Abstract
Spent coffee grounds (SCGs), deriving from the high global coffee consumption, can have an appreciable environmental impact. One of the eco-friendly reuses of SCGs is their application as a natural fertilizer. This study adopted a soilless cultivation approach to growing Cucumis sativus seedlings using, as rooting media, different coffee solutions obtained by infusing fresh coffee powder or SCGs from mocha and pods for 7 or 30 days. Seedlings’ germination, eco-physiological and biochemical traits were evaluated and the potential toxicological risks or beneficial effects of SCGs on plants were assessed. Results indicate that, although germination was unaffected, fresh coffee medium inhibited seedling development and that 7 days mocha solution stopped seedling growth and induced leaf structural alterations. Conversely, 30 days mocha and pod suspensions promoted seedling biomass, and increased leaf area, leaf polyphenol concentration, and photochemical efficiency. In conclusion, findings indicate that fresh powder can induce toxic effects while SCGs can improve cucumber from a physiological point of view by acting as a natural fertilizer. This study opens future investigations that will help manage coffee waste released into the environment by sustainable practices.
Graphical Abstract

Similar content being viewed by others
Avoid common mistakes on your manuscript.
Statement of Novelty
Our paper analyses for the first time the possibility of reusing of spent coffee from different origin, also taking into account the time of infusion for the coffee by-products.
Introduction
Characteristics like aroma, taste, smell, pleasure, and socialization make coffee one of the world’s most popular beverages. In addition, scientific evidence suggests that coffee may act as functional food thanks to its nutritional properties and health benefits. Coffee contains compounds, such as caffeine, chlorogenic acids, diterpenes, cafestol, kahweol, trigonelline, vitamin B3, magnesium, and potassium, which confer antioxidant, anti-microbial, anti-inflammatory, anti-fibrotic, and anti-cancer properties (Liang and Kitts [1]; Samoggia and Riedel [2]).
Global coffee consumption in recent years has increased considerably. The International Coffee Organization (ICO) estimated that in 2020/21, the production raised to 167.26 million bags, corresponding to a 1.9% increase compared to the previous year and a net gain of 8.6% in the last decade [3]. Such a large coffee consumption produces a considerable amount of waste, i.e., plastic cups, aluminium capsules, coffee chaff (silver skin), and spent coffee grounds (SCGs) (Stylianou et al. [4]).
The SCGs are produced in large quantities by coffee industries, but also derive from coffee-based drinks sold in cafeterias, canteens, and restaurants and from domestic use. Hot water or steam under pressure transforms 1 kg of instant coffee into 2 kg of SCGs (Mussatto et al. [5]).
The SCGs present an acidic pH, high humidity, and high content of tannins and phenolic compounds, including caffeine (Leifa et al. [6]). For such characteristics, SCGs can have an appreciable environmental impact. Indeed, caffeine is present as a significant pollutant in the environment. Resistant to sewage water treatment, it is released into aquatic environments and present in sludge (Deblonde et al. [7]; Martin et al. [8]). Having a high solubility in water (i.e. approximately 13.0 g/L in seawater), its sorption is challenging to predict also because depends on the type and abundance of the different soil components. If persisting enough, caffeine can potentially reach and contaminate superficial as well as groundwaters (Karnjanapiboonwong et al. [9]; Vieira et al. [10]). For example, caffeine concentration for lake water systems ranges from 0.019 to 37.48 µg L− 1; in rivers caffeine occurs from 0.007 to 49.60 µg L− 1 while groundwaters reported caffeine concentrations from 0.02 to 23.97 µg L− 1 (Korekar et al. [11]; Vieira et al. [10]). Besides caffeine, chlorogenic acids and tannins can act as pollutants in aquatic and terrestrial environments, inducing toxic effects on animal and vegetal organisms (Mustard [12]; Fernandes et al. [13]; McNutt and He [14]).
In this context, many ‘alternative and ecological reuses’ of SCGs are currently spreading to support more sustainable management of this waste. Promising solutions range from the production of construction materials, biodiesel, and cosmetics, to the use as heavy metals absorbents, soil amendments, and compost (Stylianou et al. [4]; McNutt and He [14]). Currently, particular attention is devoted to SCGs application to the soil as compost or fertilizer to provide consumers with food rich in bioactive compounds like phenols, carotenoids, and vitamins. This practice would help reduce the use of chemical fertilizers in agriculture and the consequent contamination of water and soil, one of the causes of a reduction in soil fertility (Kasongo et al. [15]; Cruz et al. [16, 17]). The utilization of food waste to obtain biostimulants and soil amendment is a consolidated practice (Xu and Geelen [18]) in line with the European Union (EU) waste hierarchy and the principles of ecology in organic farming (IFOAM [19]).
The effects of SCG utilization in agriculture are currently debated. Coffee residues have been applied directly to agricultural soils at different concentrations (Cruz et al. [16]; Cervera-Mata et al. [20,20,22]; Chilosi et al. [23]), increasing soil water retention but without stimulating crop growth (Cruz et al. [16]; Yamane et al. [24]; Hardgrove and Livesley [25]). It has been demonstrated that compounds present in coffee wastes, such as caffeic and chlorogenic acids, inhibit the activity of Glucose-6-phosphate dehydrogenase (G6PDH) and 6-phosphogluconate dehydrogenase (6PGHD), with adverse effects on plant metabolism (Al-Charchafchi and Al-Quadan [26]). Seed dormancy and inhibition of germination have also been observed (Alam et al. [27]; Muscolo et al. [28]; Al-Charchafchi and Al-Quadan [26]; Gomes et al. [29]).
