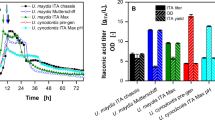
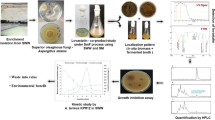
Abstract
Aspergillus niger B60 was screened for the first time toward extracellular tannase and gallic acid production by submerged fermentation using synthetic media supplemented with tannic acid as the sole carbon source at a wide concentration range (5–150 g/L). Maximum tannase (47 IU/mL) and gallic acid production (36 g/L) was obtained at initial tannic acid concentration 100 g/L. For this study, it was of interest to valorize non-sterile table olive processing wastewaters for fungal tannase production. In particular, lye and washing water effluents from Spanish-style green olive processing enriched with 100 g/L tannic acid provided effective alternative substrates for the production of tannase (21 IU/mL and 17 IU/mL, respectively) and gallic acid (22 g/L and 14 g/L, respectively). The fungal growth and tannase production kinetics were described by the Logistic and Luedeking–Piret models, respectively. The maximum dry biomass content and the maximum specific growth rate were more pronounced in the tannic acid-rich effluents (16–18 g/L and 0.5–0.6 1/h, respectively) than in the synthetic medium (11 g/L and 0.4 1/h, respectively) although in all cases tannase production was growth-associated. These novel findings cast a new light on successful biorefinery strategies of the effluents and warrant further investigation via process scaling-up and optimization.
Graphical Abstract

Similar content being viewed by others
Avoid common mistakes on your manuscript.
Statement of Novelty
Tannase, is a vital enzyme used for the production of foods, beverages, animal feed, pharmaceuticals and chemicals. Among these valuable products, tannase is primarily used in the manufacture of gallic acid that has also a plethora of commercial applications, including therapeutics, cosmetics, dyes, photography, foods, antimicrobials and radioprotection. Following the sustainability-oriented global trends, several studies have been carried out toward the exploitation of tannin-rich agricultural substrates for the production of fungal tannase. In this direction, the prospect of utilizing effluents from table olive manufacturing as raw materials in a biorefinery, although challenging, still remains largely unexplored. This study proposes a novel process for the the production of extracellular tannase by the robust Aspergillus niger B60 using tannic acid-enriched non-sterile wastewaters from the processing of Spanish−style Chalkidiki green olives. The novel findings cast a new light on successful biorefinery strategies of the effluents and warrant further investigation via process scaling-up and optimization.
Introduction
The industrial production of enzymes is of high importance worldwide toward their effective use in the processing line of high-quality foods and specialty pharmaceuticals. Specifically, the global market value of enzymes was valued at $9.8 billion in 2019 and is projected to reach $16.69 billion in 2027 with an annual growth rate of 6.9%. In 2019, about 61% of the enzyme market share was held by microorganisms. Such production systems are developed because they have simple adaptability and low production cost. Among the different types of microorganisms, fungi are the main source of industrial enzymes [1].
Tannase, also known as tannin acyl hydrolase (EC 3.1.1.20), is a vital enzyme used as a catalyst in the hydrolysis of ester and depside bonds in tannins to release gallic acid and glucose [2]. The catalytic versatility of tannase renders it suitable for the production of foods, beverages, animal feed, pharmaceuticals and chemicals. Among these valuable products, tannase is primarily used in the manufacture of gallic acid, instant tea, acorn liquor and coffee-flavored refreshing drinks. Also, the enzyme can serve as a clarifying agent in wines, beers and fruit juices [3, 4], and as a bioremediation agent in the treatment of tannery wastewaters [5]. Focusing on gallic acid, it has also a plethora of commercial applications, including therapeutics, cosmetics, dyes, photography, foods, antimicrobials and radioprotection [2]. Acid hydrolysis of tannic acid is the conventional method for gallic acid production. Because of its drawbacks in terms of product purity and yield, microbial or enzymatic hydrolysis is considered to be a prominent alternative eco-friendly method for producing gallic acid [6,7,8].
Filamentous fungi are the most widely used microorganisms for tannase production as they have a strong tolerance and biodegradation potential for tannins [5, 9]. Particularly, various strains of Aspergillus niger can survive at the highest levels of tannic acid concentrations (100–150 g/L) reported in literature and have shown the strongest biodegradation ability at these levels (up to 73% reduction of tannic acid concentration) [10,11,12]. Notably, tannase derived from A. niger belongs to the list of commercial enzymes marketed in the European Union [13].
Submerged fermentation is advantageous for the industrial production of extracellular tannase as it ensures the sterility of the process, the effective control of the process conditions (e.g. temperature, pH, agitation and aeration), the construction of accurate and feasible process kinetic models, the short fermentation time, the sufficient substrate uptake as well as the simple and efficient methods for enzyme extraction [2].
Tannase is an inducible enzyme produced by microorganisms in a strain-specific matter. In this view, related literature data are focused on the performance evaluation of different autochthonous and allochthonous tannase producing strains in media containing a wide concentration range of tannic acid that induces tannase production [2, 3]. Following the sustainability-oriented global trends, some studies have been carried out toward the combined supplementation of the liquid nutrient media with pure tannic acid (10–22 g/L) and tannin-rich solid agricultural substrates (rice flour, Emblica officinalis powder, pomegranate rind powder, leaves powder from various fruits, grape pomace) [14,15,16,17,18]. It should be pointed out that the agricultural substrates contain complex tannins that are not easily biodegradable and, thus, the simultaneous addition of pure tannic acid favors the microbial performance [17].
Effluents from olive oil and table olive manufacturing create severe environmental problems in the olive producing areas. Specifically in the Mediterranean region, large volumes of the above-mentioned wastewaters are produced seasonally in restricted geographic areas by small-sized enterprises and usually disposed untreated to inland, rivers or the sea leading to risks of surface and groundwater contamination. The prospect of utilizing these phytotoxic streams as raw materials in a biorefinery is challenging [19, 20]. Regarding the huge amounts (more than 107 m3 annually) of olive mill wastewaters generated from the olive oil manufacturing (more than 98% of global olive oil production in the Mediterranean region), a number of studies have developed biological processes using Aspergillus species to detoxify them and also produce the value-added enzyme tannase (0.3–8 IU/mL) [21,22,23]. Still, there is insufficient evidence for the production of fungal tannase in table olive processing wastewaters.
