.1002/elps.200700002
T.L. Riss, R.A. Moravec, A.L. Niles, S. Duellman, H.A. Benink, T.J. Worzella, L. Minor, Cell viability assays, 2004. http://www.ncbi.nlm.nih.gov/pubmed/23805433.
Riss TL, Moravec RA, Niles AL (2011) Cytotoxicity testing: measuring viable cells, dead cells, and detecting mechanism of cell death. Methods Mol Biol 740:103–114. doi:10.1007/978-1-61779-108-6_12
Borra RC, Lotufo MA, Gagioti SM, Barros FDM, Andrade PM (2009) A simple method to measure cell viability in proliferation and cytotoxicity assays. Braz Oral Res 23:255–262. doi:10.1590/S1806-83242009000300006
Tiberghien F, Loor F (1996) Ranking of P-glycoprotein substrates and inhibitors by a calcein-AM fluorometry screening assay. Anti-Cancer Drugs 7:568–578. doi:10.1097/00001813-199607000-00012
Schonk DM, Kuijpers HJ, van Drunen E, van Dalen CH, Geurts van Kessel AH, Verheijen R, Ramaekers FC (1989) Assignment of the gene(s) involved in the expression of the proliferation-related Ki-67 antigen to human chromosome 10. Hum Genet 83:297–299 http://www.ncbi.nlm.nih.gov/pubmed/2571566
Heimerl S, Bosserhoff AK, Langmann T, Ecker J, Schmitz G (2007) Mapping ATP-binding cassette transporter gene expression profiles in melanocytes and melanoma cells. Melanoma Res 17:265–273. doi:10.1097/CMR.0b013e3282a7e0b9
Pan S-T, Li Z-L, He Z-X, Qiu J-X, Zhou S-F (2016) Molecular mechanisms for tumour resistance to chemotherapy. Clin Exp Pharmacol Physiol 43:723–737. doi:10.1111/1440-1681.12581
Landreville S, Agapova OA, Kneass ZT, Salesse C, William Harbour J (2011) ABCB1 identifies a subpopulation of uveal melanoma cells with high metastatic propensity. Pigment Cell Melanoma Res 24:430–437. doi:10.1111/j.1755-148X.2011.00841.x
Chartrain M, Riond J, Stennevin A, Vandenberghe I, Gomes B, Lamant L, Meyer N, Gairin JE, Guilbaud N, Annereau JP (2012) Melanoma chemotherapy leads to the selection of ABCB5-expressing cells. PLoS One 7:e36762. doi:10.1371/journal.pone.0036762
Wilson BJ, Saab KR, Ma J, Schatton T, Putz P, Zhan Q, Murphy GF, Gasser M, Waaga-Gasser AM, Frank NY, Frank MH (2014) ABCB5 maintains melanoma-initiating cells through a proinflammatory cytokine signaling circuit. Cancer Res 74:4196–4207. doi:10.1158/0008-5472.CAN-14-0582
Michaelis M, Rothweiler F, Nerreter T, van Rikxoort M, Zehner R, Dirks WG, Wiese M, Cinatl J (2014a) Association between acquired resistance to PLX4032 (vemurafenib) and ATP-binding cassette transporter expression. BMC Res Notes 7:710. doi:10.1186/1756-0500-7-710
Sag D, Cekic C, Wu R, Linden J, Hedrick CC (2015) The cholesterol transporter ABCG1 links cholesterol homeostasis and tumour immunity. Nat Commun 6:6354. doi:10.1038/ncomms7354
Ejendal KF, Hrycyna CA (2002) Multidrug resistance and cancer: the role of the human ABC transporter ABCG2. Curr Protein Pept Sci 3:503–511
Brunet-Dunand SE, Vouyovitch C, Araneda S, Pandey V, Vidal LJ-P, Print C, Mertani HC, Lobie PE, Perry JK (2009) Autocrine human growth hormone promotes tumor angiogenesis in mammary carcinoma. Endocrinology 150:1341–1352. doi:10.1210/en.2008-0608
Wu Z-S, Yang K, Wan Y, Qian P-X, Perry JK, Chiesa J, Mertani HC, Zhu T, Lobie PE (2011) Tumor expression of human growth hormone and human prolactin predict a worse survival outcome in patients with mammary or endometrial carcinoma. J Clin Endocrinol Metab 96:E1619–E1629. doi:10.1210/jc.2011-1245
Perry JK, Emerald BS, Mertani HC, Lobie PE (2006) The oncogenic potential of growth hormone. Growth Hormon IGF Res 16:277–289. doi:10.1016/j.ghir.2006.09.006