Conversely, many other experiments documented the positive effects of SCGs on plant growth (Ronga et al. [30]). For example, the espresso SCGs composted in the soil can increase the dry weight and yield of lettuce plants (Cervera-Mata et al. [20]) as well as the content of antioxidant compounds (Cruz et al. [16, 17, 31]) and essential macro and micro elements in leaves (Cruz et al. [17]; Cervera-Mata et al. [21]).
Based on current and past literature, the positive or negative outcomes of SCG application on crops strictly depend on species’ intrinsic characteristics, including plant functional and structural traits. Thus, identifying and studying plant eco-physiological strategies in response to SCG application to growth medium becomes essential to assess how the potential reuse of these wastes may represent a natural resource for plants and the environment.
To give a comprehensive insight into the possible consequences of SCGs application on plants, different physiological targets were analyzed in lab tests on the model species Cucumis sativus L. This species is widely used as an effective plant test organism for the toxic effect evaluation of metals or chemical compounds (Motta et al. [32, 33]; Avallone et al. [34]; Napolitano et al. [35]). Furthermore, C. sativus represents a suitable bioindicator of soil or medium contamination. In particular, the monitoring of germination rate and plant growth, as well as the assessment of ultrastructure and analysis of photosynthesis and antioxidant compounds, may be considered a proxy of plant health status giving information about the mechanisms involved in the plant response to possible stress or benefic consequences of SCGs utilization as potential fertilizer.
This study adopted a soilless cultivation approach for cucumber seedlings’ growth and used coffee solutions as rooting media. The hydroponic provides the possibility to evaluate, on plant physiology, only the effects due to SCGs disposal in the culture mean without influences deriving from multiple soil-plant interactions. In particular, we investigated on cucumber seedlings the effects of different coffee solutions that were prepared with fresh coffee powder or spent coffee grounds from mocha and pods left in infusion for 7 or 30 days. Our specific aims were (1) to test the potential changes in cucumber eco-physiological traits following the application of SCGs obtained with different preparation procedures and rest time; (2) to identify the best SCGs preparation in promoting seed germination, photosynthesis, and bioactive compound synthesis in sprouts; (3) to assess the potential toxicological risks of SCGs application on plants.
Materials and Methods
Preparation and Characterization of Coffee Solutions
The coffee solutions (Table 1; Fig. 1) were prepared with powders from different sources: not used fresh expresso powder (NU) or dried spent grounds from expresso mocha (M) or pods (P). The powders were dissolved in mineral water at a final concentration of 17.5 g L− 1, kept in the dark at room temperature for 7 and 30 days, and then gently decanted to separate ground coffee sediment from water. Suspensions were not filtered to avoid introducing or extracting components. Simple mineral water (W), pure from any coffee powder, was used as a negative control.
Different types of coffee powders were infused in mineral water to prepare coffee solutions. From left to right mineral water (W), not used fresh coffee powder at 7 and 30 days (NU7, NU30), dried spent grounds from espresso mocha at 7 and 30 days (M7, M30), dried spent grounds from espresso pod at 7 and 30 days (P7, P30)
GC-MS Analysis for Samples Characterization and Quantification of Analytes
After 5 min of stirring, 7 mL were extracted from each sample, transferred to a Pyrex tube, and dried under a vacuum. Samples were subsequently derivatized with N,O-Bis(trimethylsilyl)acetamide (Sigma-Aldrich), dried and resuspended in 500µL of acetonitrile.
All samples were characterized with the gas-chromatography coupled to mass spectrometry (GC-MS) by using a 5390 MSD quadrupole mass spectrometer (Agilent Technologies) equipped with a gas chromatograph. The column was a Zebron ZB-5HT Inferno (5%-Phenyl-95%-Dimethylpolysiloxane) fused silica capillary column (Column 30 m × 0.32 mm × 0.10 μm) purchased by Phenomenex. The injection temperature was 250 °C. Helium was the carrier gas. GC-MS’s operating conditions were: Inlet 230 °C, gas pressure 7.55 psi, gas flow 1 mL/min, collision Energy 70 eV, mass range 30–450 m/z. The oven temperature program was initially set at 70 °C for 3 min and increased to 280 °C at 10 °C/min for 5 min, then to 300 °C at 15 °C/min for 5 min.
LC-MS/MS Analysis, Standard Solutions, and Samples Preparation
The presence of different phenolic compounds in coffee solutions samples was assessed by liquid chromatography coupled with tandem mass spectrometry (LC-MS/MS) analysis. All standards were purchased from Sigma-Aldrich (St. Louis, MO, USA). All the solutions and solvents were of the highest available purity, suitable for LC-MS analysis and purchased from J. T. Baker (Phillipsburg, NJ, USA).
The stock solutions were prepared by adding 1.00 mL aliquots of each analyte to a 10 mL volumetric flask and bringing the standard to volume with methanol to yield a standard solution with 1000 µgL-1 of each analyte. The stock solutions were stored at − 20 °C until the analysis. Quantitative analysis was performed by constructing calibration curves for a set of standard molecules selected for the different investigated analytes. Standard mixtures were prepared by series dilution as follows: 2,5, 5,0, 25, 50, 250, 500 µgL-1. Samples extracts were diluted at 1:10 in the methanol solvent and filtered and centrifuged at 10,000 × g rpm for 10 min. The supernatant was then directly transferred into an HPLC autosampler, and 1 µl of supernatant was analysed in an LC-MS/MS assay.