During the various processing stages of table olives, large quantities of water and chemicals (such as NaOH and NaCl) are used, resulting in the production of polluting and difficult to handle wastewaters. Depending on the treatment applied in each production process, these streams’ volume and composition vary greatly. Those that derive from the Spanish-style green olive processing correspond to strongly phytotoxic streams with high organic matter (average value of COD equal to 18.8 g O2/L). Special focus should be given to the lye (debittering stage) and washing water (washing stage) effluents, which correspond to the 75% of the total volume of wastewaters from Spanish-style processing, have extremely high pH values (10–12) and alkalinity, and are generated on daily basis within ~ 1 month. On the other hand, processing that is based on untreated green olives produces acidified brine effluent (average value of pH 4.0) (∼1 L/kg olives produced) also with high organic load (average value of COD equal to 32.3 g O2/L). In absolute volumes, Spanish-style processing appears to generate the largest volume of wastewaters (about 7 × 106 m3 annually at global scale), thus, priority should be given in these effluents [20]. Various biological treatment processes have been developed so far to detoxify these streams and more recently the combined detoxification and valorization of them has been studied as a very promising sustainable approach toward the synthesis of value-added products (such as methane, citric acid and β-carotene) [20, 24,25,26]. In the only available study for the production of fungal tannase using Spanish-style green table olive processing wastewater streams [27], the extracellular tannase activity, although low (0.89 IU/mL), was verified in cultures of an autochthonous strain of A. niger. However, this study was not aimed at optimizing tannase production, thus, it is suffered from certain weaknesses by being applied in wastewater streams with no optimum level of tannins that maximizes the enzyme production [10, 28]. This limitation renders difficult any explanation related to the profile of tannase production. A new approach is therefore needed for the study of optimum tannase production using table olive processing wastewaters.
In this direction, the current study aims to investigate the production of extracellular tannase by the robust A. niger B60 after enrichment of the wastewaters from the processing of Spanish-style Chalkidiki green olives with tannic acid, a hydrolyzable gallotannin. This fungal strain was chosen because it has succesfully aided in the remediation of the effluents by reducing chemical oxygen demand by up to 76% mainly due to the rapid degradation of the phytotoxic phenolic substances [24]. However, to the best of our knowledge, there are no available reports dealing with the capacity of the strain B60 to degrade hydrolyzable tannins. Thus, the objectives in this research were twofold: Firstly to study growth, tannic acid uptake as well as tannase and gallic acid yield and productivity in a synthetic medium supplemented with a wide concentration range of tannic acid as a sole carbon source. Secondly, non-sterile effluents of Spanish-style green olive processing were enriched with the highest possible level of tannic acid that serves as a supplementary carbon source and an inducer of tannase synthesis and tested as alternative feedstocks for maximum fungal tannase production. Estimation of kinetic parameters for microbial growth and product formation was carried out using different nonlinear models to evaluate the fermentation processes. The results were thoroughly discussed in comparison with the available literature data on fungal tannase production using other types of wastewaters under submerged fermentation in order to assess the competitive advantage of the proposed process.
Materials and Methods
Fungal Strain and Inoculum Preparation
Aspergillus niger strain B60 (A. niger van Tieghem, ATCC 201,573), generously provided by Prof. T. Roukas (Department of Food Science & Technology, School of Agriculture, Aristotle University of Thessaloniki), was regularly sub-cultured every 2 to 3 months on PDA plates and maintained at 4 °C. Fungal spores were suspended in sterile deionized water (2 × 107 spores/mL), mixed thoroughly with the aid of a vortex and used immediately to inoculate the fermentation substrates.
Wastewater Sampling
Representative samples of fresh lye (50 L) and washing water (50 L) effluents from Spanish-style processing of green olives (cv. Chalkidiki) were collected after olive treatment with 2% (w/v) NaOH aqueous solution for 11 h (lye) and two water changes at 8 and 16 h (washing waters). The sampling was from three different tanks (8 tons of olives) that were processed in parallel in a medium-size table olive industry located in Chalkidiki (Northern Greece) in the production season 2016–2017. The effluents were stored immediately at − 20 °C until further use (for a maximum of 6 months) [24].
Submerged Fermentation Conditions
The synthetic liquid medium used for extracellular tannase production contained NaNO3 (2.5 g/L), KH2PO4 (1 g/L), MgSO4∙7H2O (0.5 g/L), KCl (0.5 g/L) and tannic acid as the sole carbon source [29] at different concentrations (5 g/L, 10 g/L, 30 g/L, 50 g/L, 75 g/L, 100 g/L, 120 g/L, 150 g/L). The salt solution was autoclaved at 121 °C for 30 min. An appropriate amount of tannic acid was weighed and transferred into the salt solution to achieve the desired tannic acid concentration and the initial pH was adjusted to 5 with concentrated HCl (12 N). Then, the synthetic liquid medium was sterilized by filtering through a 0.22 μm pore size PTFE filter. Non-sterile lye and washing water effluents were also used as substrates after their enrichment with tannic acid at the level of 100 g/L and initial pH adjustment to 5 with 12 N HCl.
Submerged fermentation experiments were performed under aerobic conditions in Erlenmeyer flasks (250 mL) containing 50 mL of (a) synthetic liquid medium with the desired tannic acid concentration or (b) non-sterile lye or washing water effluents enriched with 100 g/L tannic acid. The inoculated flasks were incubated at 30 °C on a rotary shaker (KS 4000i control, IKA, 115 Wilmington, NC) operating at 160 rpm for 144 h. The flasks were withdrawn at the defined time points and the fermented substrate was analyzed.
Determination of Total Dry Biomass Content
For the determination of total dry biomass content (g/L), the fermented substrate (mycelium and liquid) was filtered under reduced pressure (Pump V-700, Büchi, Flawil, Switzerland) through a Whatman 1 filter paper. The collected mycelium was washed with distilled water and oven dried at 103 °C until constant mass [24]. The filtrate was used for further analysis.
Determination of Chemical Oxygen Demand, pH and Electrical Conductivity
The method of potassium dichromate was used to determine the chemical oxygen demand (COD) (g/L) in the wastewaters, using tube tests and an AL200 COD VARIO Set-Up (Aqualytic, Dortmund, Germany). The measurement of the pH value in the wastewaters was carried out with the aid of an MP 220 pH meter (Mettler-Toledo, Greifensee, Switzerland), while the electrical conductivity (mS/cm) was assessed with a portable conductivity meter CM 35 (Crison, Barcelona, Spain) [24].