Fletcher JI, Haber M, Henderson MJ, Norris MD (2010) ABC transporters in cancer: more than just drug efflux pumps. Nat Rev Cancer 10:147–156. doi:10.1038/nrc2789
Santisteban M (2010) ABC transporters as molecular effectors of pancreatic oncogenic pathways: the hedgehog-GLI model. J Gastrointest Cancer 41:153–158. doi:10.1007/s12029-010-9144-1
Michaelis M, Rothweiler F, Nerreter T, Van Rikxoort M, Sharifi M, Wiese M, Ghafourian T, Cinatl J (2014b) Differential effects of the oncogenic BRAF inhibitor PLX4032 (vemurafenib) and its progenitor PLX4720 on ABCB1 function. J Pharm Pharm Sci 17:154–168 http://www.ncbi.nlm.nih.gov/pubmed/24735766
Li S, Zhang W, Yin X, Xing S, Xie HQ, Cao Z, Zhao B (2015) Mouse ATP-binding cassette (ABC) transporters conferring multi-drug resistance. Anti Cancer Agents Med Chem 15:423–432 http://www.ncbi.nlm.nih.gov/pubmed/25929575
Nakanishi T, Ross DD (2012) Breast cancer resistance protein (BCRP/ABCG2): its role in multidrug resistance and regulation of its gene expression. Chin J Cancer 31:73–99. doi:10.5732/cjc.011.10320
Wouters J, Stas M, Gremeaux L, Govaere O, Van den Broeck A, Maes H, Agostinis P, Roskams T, van den Oord JJ, Vankelecom H (2013) The human melanoma side population displays molecular and functional characteristics of enriched chemoresistance and tumorigenesis. PLoS One 8:e76550. doi:10.1371/journal.pone.0076550
Hayashi AA, Proud CG (2007) The rapid activation of protein synthesis by growth hormone requires signaling through mTOR. AJP Endocrinol Metab 292:E1647–E1655. doi:10.1152/ajpendo.00674.2006
Jin S, Scotto KW (1998) Transcriptional regulation of the MDR1 gene by histone acetyltransferase and deacetylase is mediated by NF-Y. Mol Cell Biol 18:4377–4384 http://www.ncbi.nlm.nih.gov/pubmed/9632821
Scotto KW (2003) Transcriptional regulation of ABC drug transporters. Oncogene 22:7496–7511. doi:10.1038/sj.onc.1206950
Oldfield AJ, Yang P, Conway AE, Cinghu S, Freudenberg JM, Yellaboina S, Jothi R (2014) Histone-fold domain protein NF-Y promotes chromatin accessibility for cell type-specific master transcription factors. Mol Cell 55:708–722. doi:10.1016/j.molcel.2014.07.005
Subramanian A, Wang J, Gil G (1998) STAT 5 and NF-Y are involved in expression and growth hormone-mediated sexually dimorphic regulation of cytochrome P450 3A10/lithocholic acid 6beta-hydroxylase. Nucleic Acids Res 26:2173–2178 http://www.ncbi.nlm.nih.gov/pubmed/9547277
Kopchick JJ, List EO, Kelder B, Gosney ES, Berryman DE (2014) Evaluation of growth hormone (GH) action in mice: discovery of GH receptor antagonists and clinical indications. Mol Cell Endocrinol 386:34–45. doi:10.1016/j.mce.2013.09.004
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare that they have no conflict of interest.
Funding
This work was supported in part by the State of Ohio’s Eminent Scholar Program that includes a gift from Milton and Lawrence Goll, by the AMVETS, and the Edison Biotechnology Institute at Ohio University.
Electronic Supplementary Material
ESM 1
(PPTX 3776 kb)
Rights and permissions
About this article
Cite this article
Basu, R., Baumgaertel, N., Wu, S. et al. Growth Hormone Receptor Knockdown Sensitizes Human Melanoma Cells to Chemotherapy by Attenuating Expression of ABC Drug Efflux Pumps. HORM CANC 8, 143–156 (2017). https://doi.org/10.1007/s12672-017-0292-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12672-017-0292-7