LC–MS/MS Instrumentation and Conditions
1 µl of supernatant was analysed using an AB-sciex 5500 QTRAP® system with an HPLC chromatography system Exion LC™. The mobile phase was generated by mixing eluent A (0.1% Formic Acid in water) and eluent B (0.1% formic acid in acetonitrile) and the flow rate was 0.200 mL min-1. The chromatographic gradient was from 20 to 90% in 4 min, held for 2 min, and then returned to 20% in 1 min. Tandem mass spectrometry was performed using a Turbo VTM ion source operated in positive ion mode, and the multiple reaction monitoring (MRM) mode was used for the selected analytes. Supplementary Table S1 provides a list of precursor ions, product ions, collision energy, and declustering potential parameters. The extracted mass chromatogram peaks of metabolites were integrated using Skyline software for data processing.
Quantification of Analytes
The first step for setting mass spectral analysis consisted in the MRM detection of the analytes individually infused to establish the optimal instrument settings for each compound. Next, standard calibration curves for the selected set of molecules were constructed by plotting peak areas against concentration (µgL−1), and linear functions were applied to the calibration curves. As a result, all analytes’ coefficients of determination (R2) were greater than 0.99. Qualitative analysis and identification of the phenolic compounds contained in samples were carried out by using LC-MS/MS in negative (ESI-) ionization modes.
A set of targeted molecules were explored by mass spectrometry in multiple reaction monitoring as reported in the supplementary Table S2 taking advantage of the high performances of triple quadrupole mass spec. For each molecule, specific transitions precursor ion/fragment ion was selected. Quantitative analyses were performed by using the standard external method. Polyphenols analysis was made in triplicate.
Germination, Growth, and Experimental Design
Cucumis sativus L. seeds were sterilized by gently washing in 5% H2O2 and rinsed in distilled water. Next, they were organized in groups of 13, in Petri dishes (d 90 mm) containing two layers of filter paper and one cotton disk wetted with 8 mL of mineral water (W) or different coffee solutions: NU7, NU30, M7, M30, P7, P30.
The Petri dishes, set in triplicate, were incubated in the dark in a climatic chamber at the Department of Biology of the University of Naples Federico II (General Impianti S.A.S., Naples, Italy), at a fixed temperature of 23 ± 2 °C and relative humidity (RH) of 50 ± 5%. When necessary, a few drops of water were added to the paper to maintain the humidity. Petri dishes were monitored daily: the effect of the different coffee solutions on the germination process was evaluated after 4 days of incubation until a constant count of germinated seeds was obtained.
The germination percentage (G%), the days to 50% emergence (E50) and the emergence index (EI) were estimated as reported in Noman et al. [36] using the formulas:
where E50 indicates the rapidity in terms of days to obtain 50% germination, N = final number of germinated seeds; ni = number of seeds emerged by count at time ti when ni< N/2; nj = number of seeds emerged by count at time tj when N/2 < nj;
The germination test was repeated in triplicate for a total of 117 observations (13 seeds × 3 Petri × 3 replicates).
After the germination, sprouts were disposed in vials with sole mineral water (W) or coffee solutions (NU7, NU30, M7, M30, P7, P30) and moved under a Photosynthetic Photon Flux Density (PPFD) of 100 ± 10 µmol photons m− 2s− 1, provided by white LEDs (Light Emitting Diodes), under a photoperiod of 12 h. Temperature (T) and relative humidity (RH) were maintained respectively at 23 ± 2 °C and 50 ± 5%. The cucumber sprouts were grown until 40 days after sowing (DAS), at the complete development of the first true leaves. During the experimental period, the vials were refilled with opportune solutions to reintegrate the water lost by evapotranspiration.
We used a multidisciplinary approach to assess the suitability of different coffee solutions to be utilized as an enhancer for growth in cucumber seedlings considering changes in morphological parameters and leaf anatomy, functional characteristics such as photosynthetic activity and biochemical traits including photosynthetic pigment content and antioxidant capacity as previously studied for several species subjected to other abiotic stress (Arena et al. [37]; Vitale et al. [38, 39]).
Morphological Parameters
At 7, 30 or 40 DAS, cucumber seedlings were compared for morphological parameters, namely total fresh biomass, length, and leaf area. All measurements were performed on 10 seedlings per condition, considering one seedling as one replica. The biomass was determined on fresh weight bases (g FW plant− 1). The total length (cm plant− 1) and the total leaf area (cm2 plant− 1) were measured by digital images of randomly chosen control and treated seedlings and analyzed by ImageJ software (Image Analysis Software, Rasband, NIH, Bethesda, Maryland, USA).
Leaf Anatomical Structure
For light microscopy, cotyledon leaves were collected at 7 DAS from seedlings sprouted from seeds irrigated with 7-day coffee solutions (NU7, M7, P7) or water (W). After excision, cotyledon leaves were immediately fixed in Bouin’s solution, dehydrated in graded ethanol, and processed for paraffin embedding according to routine protocols. Section (6 μm) were stained with hemalum eosin to show the general morphology (Motta et al. [33]). Images were acquired by a ZEISS Axiocam Microscope Camera applied to a Zeiss Axioskop microscope.