Determination of Total Solids, Total Dissolved Solids and Total Suspended Solids
The determination of total solids (g/L), total dissolved solids (g/L) and total suspended solids (g/L) in the wastewaters was accomplished following the procedure presented in Standard Methods [30].
Determination of Ash and Metals Content
The assessment of ash content (g/L) in the wastewaters was carried out with the following steps: sample drying (105 °C), acidification (HCl 6 N), burning over a Bunsen burner and incineration (550 °C) [25]. The ash was further analyzed through inductively coupled plasma atomic emission spectrometry to determine the content of Cu (mg/L), Mg (mg/L), Mn (mg/L), Fe (mg/L) and Zn (mg/L) in the wastewaters, according to the protocol described by Papadaki and Mantzouridou [25].
Determination of Total Nitrogen Content
The persulfate digestion method was used to determine the total nitrogen content (mg/L) in the wastewaters with the aid of a total nitrogen kit LCK 338 and a DR 3900 spectrophotometer (Düsseldorf, Germany, Hach Lange) [24].
Determination of Soluble Sugar Content
Total soluble sugar content in the wastewaters was assessed spectrophotometrically using the phenol-sulfuric acid method [31] and the results were expressed as glucose equivalents (g/L). Glucose (g/L) and fructose (g/L) were quantified by high-performance liquid chromatography (HPLC) analysis as described elsewhere [24].
Determination of Polar Phenolic Compound Content
The polar extract of the wastewaters was obtained according to the liquid-liquid extraction procedure described by Papadaki et al. [24]. Folin–Ciocalteu assay [32] was used to determine the total polar phenol content in the extracts and the results were expressed as caffeic acid equivalents (mg/L). Individual phenolic compounds in the extracts were quantified by high-performance liquid chromatography (HPLC) analysis following the protocol of Papadaki et al. [24].
Determination of Tannase Activity and Gallic Acid Concentration
The method of Sharma et al. [33] was used for the determination of extracellular tannase activity using methyl gallate (0.01 M) as a substrate in citrate buffer (0.05 M, pH 5.0) based on the formation of chromogen between gallic acid and rhodanine. Briefly, 0.25 mL of the substrate solution was incubated with 0.25 mL of enzyme sample (reaction mixture) at 30 °C for 5 min followed by subsequent addition of 0.3 mL of methanolic rhodanine (0.667%; w/v) and 0.2 mL of KOH (0.5 N) every 5 min (at 30 °C). Finally, the mixture was diluted with 4 mL of distilled water and incubated at 30 °C for 10 min. The absorbance was recorded at 520 nm using a spectrophotometer (UV-1601, Shimadzu, Kyoto, Japan). One international unit of activity (IU) was defined as the amount of enzyme required to release 1 µmol of gallic acid per min under assay conditions. Tannase activity was expressed in IU per mL of substrate (IU/mL).
Gallic acid concentration (g/L) in the culture filtrates was also measured by the methanolic rhodanine spectrophotometric method [29].
The yield of tannase and gallic acid was expressed as product per dry biomass content (IU/g and g/g, respectively). The productivity of tannase and gallic acid was expressed as product per substrate volume per time (IU/mL/h and g/L/h, respectively).
Determination of Total Tannin Concentration
The protein precipitation method of Hagerman and Butler [34] was used for the determination of total tannin concentration (g/L) using bovine serum albumin (BSA) to precipitate and quantify tannins. The uptake of tannic acid was expressed as mass of tannic acid consumed per initial mass of tannic acid (%, w/w).
Determination of Kinetic Parameters of Microbial Growth
Among the different models (Logistic, Gompertz, and Richards) tested to describe the growth kinetics of A. niger in the streams, the modified Logistic model (Eq. 1) was the most appropriate one in describing the fungal growth dynamics in the synthetic liquid medium and the table olive processing wastewaters streams containing the optimum tannic acid concentration for maximum tannase production [24].
where X is the dry biomass content (g/L) at time t (h), Xm is the maximum dry biomass content (g/L), µm is the maximum specific growth rate (1/h), λB is the growth lag time (h) and t is the fermentation time (h).
The biomass productivity PB (g/L/h) was calculated by:
where X1 and X2 were the dry biomass content (g/L) at time t1 (starting point of cultivation, h) and t2 (timepoint of maximum dry biomass content, h), respectively.
Determination of Kinetic Parameters of Tannase Production
The Luedeking–Piret equation (Eq. 3) [35] was used to find growth associated parameter ‘α’ and non-growth associated parameter ‘β’ by fitting tannase production data from the synthetic liquid medium and the table olive processing wastewaters containing the optimum tannic acid concentration for maximum tannase production.
where Ptan is the tannase activity (IU/mL) at time t (h), X is the dry biomass content (g/L) at time t (h), α is the growth associated product formation constant (IU/mL), β is the non-growth associated product formation constant (1/h) and t is the fermentation time (h).
Statistical Analysis
All measurements and treatments were performed in triplicate. Statistical comparisons of the mean values were performed by one-way ANOVA followed by Duncan’s test (p < 0.05 confidence level) using the SPSS 20.0 software (SPSS Inc., Chicago, IL, USA). The kinetic models were fitted to the experimental data with Microsoft Excel spreadsheet using the Solver function (Microsoft Corp., Redmond, WA, USA) in order to find the values of the kinetic parameters that result in the minimum level for sum chi-squared.
Results
Growth, Gallic Acid and Tannase Production in Synthetic Media
The ability of A. niger B60 to grow as well as to produce extracellular tannase and gallic acid was evaluated under submerged fermentation conditions in synthetic media containing tannic acid as a sole carbon source in a wide range of concentrations (5 g/L to 150 g/L). The curves of biomass, tannase activity and gallic acid content versus time in the different culture media are presented in Fig. 1. Tannic acid uptake as well as maximum values of tannase and gallic acid yield (based on biomass) and productivity under the different initial tannic acid levels are given in Table 1.
According to data in Fig. 1a, tannic acid favored fungal growth and biomass gradually increased with the increase of tannic acid concentration. Tannic acid was almost completely assimilated (98.2–99.8%) at up to 75 g/L initial level of the substrate. Notably, even at the highest level of tannic acid tested (150 g/L), the percent uptake of the compound was high (90.5%) (Table 1). Under the latter fermentation conditions, it was reported the maximum biomass content (16.9 g/L) (Fig. 1a). The above findings highlight the strong affinity of the strain for tannic acid uptake thus accelerating the growth of the strain.
