Abstract
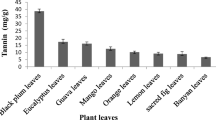
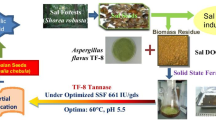
Gallic acid (3,4,5-trihydroxybenzoic acid) is an industrially important organic acid that is found in plants as secondary metabolite. It possesses wide range of applications in healthcare, food and pharmaceutical industry, in manufacturing inks, paints, dyes and also in cinematography. The annual consumption of gallic acid in Pakistan is 8000 tons which is mainly met by importing this item from developed countries. This study was planned to assess the potential of various tannin rich bio-wastes [e.g., peels (banana, pomegranate, apple, and mango) and seeds (black plum, mango, apple, and tamarind)] from fruit processing industries to produce gallic acid by using Aspergillus niger via solid state fermentation. Different physical and chemical parameters were optimized to get the optimum yield of gallic acid. Among all bio-wastes, black plum seed powder gave highest yield of gallic acid i.e. 13.31 mg/g of substrate; the parameters being: substrate water ratio of 1:3, 72 h of incubation period, 2 mL of inoculum, pH 5 and temperature of 30 °C. Carbon source supplementation i.e., glucose increased the synthesis of gallic acid to 14.5 mg/g of substrate while addition of nitrogen sources had negative effect. Extraction of gallic acid was done by using Soxhlet extraction apparatus while FTIR was used for characterization. The solid state fermentation protocol for the production of gallic acid from tannin rich biowastes has been developed and proved to be cost-effective method. The results presented can be optimized further on large scale for industrial production of gallic acid.
Graphic Abstract









Similar content being viewed by others
References
M.Z. El-Fouly, Z. El-Awamry, A.A.M. Shahin, H.A. El-Bialy, E. Naeem, G.E. El-Saeed: Gallic acid formation from gallotannins-rich agricultural wastes using Aspergillus niger AUMC 4301 or its tannase enzyme. Ar. J. Nucl. Sci. Appl.. 45, 489–496 (2012)
S.N.A. Shah, H. Li, J.M. Lin: Enhancement of periodate-hydrogen peroxide chemiluminescence by nitrogen doped carbon dots and its application for the determination of pyrogallol and gallic acid. Talanta 153, 23–30 (2016)
V. Subramanian, B. Venkatesan, A. Tumala, E. Vellaichamy: Topical application of Gallic acid suppresses the 7, 12-DMBA/Croton oil induced two-step skin carcinogenesis by modulating anti-oxidants and MMP-2/MMP-9 in Swiss albino mice. Food Chem. Toxicol. 66, 44–55 (2014)
D. Brahmbhatt, H.A. Modi, N.K. Jain: Preliminary isolation and screening of tannase producing bacteria and fungi. Int. J. Curr. Microbiol. Appl. Sci. .3, 193–203 (2014)
S. Sharma, T.K. Bhat, R.K. Dawra: A spectrophotometric method for assay tannase using rhodanine. Anal. Biochem. 279, 85–89 (2000)
C.M. Furlan, L.B. Motta, D. Santos: Tannins: What Do They Represent in Plant Life? Tannins: Types, Foods Containing, and Nutrition. Nova Science Publishers Inc., New York, 329 (2011)
C. Roberta, S. de Lima Juliana, J. Fonseca, J.E.G. Gomes: Promising substrates to increase the production of tannase under solid state fermentation (SSF) by Penicillium spp. Afr. J. Biotechnol. 16, 2121–2126 (2017)
S. Martins, S.I. Mussatto, G. Martínez-Avila, J. Montañez-Saenz, C.N. Aguilar, J.A. Teixeira: Bioactive phenolic compounds: production and extraction by solid-state fermentation. A review. Biotechnol. Adv. 29, 365–373 (2011)
R. Arshad, A. Mohyuddin, S. Saeed, A.U. Hassan: Optimized production of tannase and gallic acid from fruit seeds by solid state fermentation. Trop. J. Pharm. Res. 18, 911–918 (2019)
R. Kumar, A. Kumari, S. Sheoran, A. Sindhu: Production of tannase under solid-state fermentation using amla and jamun leaves. Ann. Biol. 25, 7–12 (2009)
R. Kumar, J. Sharma, R. Singh: Production of tannasefrom Aspergillusruber under solid-state fermentation using jamun(Syzygiumcumini) leaves. Microbiol. Res. 162, 384–390 (2007)