Fluorescence Measurements and Photosynthetic Pigment Content
The functionality of the photosynthetic apparatus of the cucumber seedlings exposed to different coffee solutions was evaluated at 40 DAS through chlorophyll fluorescence emission measurements in vivo and quantification of photosynthetic pigment content. Fluorescence analysis was carried out on the first true leaf of ten seedlings per treatment by a portable fluorometer equipped with a light sensor (FluorPen FP100, Photon System Instruments, Brno, Czech Republic). The ground fluorescence signal (F0) was induced on 30’ dark-adapted leaves, by an internal blue LED light of about 1–2 µmol photons m− 2s− 1. The maximal fluorescence signal in the dark-adapted state (Fm) was induced by a saturating light pulse of 3000 µmol photons m− 2s− 1. The PSII maximal photochemical efficiency (Fv/Fm) was calculated by the formula (Fm – F0)/Fm. The steady-state fluorescence signal (Ft) was measured under plant growth irradiance (PPFD of 190 ± 10 µmol photons m− 2s− 1), while the maximum fluorescence (Fm’) in the light-adapted state was determined by applying a saturating pulse of 6000 µmol photons m− 2 s− 1. The quantum yield of PSII electron transport (ΦPSII) was calculated as (Fm’-Ft)/Fm’ as indicated by Genty et al. [40]. The electron transport rate (ETR) was determined according to Maxwell and Johnson [41] as ΦPSII * PFD * 0.5 * 0.84. Finally, the non-photochemical quenching (NPQ) was expressed as (Fm-Fm’)/Fm’ following Bilger and Björkman [42].
After fluorescence measurements, the same leaves were collected to determine the concentration of total chlorophylls (a + b) and carotenoids (x + c), a/b and (a + b)/(x + c) ratios according to Lichtenthaler [43]. Pigments were extracted from disks of known area using ice-cold 100% acetone. Samples were centrifuged (Labofuge GL, Heraeus Sepatech, Hanau, Germany) at 5000 rpm for 5 min. The absorbance of supernatants was measured by spectrophotometer (Cary 100 UV-VIS, Agilent Technologies, Santa Clara, CA, USA) at wavelengths of 470, 645 and 662 nm. The pigment concentration was expressed in µg cm− 2.
Total Polyphenols and Antioxidant Capacity
At 40 DAS, total polyphenols and the antioxidant capacity were evaluated on the first true leaves of ten seedlings per treatment.
Fresh samples (0.02 g), preventively powdered in liquid nitrogen, were extracted in methanol at 4 °C and centrifuged at 11,000 rpm for 5 min. Supernatants were mixed with 1:1 (v/v) Folin-Ciocâlteau reagent and, after 3 min, the 700 mM Na2CO3 solution was added to the resulting mixture in 5:1 (v/v). After an incubation of 2 h in darkness, the absorbance of the samples was read at 765 nm by a spectrophotometer (UV-VIS Cary 100, Agilent Technologies, Palo Alto, CA, USA). The total polyphenol content was calculated by a calibration curve using gallic acid as standard and expressed as mg of gallic acid equivalents per g of fresh weight (mg GAE g− 1 FW).
The determination of the total antioxidant capacity was carried out by the ferric reducing/antioxidant power assay (FRAP) according to George et al. [44]. Briefly, fresh samples (0.250 g) preventively powdered in liquid nitrogen, were homogenized in 60:40 (v/v) methanol/water solution and centrifuged at 14.000 rpm for 15 min at 4 °C. The extracts were mixed with FRAP reagents in the following ratio (v/v): 300 mM acetate buffer pH 3.6 (1:16.6); 10 mM tripyridyltriazine (TPTZ; 1:1.6); 40 mM HCl and 12 mM FeCl3 (1:1.6). After 1 h in darkness, samples absorbance was measured at 593 nm with a spectrophotometer (UV-VIS Cary 100, Agilent Technologies, Palo Alto, CA, USA). Trolox (6-hydroxy-2,5,7,8- tetramethylchroman-2-carboxylic acid) was used as a standard to prepare a calibration curve and determine the total antioxidant capacity expressed as µmol Trolox equivalents per g of fresh weight (µmol Trolox eq g-1 FW).
Statistical Analysis
All data were analyzed using the Sigma Plot 12 software (Jandel Scientific, San Rafael, CA, USA). The one-way analysis of variance (ANOVA) was performed to compare the effect of different coffee solutions on seed germination and morphological, anatomical, and ecophysiological traits of cucumber seedlings. The Shapiro-Wilk test was applied to check the normal distribution of data; the Holm-Sidak test was used for all multiple comparison tests with a significance level of P < 0.05. Data are reported as mean values ± standard error (n = 10).
The overall data collected for seedlings monitored until 40 days after sowing were subjected to the heatmap function using the ClustVis program package (https://biit.cs.ut.ee/clustvis/online). The Euclidean distance and the average linkage method were used to cluster both rows and columns.
The correlations between the parameters were investigated using Pearson’s correlation test and the coefficients were represented in a correlation matrix using the STHDA Statistical tools for high-throughput data analysis (http://www.sthda.com/english/rsthda/correlation-matrix.php).
The color scale used for the heatmap, and the correlation matrix indicates the increment of the values proceeding from red to blue.