The uptake of tannic acid was associated with tannase production by the fungus. Figure 1b reveals that secretion of extracellular tannase activity increased with increasing tannic acid concentrations at up to 100 g/L. In particular, tannase activity detected in the culture medium containing the lowest initial content of tannic acid (5 g/L) was up to 2.4 IU/mL after cultivation for 48 h. Experiments in the culture media supplemented with 10 to 75 g/L tannic acid showed variation in the maximum tannase activity from 10.4 to 32.7 IU/mL at 96 h of cultivation. Interestingly, the highest enzyme activity of 47.5 IU/mL was recorded after 72 h of cultivation in the medium supplemented with 100 g/L tannic acid, which corresponded to a production yield of 4.3 IU/g and a productivity of 0.5 IU/mL/h (Fig. 1b; Table 1). However, a reduction in tannase activity, yield and productivity was evident by increasing the initial concentration of tannic acid from 100 g/L to 120 g/L and 150 g/L (Fig. 1b; Table 1). The latter observation indicates that A. niger B60 tolerates tannic acid concentrations as high as 100 g/L without having a deleterious effect on both growth and enzyme production.
Next, gallic acid co-production capability of the fungus cultivated in media with different concentrations of tannic acid and incubation times was evaluated to determine also the highest level of tannic acid bioconversion to gallic acid. As depicted in Fig. 1c, maximum gallic acid production of 36.1 g/L from 100 g/L tannic acid was detected after 48 h of cultivation, which corresponded to a product yield of 3.6 g/g and a productivity of 0.75 g/L/h (Table 1). When the fungus was cultivated in media supplemented with 75, 120 or 150 g/L initial tannic acid, gallic acid was satisfactory obtained (35.0, 33.1 or 31.8 g/L, respectively). A decrease in the initial tannic acid level below 75 g/L was followed by a decrease in maximum gallic acid production (2.3–13.1 g/L). It should be noted that, in all cases, gallic acid content declined after reaching a maximum value, revealing the ability of the fungal strain to catabolize gallic acid via gallic acid decarboxylase into pyrogalloll which enters the TCA cycle as pyruvic acid, cis-aconitic acid, and 3-hydroxy-5-oxo hexanoate [2]. Regarding glucose, the other product generated along with gallic acid via the action of tannase, not any level of this sugar was detected under all the initial tannic acid conditions tested (data not shown). This highlights the instant consumption of glucose by the fungus after the biodegradation of tannic acid.
Growth, Gallic Acid and Tannase Production in Table Olive Processing Wastewaters
A further research question was whether non-sterile table olive processing wastewaters (lye and washing waters) enriched with tannic acid have a strong potential for production of fungal extracellular tannase in order to improve the economics of the bioprocess and to reduce the environmental issues associated with water resource management and wastewater disposal.
The main physicochemical characteristics of the lye and washing water effluents used in the current studies are displayed in Table 2. It is clearly evident from the results that these streams did not contain any tannic acid, thus, their pre-enrichment with tannic acid was the only way for rendering possible the production of fungal tannase. The level of 100 g/L of tannic acid chosen for the enrichment of the wastewaters was the optimum one found in the experiments carried out under submerged fermentation conditions in synthetic media containing tannic acid as a sole carbon source in a wide range of concentrations (5 to 150 g/L) to maximize enzyme activity. The initial level of total sugars (glucose and fructose) in the effluents was 6 g/L, consisting of glucose and fructose at a ratio of ~ 0.9. Simple phenolic compounds were also present in lye (0.4 g/L) and washing waters (0.7 g/L). In addition, the effluents contained growth factors like nitrogen and metals. The ash content (inorganic matter) of lye and washing waters resulted in high electrical conductivity level. The latter was more pronounced in lye due to the use of NaOH in the debittering stage of the olive fruit [20]. Also, the strong alkalinity of both effluents led to the adjustment of their initial pH value to 5 as a mandatory pretreatment step to enable the fungal growth.
Figure 2 illustrates the evolution of biomass formation as well as tannase activity, tannic and gallic acid production during the treatment of the enriched lye and washing waters with A. niger B60. The biomass content reached 16.6 g/L in the lye and 18.9 g/L in the washing waters. The easily assimilable sugars, glucose and fructose, were completely catabolized by the fungus within the first 24 h of treatment (data not shown), while tannic acid was constantly used as the main carbon source. The tannic acid consumption and fungal growth in the lye resulted in a maximum extracellular tannase activity of 21.5 IU/mL at 72 h, which corresponded to yield and productivity values of 1.30 ± 0.11 IU/g and 0.30 ± 0.02 IU/mL/h, respectively. The maximum extracellular tannase activity in the washing waters was 17.3 IU/mL at 48 h corresponding to a yield of 1.05 ± 0.06 IU/g and a productivity of 0.36 IU/mL/h ± 0.01 IU/mL/h. This enzymatic activity led to the release of gallic acid in the streams. Specifically, the maximum gallic acid content detected in lye and washing waters was 21.6 g/L (yield 1.30 ± 0.12 IU/g and productivity 0.45 ± 0.03 IU/mL/h) and 14.1 g/L (yield 0.85 ± 0.03 IU/g and productivity 0.29 ± 0.02 IU/mL/h), respectively, after 48 h of incubation. It should be also noted that after 60 h of fermentation, the tannic acid was almost depleted (Fig. 2), i.e. tannic acid reduction of 75% in lye and 77% in washing waters. As a result, the fungus started to catabolize the other carbon sources present in the streams, leading to the gradual reduction of gallic acid content (Fig. 2) as well as the complete biodegradation of the simple phenolic compounds, hydroxytyrosol, methoxy derivative of hydroxytyrosol, tyrosol, caffeic acid, luteolin-7-O-glucoside and p-coumaric acid (data not shown).
Kinetics of Fungal Growth and Tannase Production
The experimental data on biomass formation and tannase activity in synthetic medium and non-sterile effluents (lye and washing waters) containing initial tannic acid concentration of 100 g/L were interpreted using the modified Logistic sigmoid function and the Luedeking–Piret models, respectively, to characterize the fermentation processes. The derived biological parameters are given in Tables 3 and 4.
In the case of fungal growth in the synthetic medium, findings demonstrated that the non-linear models can be successfully used to predict the kinetic parameters of growth (R2 = 0.99) (Table 3) and tannase production (R2 = 0.97) (Table 4). Specifically, after a lag time (λB) of 15 h, fungal biomass was formed with a maximum specific growth rate (µm) of 0.369 1/h, reaching up to 11 g/L (Xm) (Table 3). It seems that fungal growth influenced strongly the production of extracellular tannase during the incubation period as the value of α was 20-fold higher than that of β (Table 4).