D. Lal, J.J. Gardner: Production, characterization and purification of tannase from Aspergillus niger. Eur. J. Exp. Biol. 2, 1430–1438 (2012)
A. Xiao, Y. Huang, H. Ni, H. Cai, Q. Yang: Statistical optimization for tannase production by Aspergillus tubingensis in solid-state fermentation using tea stalks. Electron. J. Biotechnol. 18, 143–152 (2015)
M.K. Selwal, A. Yadav, K.K. Selwal, N.K. Aggarwal, R. Gupta, S.K. Gautam: Tannase production by Penicillium tomentosum KM under SSF and its applications in wine clarification and tea cream solubilization. Braz. J. Microbiol. 42, 374–387 (2011)
H.A. Murad, A.M.A. El-Tawab, A.M. Kholif, S.A. El-Nor, O.H. Matloup, M.M. Khorshed, H.M. El-Sayed: Production of tannase by Aspergillus niger from palm kernel. Biotechnology 13, 68–73 (2014)
V. Beniwal, V. Chhokar, N. Singh, J. Sharma: Optimization of process parameters for the production of tannase and gallic acid by Enterobacter cloacae MTCC 9125. J. Am. Sci. 6, 389–397 (2010)
N. Shanmugapriya, S. Ramganesh, N. Labhane: Production, Purification and characterization of Tannase from native Aspergillus sp. using Syzygium cumini. Gold. Res. Thoughts .3, 1–12 (2014)
M.N. Hasmida, A.R.N. Syukriah, L.M. Salleh, C.Y.M. Azizi: Effect of different extraction techniques on total phenolic content and antioxidant activity of Quercusinfectoriagalls. Int. Food Res. J. 21, 1039–1079 (2014)
U. Balyan, B. Sarkar: Aqueous extraction kinetics of phenolic compounds from jamun(Syzygiumcuminii L.) seeds. Int. J. Food Prop. 20, 372–389 (2017)
P. Ghosh, R. Pradhan, S. Mishra, A.S. Patel, A. Kar: Physicochemical and nutritional characterization of jamun(Syzygium cuminii). Curr. Res. Nutr. Food Sci. 5, 1–25 (2017)
K.E. Nandini, A. Gau, S.K. Sundari: The suitability of natural tannins from food and agricultural residues (FAR) for producing industrially important tannase and gallic acid through microbial fermentation. Int. J. Agric. Sci. Food Technol. 4, 1–999 (2013)
A. Sabu, A. Pandey, M.J. Daud, G. Szakacs: Tamarind seed powder and palm kernel cake: two novel agro residues for the production of tannase under solid state fermentation by Aspergillusniger ATCC 16620. Bioresour. Technol. 96, 1223–1228 (2005)
S.K. Hota, J.R. Dutta, R. Banerjee: Immobilization of tannase from Rhizopusoryzae and its efficiency to produce gallic acid from tannin rich agro-residues. Indian J. Biotechnol. 6, 200–204 (2017)
A. Srivastava, R. Kar: Characterization and application of tannase produced by AspergillusnigerITCC 6514.07 on pomegranate rind. Braz. J. Microbiol. 40, 782–789 (2009)
H.M. El-Shora, O.A. Awadalla, S.M. Metwally: Optimization of tannase production by Aspergillusflavus. Egypt. J. Exp. Biol. 11, 121–127 (2015)
G. Mukherjee, R. Banerjee: Biosynthesis of tannase and gallic acid from tannin rich substrates by Rhizopus oryzae and Aspergillus foetidus. J. Basic Microbiol. 44, 42 (2004)
D. Banerjee, S. Mahapatra, B.R. Pati: Gallic acid production by submerged fermentation of Aspergillus aculeatus DBF9. Res. J. Microbiol. 2, 462–468 (2007)
C.N. Aguilar, A. Aguilera-Carbo, A. Robledo, J. Ventura, R. Belmares, D. Martinez, R. Rodríguez-Herrera, J. Contreras: Production of antioxidant nutraceuticals by solid-state cultures of pomegranate (Punica granatum) peel and creosote bush (Larreatridentata) leaves. Food Sci. Biotechnol. 46, 218–222 (2008)
N. Lokeswari, K.J. Raju: Optimization of gallic acid production from Terminaliachebula by Aspergillus niger. J. Chem. 4, 287 (2006)
Author information
Authors and Affiliations
Corresponding authors
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Saeed, S., Aslam, S., Mehmood, T. et al. Production of Gallic Acid Under Solid-State Fermentation by Utilizing Waste from Food Processing Industries. Waste Biomass Valor 12, 155–163 (2021). https://doi.org/10.1007/s12649-020-00980-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12649-020-00980-z