Results
Extract Characterization and Quantitative Analysis of the Phenolic Compounds
The results of the quantitative analysis performed by GC-MS on NU7, NU30, M7, M30, P7, and P30 solutions were reported in Supplementary Table S1. Table S1 did not include the water control sample (W) because no organic compounds were identified.
The GC-MS quantitative analysis evidenced that most analytes are present in either the 7-day solutions or the 30-day solutions. The analytes common to NU7 and NU30 suspensions are 1,2-Benzenediol bis(trimethylsilyl) ether; 9,12,15-Octadecatrienoic acid; 2-[(trimethylsilyl)oxy]-1-[[(trimethylsilyl)oxy]methyl; Sugar; Hexadecanoic acid, methyl ester; n-Hexadecanoic acid and Caffeine.
M7 and M30 suspensions show as common analyte only caffeine. On the other hand, P7 and P30 showed more common analytes: 5-Phenyl-2,4-pyrimidinediamine tritms; 5-Pregnen-3ß-ol-20-one, trifluoroacetate; 9,12,15-Octadecatrienoic acid, 2-[(trimethylsilyl)oxy]-1-[[(trimethylsilyl)oxy]methyl; 9-Octadecenoic acid (Z)-, methyl ester; Sugar; Benzenepropanoic acid, 4-hydroxy-a-phenyl-, methyl ester; Eicosanoic acid, methyl ester; Hexadecanoic acid, methyl ester; Hexanedioic acid, bis(2-ethylhexyl) ester; L-Proline, 5-oxo-1-(trimethylsilyl)-, trimethylsilyl ester; Methyl stearate; Pyridine; Caffeine.
Irrespective of the coffee source, caffeine always has the most significant area peak among all the analytes. Concerning the preparation time, the peak area is higher in 30 days solutions than in 7 days.
The quantitative analysis of the 83 phenolic compounds identified by MRM mass spectrometry analyses in the coffee solutions is shown in Table 2. The analysis of the water control sample (W) is not included since no organic compounds were detected.
In NU7 and NU30 suspensions, phenols exceeding the concentration of 100 µg L‒1 are gallic acid, vanillic acid, caffeic acid, caffeoylquinic acid derivative, Chlorogenic Acid, Caffeoyliquinic Acid Lactone, 3PCoumaroylquinic Acid, Orientin, and QuercetinORhamnoside. In particular, NU7 showed a higher concentration of gallic acid, vanillic acid, caffeic acid, Chlorogenic Acid and Caffeoyliquinic Acid Lactone. In comparison, lower concentrations of caffeoylquinic acid derivative, 3PCoumaroylquinic Acid, Orientin and QuercetinORhamnoside are observed.
In M7 and M30 solutions, phenols exceeding the concentration of 100 µg L‒1 are gallic acid, caffeoylquinic acid derivative, Chlorogenic Acid, 3PCoumaroylquinic Acid, and Orientin. All of them are in higher concentrations in M30 than M7, except for gallic acid.
In P7 and P30 suspensions, phenols exceeding the concentration of 100 µg L‒1 are gallic acid, vanillic acid, caffeoylquinic acid derivative, Chlorogenic Acid, Caffeoyliquinic Acid Lactone, 3PCoumaroylquinic Acid. Vanillic acid, caffeoylquinic acid derivative and Caffeoyliquinic Acid Lactone are most abundant in P30 while the remaining in P7.
Among all phenolic compounds, the amount of caffeine is higher in NU compared to M and P solutions and the highest value is observed in NU7 suspension.
Effect on Germination
Data presented in Table 3 shows that the different coffee solutions did not significantly affect seed germination, namely G%, E50, and EI.
Effect on Seedling Growth
As shown in Fig. 2, at 4 DAS, all seedlings were moved in vials containing different coffee solutions. Starting from this moment, seedlings showed a different development depending on the treatment. More specifically, NU7 and NU30 seedlings stopped their development at 7 DAS, most of them without the emergence of the cotyledons. M7s were maintained until 30 DAS and correctly developed the cotyledons but did not complete the expansion of the first true leaves. Conversely, seedlings irrigated with Water, M30, P7 and P30 solutions were monitored until 40 DAS and showed the complete development of the first true leaves.
Growth of cucumber seedlings treated with different types of coffee powders from the germination to 7 DAS prior the development of cotyledons (NU7 and NU 30), to 30 DAS with the development of cotyledons and prior the expansion of the first true leaves (M7), to 40 DAS showing completely developed first true leaves (W, M30, P7, P30). W: mineral water; NU7: not used coffee 7 days; NU30: not used coffee 30 days; M7: mocha seven days; M30: mocha 30 days; P7: pod 7 days; P30: pod 30 days
The length and the fresh biomass (Fig. 3) of cucumber seedlings were measured at 7, 30, and 40 DAS, final developmental stages. Concerning length (Fig. 3A), at 7 DAS, seedlings treated with NU7 and NU30 solutions were shorter (P < 0.001) and P7 seedlings longer (P < 0.05) than those found in W and M7 samples. At 30 DAS, P7 seedlings confirmed being the longest (P < 0.01), while at 40 DAS, the longest (P = 0.002) were M30 seedlings followed by P7.
At 7 DAS, biomass is significantly reduced (P ≤ 0.01) in NU7 and NU30 seedlings (Fig. 3B). At 30 DAS, P30 seedlings were heavier than P7 ones (P = 0.008) and at 40 DAS, in all treatments, namely M30, P7, and P30, seedlings exhibited a greater biomass (P < 0.001) than control-treated seedlings.