The kinetics of fungal growth and extracellular tannase production in lye and washing waters were also adequately described by the modified Logistic (R2 = 0.99) (Table 3) and the Luedeking–Piret models (R2 = 0.96–0.97) (Table 4), respectively. In both effluents, a lag period of 15 h was evident prior to the exponential growth phase. The biomass formation was faster in the washing waters (µm = 0.594 1/h) as compared with the lye (µm = 0.540 1/h), leading to a significantly higher maximum biomass content (18 g/L vs. 16 g/L) (Table 3). The recorded tannase activity during the treatment of the effluents was growth-associated; a finding that was more evident in the lye (α/β = 10) than in the washing waters (α/β = 5) (Table 4).
Discussion
Evaluating the Performance of A. niger B60 in Synthetic Media
A. niger B60 followed a normal pattern of growth and metabolic activity over a wide concentration range of tannic acid in synthetic medium (Fig. 1). The maximum attainable biomass concentration increased with the increase of the initial tannic acid concentration, and they were correlated in a linear relationship (correlation coefficient of 0.9919). Moreover, the stationary phase in biomass production in media containing high initial tannic acid content (> 100 g/L) occurred almost at the same fermentation time point (i.e. 48 h after inoculation). This resulted in higher biomass productivity under higher initial tannic acid concentration (0.095 g/L/h vs. 0.1408 g/L/h at an initial tannic acid concentration of 100 g/L and 150 g/L, respectively).
From the mechanistic point of view, the fungal cells produced tannase to hydrolyze tannic acid into gallic acid and glucose [2]. At the stationary phase of fungal growth, tannase activity decrease was associated with the depletion of tannic acid, while the reduction of gallic acid concentration was attributed to the catabolic activity of the fungus [36, 37]. End-product repression by gallic acid and catabolite repression by glucose might as well have been involved in the decline of tannase activity during the submerged fermentation [10, 28, 37].
When comparing our results to those of previous studies using other strains of Aspergillus species and the fungal genera Penicillium, Trichoderma and Fusarium, maximum tannase activity and accumulated gallic acid were of similar values under submerged fermentation with initial level of tannic acid 5 g/L. In particular, Bajpai and Patil [38] reported values for A. niger MTCC 282 (1.6 IU/mL and 2.3 g/L, respectively), A. niger MTCC 404 (1.6 IU/mL and 2.3 g/L, respectively), A. aureus MTCC 151 (1.7 IU/mL and 2.4 g/L, respectively), A. parasiticus MTCC 411 (2.3 IU/mL and 3.3 g/L, respectively), Penicillium chrysogenum MTCC 161 (2.3 IU/mL and 3.3 g/L, respectively), T. viride MTCC 167 (2.4 IU/mL and 3.5 g/L, respectively) and F. solani MTCC 350 (2.5 IU/mL and 3.7 g/L, respectively) under submerged fermentation in synthetic medium with tannic acid 5 g/L. Extracellular tannase production by A. niger B60 at an initial tannic acid concentration of 10 g/L (10.4 IU/mL) was considerably higher than those reported for other A. niger strains under the submerged fermentation using the same tannic acid concentration. For example, A. niger FETL FT3 free and immobilized cells produced 2.8 and 4.0 IU/mL of tannase, correspondingly [29], while (A) niger Van Tieghem released 6.2 IU/mL of the enzyme [39]. The results were even better than those reported for the bacterial species Bacillus licheniformis KBR6 (0.4 IU/mL) [40], (B) subtilis AM1 (1.4 IU/mL) and Lactobacillus plantarum CIR1 (1.2 IU/mL) under anaerobic submerged fermentation [41]. However, Iqbal and Kapoor [42] reported higher tannase activity of the fungus T. harzianum MTCC 10,841 (32.3 IU/mL).
The ability of A. niger B60 to grow well and produce extracellular tannase in the media with high initial tannic acid concentrations (50–150 g/L) (Fig. 1) is a characteristic of the species [36, 43]. Tannase activity of the strain B60 in the presence of 50 g/L initial tannic acid concentration (25.4 IU/mL) was superior to those reported for the strains Aa-20 (2.7 IU/mL) [28], GH1 (0.35 IU/mL), GH2 (0.50 IU/mL), NH4 (0.50 IU/mL) and PSH (0.35 IU/mL) [12]. However, even higher tannase activity was detected when using 100 g/L tannic acid (47.5 IU/mL) (Fig. 1b), which was about 20-fold greater than that reported for the strain Aa-20 (2.4–2.6 IU/mL) [10, 28]. These differences seem to be related to the ability of strain B60 to uptake efficiently the compound by 95% (Table 1) in contrast with the limited hydrolytic performance of the strains Aa-20 (54–65%), GH1 (67%) and PSH (70%) [10, 12].
Evaluating the Performance of A. niger B60 in Enriched Table Olive Processing Wastewaters
Sterilization has been considered a significant part of the costs of A. niger biorefinery for the production of hydrolytic enzymes [44]. Thus, in an attempt to render the fermentation process cost effective and resource efficient, non-sterile lye and washing waters from Spanish-style green olive processing (Table 2) were enriched with the highest possible level of tannic acid (i.e. 100 g/L) for extracellular tannase production using the robust A. niger B60. Fungal growth was favored in lye and washing waters as Xm (16 and 18 g/L, respectively) and µm values (0.540 1/h and 0.594 1/h, respectively) were about 1.5-fold higher than those estimated in the synthetic medium with the same initial tannic acid concentration (Xm = 11 g/L and µm = 0.369 1/h). It is important to note, that the present evidence relies on the presence of metals in the effluents (e.g. Mg, Fe, Cu, Zn and Mn) (Table 2) acting as fungal growth factors through the catalysis of redox reactions, the activation of enzyme systems and/or their participation in the molecular structure of the enzymes [26, 45]. Biomass formation became more pronounced in the washing waters than in the lye. This could be associated to the elevated content of NaOH in lye than in washing waters [20], leading to higher salinity levels (ash content 7 g/L in lye vs. 4 g/L in washing waters) (Table 2) that cause nutritional imbalance and osmotic stress as well as to higher alkalinity that decreases the solubility of the nutrients [24]. It should be also taken into consideration that the total suspended solids, which were more pronounced in the lye (5 g/L) than in the washing waters (3 g/L) (Table 2), tend to increase the turbidity of the medium and decrease the available dissolved oxygen concentration for the microbial growth [24, 46]. In addition, the lag phase of growth (15 h) did not differ within the three substrates signifying that the fungal cells were easily adapted in the effluents. The growth kinetic parameters of A. niger B60 in this research were better than those reported for the same strain in lye (µm = 0.061 1/h, Xm = 3 g/L) and washing waters (µm = 0.085 1/h, Xm = 3 g/L) without pre-enriched step [24]. Alternatively, a similar rate of growth (µm = 0.458 1/h) was observed for the strain B60 in a lye/washing water mixture enriched with 100 g/L sugars (glucose and fructose) of white grape pomace for citric acid production [25]. On the other hand, the fungal growth rate (µm = 0.146 1/h) of A. niger Aa-20 in a synthetic medium containing 100 g/L tannic acid was 2.5-fold lower than the respective one estimated for A. niger B60 providing, however, a similar maximum value for biomass (Xm = 12 g/L) [28].