Total length (A) and total fresh biomass (B) of cucumber seedlings treated with W: mineral water; NU7: not used coffee 7 days; NU30: not used coffee 30 days; M7: mocha seven days; M30: mocha 30 days; P7: pod 7 days; P30: pod 30 days. Data are mean ± se (n = 10). Different letters indicate statistically significant differences according to one-way ANOVA (P < 0.05)
At 40 DAS, the leaf number and the total leaf area were measured in seedlings that developed the first true leaves (Figs. 2, 3 and 4). The number of leaves was comparable among all treatments (Fig. 4A), whereas the total leaf area was higher (P ≤ 0.031) in M30, P7 and P30 seedlings compared to control W (Fig. 4B).
Effects on Leaf Anatomy
In W leaves (Fig. 5A), palisade mesophyll was a bilayer with closely disposed cylindrical cells, about 60 ± 4.5 μm high. The cytoplasm was filled with large protein bodies, intensely stained by eosin; the nucleus was centrally located, immersed in a dense cytoplasm rather intensely stained with hemalum.
Sections of cotyledonary leaves collected 7 DAS from cucumber seedlings sprouted from seeds irrigated with7-day coffee solutions. A Control waterW. Double layer of cylindrical palisade cells (p) filled with large protein bodies (arrow). Upper epidermis (e). B Mocha M7; bilayer of elongated palisade cells (p) on a third discontinuous layer (*). Spongy mesophyll (s). C Mocha M7; disorganized area (*) in the external palisade layer. Cells show few protein bodies dispersed in a very pale cytoplasm (*). D Pod P7; palisade cells with very large protein bodies (arrow). Hemalum eosin staining; bars: 25 μm (A and D), 50 μm (B and C)
Treatment with Mocha M7 (Fig. 5B, C) induced a moderate lengthening of palisade cells that reached an average of 75 ± 3.3 μm in height. A third, discontinuous deep layer of shorter cells was occasionally also noticed. In the cell’s cytoplasm, the protein bodies were reduced in size and number so they appeared dispersed in a less dense cytoplasm. Patches of anomalous cells could also be recognized (Fig. 5C). Here, cells appeared markedly shorter than in the surrounding areas, the protein bodies were very reduced in size and number, and the cytoplasm was extremely pale, almost unstained. After exposure to pods P7, leaves (Fig. 5D) leaves showed a regularly organized palisade mesophyll, but the protein bodies were fused forming large masses at the two poles of the cell.
Photosynthetic Pigment Content and PSII Photochemistry
Chlorophyll a fluorescence measurements were performed at 40 DAS on the first true leaves. Results are displayed in Fig. 6. Concerning the quantum yield of PSII electron transport (ΦPSII), a significant difference between control-W seedlings and those exposed to P7 solution showed a higher ΦPSII (P = 0.043). No differences were observed among the other treatments (Fig. 6A). The ETR was higher (P ≤ 0.042) in M30 and P7 seedlings compared to control-W (Fig. 6B). Seedlings irrigated with P7 solution exhibited a lower NPQ (P = 0.032) than those treated with P30. No significant changes emerged among the remaining conditions (Fig. 6B). Finally, all coffee solutions, namely M30, P7 and P30, induced a considerable increment of Fv/Fm (P < 0.001) compared to control-W (Fig. 5D).
Quantum yield of PSII electron transport, ΦPSII (A), electron transport rate, ETR (B), non-photochemical quenching, NPQ (C), maximum PSII photochemical efficiency, Fv/Fm (D) of true leaves of cucumber seedlings at 40 DAS treated with W: mineral water; M30: mocha 30 days; P7: pod 7 days; P30: pod 30 days. Data are mean ± se (n = 10). Different letters indicate statistically significant differences according to one-way ANOVA (P < 0.05)
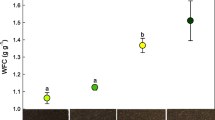
Total chlorophylls and carotenoids decreased (P ≤ 0.008, P ≤ 0.01) in M30, P7 and P30 seedlings compared to those treated with control water W (Fig. 7A, B). More specifically, P7 seedlings showed the lowest values. In addition, M30 and P30 coffee solutions determined the lowest (P ≤ 0.037) a/b ratio (Fig. 7C) while the highest (P ≤ 0.034) (a + b)/(x + c) ratio was registered in P7 seedlings (Fig. 7D).
Total chlorophylls a + b (A), total carotenoids x + c (B), a/b ratio (C), (a + b)/(x + c) ratio of true leaves of cucumber seedlings at 40 DAS treated with W: mineral water; M30: mocha 30 days; P7: pod 7 days; P30: pod 30 days. Data are mean ± se (n = 10). Different letters indicate statistically significant differences according to one-way ANOVA (P < 0.05)
Polyphenols and Antioxidant Capacity
At 40 DAS, the total polyphenol content was higher (P ≤ 0.006) in the first true leaves of P7 and P30 seedlings compared to those treated with W orM30 solutions (Fig. 8A). However, an opposite trend was observed for the total antioxidant capacity, which decreased (P ≤ 0.009) in P7 and P30 seedlings compared to W and M30 ones (Fig. 8B).
Total polyphenol content (A) and total antioxidant capacity (B) in the first true leaves of cucumber seedlings at 40 DAS treated with W: mineral water; M30: mocha 30 days; P7: pod 7 days; P30: pod 30 days. Data are mean ± se (n = 10). Different letters indicate statistically significant differences according to one-way ANOVA (P < 0.05)
Heatmap and Correlation’s Matrix
The heatmap (Fig. 9A) identified two main clusters: the first (I) composed of W-seedlings, characterized by higher values of pigments (Chl a + b and Car x + c), and the second (II) that grouped seedlings treated with different coffee solutions. In particular, the II cluster divided M30 seedlings from those exposed to P7 and P30, identifying the coffee source as the main factor and the coffee solution’s preparation time as the second factor. As a result, M30 seedlings exhibited higher length and antioxidant capacity; P7 seedlings showed the highest Fv/Fm and (a + b)/(x + c) ratios, whereas P30 seedlings returned greater NPQ and polyphenol amounts.
Cluster heatmap analysis (A) summarizing biometrical, physiological, and biochemical traits of cucumber seedlings grown until 40 DAS. Sample groups are clustered in the coffee solution factor columns, W: mineral water; M30: mocha 30 days; P7: pod 7 days; P30: pod 30 days. Coefficients of correlation (Pearson’s test) representation (B) among investigated parameters at 40 DAS. On the basis of the color scale, blue and red boxes indicate increasing and decreasing values respectively
The correlations among the investigated parameters are represented in Fig. 9 B. The ΦPSII was positively correlated to ETR (r = 0.95), Fv/Fm (r = 0.45), and length (r = 0.4), and negatively correlated to NPQ (r = − 0.49). The ratio Fv/Fm was also positively correlated to ETR (r = 0.44), length (r = 0.48), biomass (r = 0.49), and total leaf area (r = 0.46), and negatively correlated to Chl a + b and Car x + c (r = − 0.54). The NPQ was also negatively correlated to ETR (r = − 0.45). The total leaf area was positively correlated to biomass (r = 0.43) and leaf number (r = 0.49), and negatively correlated to Chl a + b (r = − 0.40) and Car x + c (r = − 0.41). The ratio a/b was negatively correlated to biomass (r = − 0.44) and total leaf area (r = − 0.46). The ratio (a + b)/(x + c) was negatively correlated to Chl a + b (r = − 0.73) and Car x + c (r = − 0.81). Finally, polyphenols were negatively correlated to antioxidant capacity (r = − 0.53), Chl a + b (r = − 0.53), and Car x + c (r = − 0.56).
Discussion
Coffee grounds waste is often used as an eco-friendly resource for soil amendment and fertilization. This by-product however cannot be considered safe since it is well known that potentially releases leachates that can impact soil and water quality. The analysis carried out by LC-MS/MS demonstrated how many polyphenols may be present in such leachates and gave their putative concentrations. As expected, the fresh, not-used coffee solution is the richest, for quantities and variety of compounds but composition markedly varies depending on the time of infusion, 7 or 30-days, and on the type of coffee, Mocha or Pods.
In all, the chemical analyses indicated that coffee waste should be used with some caution. Polyphenols in fact are reported to cross seed teguments and interfere with embryo development (Tanti et al. [45]), eventually inhibiting the germination process (Khan and Ungar [46]; Muscolo et al. [28]; Al-Charchafchi and Al-Quadan [27]; Li et al. [47]; Gomes et al. [29]). Although such potential toxic components were present, the different coffee solutions did not alter the G% of cucumber seeds, nor E50 or EI compared to the controls.
However, it was not possible to conclude that no harm was exerted since evident effects were registered on later development, in terms of growth, leaf anatomy, photosynthesis, and antioxidant content. The fresh coffee powder (NU) arrested the development at the rooting stage; Mocha 7-day solution resulted in more moderate effects since allowed the formation of cotyledonary leaves that, however, showed morphological alteration in the palisade mesophyll. Only Mocha 30-days and Pods P7 and P30 seedlings apparently developed normally.
Caffeine, more abundant in NU than in spent Mocha and Pod coffees, can be considered the most probable responsible for the observed effects. By interacting with phospholipids, it inhibits in a dose-dependent manner the embryo water uptake capability, the extensibility of cell walls, and the rigidity of the plasma membranes (Tanti et al. [45]). Moreover, caffeine limits root development and elongation by reducing the mitotic index, destabilizing protein metabolism, and decreasing protein content in the root apical tips (Batish et al. [48]; Silva et al. [49]). On the other hand, other chemicals present in solutions, like benzoic, vanillic, or ferulic acids may have contributed by increasing cell permeability and lipid peroxidation, limiting nutrient absorption from the surrounding environment with consequences on plant growth (Politycka [50]; Yu et al. [51]). For example, previous studies demonstrated that benzoic acid hampers germination and root and shoot growth in radish (Andriana et al. [52]), while after only 7-day treatment, it inhibited root elongation in Eutrema wasabi plants (Li et al. [47]). Further evidence comes directly from cucumber seedlings, in which solutions containing derivates of benzoic, caffeic, gallic, or vanillic acids strongly decreased gas exchanges (Yu et al. [51]). In our case, the benzoic acid has been found in NU7 and not in NU30 suspension, while the vanillic acid is more abundant in NU7 than N30. These results may explain why the biomass is more reduced in seedlings exposed to NU7 than in the NU30 solution.