Extracellular tannase production was efficient in lye (21.5 IU/mL) and washing waters (17.3 IU/mL) but 2.2- and 2.7-fold lower, respectively, than in the synthetic medium with an initial tannic acid concentration of 100 g/L (47.5 IU/mL). This might be caused by the metals contained in the effluents (such as Mg, Fe, Cu, Zn and Mn) [25], which have been acknowledged as inhibitors of tannase activity by Aspergillus species [17, 37]. On the other hand, the low level of soluble sugars in both effluents (6 g/L) was not expected to contribute significantly to the fungal tannase activity repression [10, 28]. In any case, the negative influence of the olive-derived phenolic compounds on fungal performance should not be precluded [22] as the simple phenolic compounds, hydroxytyrosol, methoxy derivative of hydroxytyrosol, tyrosol, caffeic acid, luteolin-7-O-glucoside and p-coumaric acid were completely catabolized by the fungus. Yet, the tannase activity levels obtained by A. niger B60 after the enrichment of the effluents with tannic acid were found to be antagonistic to those of other Aspergillus strains using different wastewaters and residues from food processing. Submerged fermentation of undiluted olive mill wastewaters by A. niger MUM 03.58 led to an extracellular tannase production as low as 0.3 IU/mL [23], while tannase activity after fermentation of the diluted streams with A. niger HA37 was 0.6 IU/mL [21]. The maximum tannase activity (8 IU/mL) was achieved only after oxidation and further dilution of the olive mill wastewaters to minimize the inhibitory effect of phenolic compounds on the growth of Aspergillus flavus F2 [22], although these levels were 2.7- and 2.2-fold lower than those obtained by A. niger B60 in the enriched lye and washing waters, correspondingly. Comparable levels with the current study were obtained after supplementation of liquid nutrient media with tannin-rich pomegranate rind (29.2 IU/mL by A. niger ITCC 6514.07) [17] and or Emblica officinalis powder (35.6 IU/mL by Aspergillus sp. from soil) [16]. It should be noted that the growth-associated nature of tannase production by A. niger B60 in the effluents and the synthetic medium (α >> β) coincides with the literature data on the growth of A. flavus MTCC 3783 (α = 18.02 1/h, β = 0.041 1/h) [35] or Aspergillus foetidus MTCC 3557 (α = 21.20 1/h, β = 0.035 1/h) in redgram husk [47], Bacillus cereus M1GT (α = 0.58 1/h, β = ‒0.002 1/h) in Triphala residue [48] and Lb. plantarum MTCC 1407 (α = 5.50 1/h, β = 0.685 1/h) in synthetic medium [49].
Conclusion
Overall, submerged fermentation is the preferred method for industrial tannase production at a global level. Thus, efforts to optimize the process are currently needed. Maximum extracellular tannase production by A. niger B60 (47.5 IU/mL) was obtained in a synthetic medium with an initial tannic acid concentration of 100 g/L. The obtained value was found to be competitive with those reported in literature for other fungal as well as bacterial strains. Gallic acid was also produced at satisfactory levels as a secondary product. Tannic acid degradation was almost complete and considerably higher than other A. niger strains studied previously, signifying the strong potential of strain B60. Replacement of process water and growth factors with non-sterile lye and washing waters from Spanish-style green olive processing provides a cost-effective advantage to the process and promotes environmental protection. Remarkably, effluents enrichment with 100 g/L tannic acid followed by submerged fermentation with A. niger B60 was effective for fungal growth and extracellular tannase production. The latter was considered a process with competitive advantage to other wastewaters and residues from food processing proposed as alternative feedstocks for the production of fungal tannase. However, a major source of limitation is due to the fact that commercial tannic acid is a costly substrate. These findings seem encouraging toward the direction of scale up in bioreactors that should be carried out together with optimization studies using cost-effective tannin-rich agro-residual substrates and provision of process economics.
Data Availability
The datasets generated during and/or analysed during the current study are available from the corresponding author on reasonable request.
References
Emergen Research:. Enzymes Market Size, Share, Trends, By Source (Animals, Microorganisms, Plants), By Product (Proteases, Carbohydrase, Polymerases & Nucleases, Lipases), By Application (Specialty Enzymes, Industrial Enzymes), Forecasts to 2027. 250 (2020) ER_0069
Dhiman, S., Mukherjee, G., Singh, A.K.: Recent trends and advancements in microbial tannase-catalyzed biotransformation of tannins: a review. Int. Microbiol. 21, 175–195 (2018). https://doi.org/10.1007/s10123-018-0027-9
Aguilar, C.N., Rodríguez, R., Gutiérrez-Sánchez, G., Augur, C., Favela-Torres, E., Prado-Barragan, L.A., Ramírez-Coronel, A., Contreras-Esquivel, J.C.: Microbial tannases: advances and perspectives. Appl. Microbiol. Biotechnol. 76, 47–59 (2007). https://doi.org/10.1007/s00253-007-1000-2
de las Rivas, B., Rodríguez, H., Anguita, J., Muñoz, R.: Bacterial tannases: classification and biochemical properties. Appl. Microbiol. Biotechnol. 103, 603–623 (2019). https://doi.org/10.1007/s00253-018-9519-y
Prigione, V., Trocini, B., Spina, F., Poli, A., Romanisio, D., Giovando, S., Varese, G.C.: Fungi from industrial tannins: potential application in biotransformation and bioremediation of tannery wastewaters. Appl. Microbiol. Biotechnol. 102, 4203–4216 (2018). https://doi.org/10.1007/s00253-018-8876-x
Kanpiengjai, A., Khanongnuch, C., Lumyong, S., Haltrich, D., Nguyen, T.H., Kittibunchakul, S.: Co-production of gallic acid and a novel cell-associated tannase by a pigment-producing yeast, Sporidiobolusruineniae A45.2. Microb. Cell Fact. 19, 95 (2020). https://doi.org/10.1186/s12934-020-01353-w
Saeed, S., Aslam, S., Mehmood, T., Naseer, R., Nawaz, S., Mujahid, H., Firyal, S., Anjum, A.A., Sultan, A.: Production of gallic acid under solid-state fermentation by utilizing waste from food processing industries. Waste Biomass Valoriz. 12, 155–163 (2021). https://doi.org/10.1007/s12649-020-00980-z
Borah, A., Selvaraj, S., Murty, V.R.: Production of gallic acid from Swieteniam acrophylla using tannase from Bacillus gottheilii M2S2 in semi-solid state fermentation. Waste Biomass Valoriz. (2023). https://doi.org/10.1007/s12649-022-02023-1
Prigione, V., Spina, F., Tigini, V., Giovando, S., Varese, G.C.: Biotransformation of industrial tannins by filamentous fungi. Appl. Microbiol. Biotechnol. 102, 10361–10375 (2018). https://doi.org/10.1007/s00253-018-9408-4
Aguilar, C.N., Augur, C., Favela-Torres, E., Viniegra-González, G.: Production of tannase by Aspergillus niger Aa-20 in submerged and solid-state fermentation: influence of glucose and tannic acid. J. Ind. Microbiol. Biotechnol. 26, 296–302 (2001). https://doi.org/10.1038/sj.jim.7000132
Bhat, T.K., Makkar, H.P.S., Singh, B.: Preliminary studies on tannin degradation by Aspergillus niger van tieghem MTCC 2425. Lett. Appl. Microbiol. 25, 22–23 (1997). https://doi.org/10.1046/j.1472-765x.1997.00164.x
Cruz-Hernandez, M., Contreras-Esquivel, J.C., Lara, F., Rodriguez, R., Aguilar, C.N.: Isolation and evaluation of tannin-degrading fungal strains from the mexican desert. Z. Naturforsch. C 60, 844–848 (2005). https://doi.org/10.1515/znc-2005-11-1205
Amfep (2015). List of Commercial Enzymes. Brussels, Belgium: Association of Manufacturers & Formulators of Enzyme Products (Amfep). Available at: https://amfep.org/_library/_files/Amfep_List_of_Enzymes_update_May_2015.pdf
Jin, W., Nie, G., Liu, H., Xiaoran, Y., Gong, G., Wang, L., Zheng, Z.: Improving Aspergillus niger tannase yield by N+ ion beam implantation. Braz. Arch. Biol. Technol. 56, 135–142 (2013). https://doi.org/10.1590/S1516-89132013000100018
Kumar, M., Singh, A., Beniwal, V., Salar, R.K.: Improved production of tannase by Klebsiella pneumoniae using indian gooseberry leaves under submerged fermentation using Taguchi approach. AMB Express. 6, 46 (2016). https://doi.org/10.1186/s13568-016-0217-9
Sharma, P., Chaturvedi, A., Sharma, L.: Parametric optimization for extracellular tannase production in submerged fermentation by isolated Aspergillus species. Int. J. Curr. Microbiol. Appl. Sci. 4, 232–239 (2015)
Srivastava, A., Kar, R.: Characterization and application of tannase produced by Aspergillus niger ITCC 6514.07 on pomegranate rind. Braz. J. Microbiol. 40, 782–789 (2009). https://doi.org/10.1590/S1517-83822009000400008
Meini, M.R., Ricardi, L.L., Romanini, D.: Novel routes for valorisation of grape pomace through the production of bioactives by Aspergillus niger. Waste Biomass Valoriz. 11, 6047–6055 (2020). https://doi.org/10.1007/s12649-019-00844-1
Rocha, C., Soria, M.A., Madeira, L.M.: Olive mill wastewater valorization through steam reforming using multifunctional reactors: challenges of the process intensification. Energies 15, 920 (2022). https://doi.org/10.3390/en15030920
Papadaki, E., Mantzouridou, F.T.: Current status and future challenges of table olive processing wastewater valorization. Biochem. Eng. J. 112, 103–113 (2016). https://doi.org/10.1016/j.bej.2016.04.008
Aissam, H., Errachidi, F., Penninckx, M.J., Merzouki, M., Benlemlih, M.: Production of tannase by Aspergillus niger HA37 growing on tannic acid and olive mill waste waters. World J. Microbiol. Biotechnol. 21, 609–614 (2005). https://doi.org/10.1007/s11274-004-3554-9
Kachouri, S., Halaouli, S., Lomascolo, A., Asther, M., Hamdi, M.: Decolourization of black oxidized olive-mill wastewater by a new tannase-producing Aspergillus flavus strain isolated from soil. World J. Microbiol. Biotechnol. 21, 1465–1470 (2005). https://doi.org/10.1007/s11274-005-6810-8
Salgado, J.M., Abrunhosa, L., Venâncio, A., Domínguez, J.M., Belo, I.: Combined bioremediation and enzyme production by Aspergillus sp. in olive mill and winery wastewaters. Int. Biodeterior. Biodegrad. 110, 16–23 (2016). https://doi.org/10.1016/j.ibiod.2015.12.011
Papadaki, E., Tsimidou, M.Z., Mantzouridou, F.T.: Changes in phenolic compounds and phytotoxicity of the spanish-style green olive processing wastewaters by Aspergillus niger B60. J. Agric. Food Chem. 66, 4891–4901 (2018). https://doi.org/10.1021/acs.jafc.8b00918
Papadaki, E., Mantzouridou, F.T.: Citric acid production from the integration of spanish-style green olive processing wastewaters with white grape pomace by Aspergillus niger. Bioresour. Technol. 280, 59–69 (2019). https://doi.org/10.1016/j.biortech.2019.01.139
Papadaki, E., Mantzouridou, F.T.: Natural β-carotene production by Blakeslea trispora cultivated in spanish-style green olive processing wastewaters. Foods 10, 327 (2021). https://doi.org/10.3390/foods10020327
Ayed, L., Chammam, N., Asses, N., Hamdi, M.: Optimization of biological pretreatment of green table olive processing wastewaters using Aspergillus niger. J. Bioremediat. Biodegrad. 5, 212 (2013). https://doi.org/10.4172/2155-6199.1000212
Aguilar, C.N., Augur, C., Favela-Torres, E., Viniegra-González, G.: Induction and repression patterns of fungal tannase in solid-state and submerged cultures. Process. Biochem. 36, 565–570 (2001). https://doi.org/10.1016/S0032-9592(00)00251-X
Darah, I., Sumathi, G., Jain, K., Lim, S.H.: tannase enzyme production by entrapped cells of Aspergillus niger FETL FT3 in submerged culture system. Bioprocess. Biosyst. Eng. 34, 795–801 (2011). https://doi.org/10.1007/s00449-011-0529-8
American Public Health Association, American Water Works Association: Water environment federation: physical and aggregate properties: Solids (methods 2540). In: Baird, R.B., Eaton, A.D., Rice, E.W. (eds.) Standard methods for the examination of water and wastewater. APHA, Washington, DC (2017)
Dubois, M., Gilles, K.A., Hamilton, J.K., Rebers, P.A., Smith, F.: Colorimetric method for determination of sugars and related substances. Anal. Chem. 28, 350–356 (1956)
Singleton, V.L., Orthofer, R., Lamuela-Raventós, R.M.: Analysis of total phenols and other oxidation substrates and antioxidants by means of folin–ciocalteu reagent. Methods Enzymol. 299, 152–178 (1999)
Sharma, S., Bhat, T.K., Dawra, R.K.: A spectrophotometric method for assay of tannase using rhodanine. Anal. Biochem. 279, 85–89 (2000). https://doi.org/10.1006/abio.1999.4405
Hagerman, A.E., Butler, L.G.: Protein precipitation method for the quantitative determination of tannins. J. Agric. Food Chem. 26, 809–812 (1978). https://doi.org/10.1021/jf60218a027
Mohan, S.K., Viruthagiri, T., Arunkumar, C.: Kinetic studies on microbial production of tannase using redgram husk. Int. J. Biol. Biomol. Agric. Food Biotechnol. Eng. 8, 1077–1080 (2014)
Knudson, L.: Tannic acid fermentation I. J. Biol. Chem. 14, 159–184 (1913). https://doi.org/10.1016/S0021-9258(18)88611-2
Bradoo, S., Gupta, R., Saxena, R.K.: Parametric optimization and biochemical regulation of extracellular tannase from Aspergillus japonicus. Process. Biochem. 32, 135–139 (1997). https://doi.org/10.1016/S0032-9592(96)00056-8
Bajpai, B., Patil, S.: Tannin acyl hydrolase (EC 3.1.1.20) activity of Aspergillus, Penicillium, Fusarium and Trichoderma. World J. Microbiol. Biotechnol. 12, 217–220 (1996). https://doi.org/10.1007/BF00360918
Aboubakr, H.A., El-Sahn, M.A., El-Banna, A.A.: Some factors affecting tannase production by Aspergillus niger Van Tieghem. Braz. J. Microbiol. 44, 559–567 (2013). https://doi.org/10.1590/S1517-83822013000200036
Das Mohapatra, P.K., Maity, C., Rao, R.S., Pati, B.R., Mondal, K.C.: Tannase production by Bacillus licheniformis KBR6: optimization of submerged culture conditions by Taguchi DOE methodology. Food Res. Int. 42, 430–435 (2009). https://doi.org/10.1016/j.foodres.2009.02.013
Aguilar-Zárate, P., Cruz, M.A., Montañez, J., Rodríguez-Herrera, R., Wong-Paz, J.E., Belmares, R.E., Aguilar, C.N.: Gallic acid production under anaerobic submerged fermentation by two bacilli strains. Microb. Cell Fact. 14, 209 (2015). https://doi.org/10.1186/s12934-015-0386-2
Iqbal, H., Kapoor, A.: Culture conditions for the production of Tannase from Trichoderma harzianum MTCC 10841. Int. J. Sci. Technol. 1, 584–595 (2012)
Van Diepeningen, A.D., Debets, A.J.M., Varga, J., Van Der Gaag, M., Swart, K., Hoekstra, R.F.: Efficient degradation of tannic acid by black Aspergillus species. Mycol. Res. 108, 919–925 (2004). https://doi.org/10.1017/S0953756204000747
Papadaki, E., Kontogiannopoulos, K.N., Assimopoulou, A.N., Mantzouridou, F.T.: Feasibility of multi-hydrolytic enzymes production from optimized grape pomace residues and wheat bran mixture using Aspergillus niger in an integrated citric acid-enzymes production process. Bioresour. Technol. 309, 123317 (2020). https://doi.org/10.1016/j.biortech.2020.123317
Foster, J.W.: The heavy metal nutrition of fungi. Bot. Rev. 5, 207–239 (1939)
Bilotta, G.S., Brazier, R.E.: Understanding the influence of suspended solids on water quality and aquatic biota. Water Res. 42, 2849–2861 (2008). https://doi.org/10.1016/j.watres.2008.03.018
Mohan, K., Viruthagiri, T., Arun Kumar, C.: Kinetics and modeling of tannase production using Aspergillus foetidus in batch fermentation. Int. J. Pharm. Pharm. Sci. 7, 64–67 (2015)
Selvaraj, S., Natarajan, K., Nowak, A., Murty, V.R.: Mathematical modeling and simulation of newly isolated Bacillus cereus M1GT for tannase production through semi-solid state fermentation with agriculture residue Triphala. South Afr. J. Chem. Eng. 35, 89–97 (2021). https://doi.org/10.1016/j.sajce.2020.10.001
Kannan, N., Aravindan, R., Viruthagiri, T.: Effect of culture conditions and kinetic studies on extracellular tannase production by Lactobacillus plantarum MTCC 1407. Indian J. Biotechnol. 10, 321–328 (2011)
Acknowledgements
We express our sincere thanks to LADAS S.A. (Simantra, Chalkidiki, Greece) for the supply of Spanish-style green olive processing wastewaters.
Funding
Open access funding provided by HEAL-Link Greece. The authors have not disclosed any funding.
Author information
Authors and Affiliations
Contributions
EP conducted experiments, analyzed data and wrote the manuscript. FM conceived of the study and revised the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Papadaki, E., Mantzouridou, F.T. Αpplication of Aspergillus niger for Extracellular Tannase and Gallic Acid Production in Non-sterile Table Olive Processing Wastewaters. Waste Biomass Valor 15, 1199–1212 (2024). https://doi.org/10.1007/s12649-023-02242-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12649-023-02242-0