Interestingly, the seedlings exposed to M7 but not to P7 solutions developed altered cotyledonary leaves with a thickening of the palisade cell layer, the formation of an additional third discontinuous palisade layer, and reduced protein content. No information is available on the effects of coffee compounds on plant structural traits. Caffeine, present in both samples in similar amounts, can injure vascular tissues, epidermal and palisade cells, and plasma and thylakoid membranes. It can also induce the production of large starch grains, interfering with photosynthesis (Alkhatib et al. [53]). On the other hand, in unaltered P7 samples, there are higher levels of chlorogenic acids, cause of epithelial disorganization and membrane lysis in animals (Rey et al. [54]; Bahrami et al. [55]) and of ferulic acid, known to inhibit amino acid transport and protein synthesis (Li et al. [47]). In the alternative, the negative effect observed on leaf structure in M7 samples can rather depend on the high levels of gallic acid (Muzaffar et al. [56]) or on the synergic effect of specific coffee compounds. Consistent with our hypothesis it has been demonstrated that cucumber seedlings exposed to higher concentrations of gallic acid, showed reduced growth, stem elongation, biomass, and polyphenol content (Muzaffar et al. [56]). Since there was no direct contact between leaves and coffee solutions, the cotyledonary alterations suggest that polyphenols were absorbed by the roots and, by exerting a systemic effect, altered photosystem functionality and seedling growth. Indeed, seedlings exposed to M7 solution up to 30 DAS did not exhibit significant morphological alterations compared to the control.
Based on these results, it may be supposed that the main discriminating factor in inducing specific physiological outcomes in seedlings is the type of powder used for the preparation of the coffee solutions. The clustering provided by the heatmap supports this hypothesis since the control is separated from M30 seedlings and P7 and P30 clusters. In addition, a second discriminating factor can be identified in the time the coffees were maintained in infusion: the 7-day solutions had a more pronounced impact, and such information may be relevant to managing potential damages induced by inappropriate coffee waste released into the environment.
From the results it is evident that M30, P7, and P30 coffee solutions determined a different effect, allowing seedling growth and increasing photochemical efficiency. In particular, at 30 DAS, a significant elongation of P7 seedlings was observed together with a biomass decrease compared to P30s. Furthermore, at 40 DAS, all seedlings in M30, P7 and P30 solutions exhibited a consistent increase in length, fresh biomass, and total leaf area, suggesting that the time of infusion is likely responsible of an altered bioavailability of specific components released in the solutions which may elicit stimulatory effects on cucumber growth.
Seedlings exhibited a consistent increase in length, fresh biomass, and total leaf area suggesting a moderate stimulatory effect. It is likely to suppose that leaf expansion compensated for the lower chlorophyll and carotenoid content, so ensuring the light-harvesting for photosynthesis. This hypothesis is consistent with the observation that all seedlings exposed to coffee solutions showed a higher Fv/Fm than Ws, indicating a better physiological performance of photosystem II. Interestingly, P7 seedlings promoted the synthesis of chl b to a (lower a/b ratio) and favored chlorophyll compared to carotenoid production (higher a + b/x + c ratio). These adjustments support the high photochemical performance in these leaves. Moreover, the higher ΦPSII and the lower NPQ values indicated that the photochemical reactions are promoted instead of thermal dissipation processes. Our data clearly indicate that photochemical and morphological parameters were positively correlated, suggesting that coffee-based solutions promoted photosynthesis and consequently stimulated cucumber growth. Although some studies report adverse effects of coffee compounds on pigment content, leaf expansion, and stomatal conductance with negative effects on photosynthesis and plant growth (Patterson et al. [57]), other studies demonstrated the opposite making it interesting to enlarge the knowledge on a possible re-utilization of coffee wastes. Furthermore, it has clearly demonstrated that SCGs promoted yield, the production of chlorophylls and carotenoids, ascorbic acid, tocopherols, minerals and phenols in lettuce plants (Cruz et al. [16, 31]; Cervera-Mata [21]), supporting the application of coffee residues as a direct, simple and safe approach to obtain beneficial effects on plant physiological traits while reducing food waste disposal.
An interesting aspect regards how the plant’ antioxidants are affected by SCGs. Notoriously, phenolic compounds contribute to the plant’s total antioxidant capacity, and generally, a strong correlation between polyphenols and radical scavenging activity is found in a concentration-dependent manner. However, as observed for R. canina infusions, the scavenging activity can decrease with increasing concentrations, suggesting that plant extracts with an improved antioxidant capacity may also exert a prooxidant capacity in different test systems depending on concentration (Kılıçgün and Altıner [58]). Moreover, some polyphenols have both antioxidant and prooxidant properties depending on their metal-reducing potential, chelating behavior, pH, and solubility characteristics (Babich et al. [59]; Morikawa [60]). Some examples are gallic acid, protocatechuic acid, vanillic acid, caffeic acid, coumaric acid, chlorogenic acid, ferulic acid, myricetin, quercetin, rutin, kaempferol, catechin, epicatechin, delphinidin, malvidin, and theobromine (Azam et al. [61]; Babich et al. [59]; Morikawa [60]).
Interestingly, our study observed a negative correlation between polyphenols and antioxidant capacity. Polyphenols significantly increased in P7 and P30 seedlings compared to Ws and M30s, while the leaf antioxidant capacity decreased. The source of spent coffee grounds, in this case, pods, the preparation procedure, including the infusion time, and polyphenols’ solubility and concentration may have elicited a prooxidant activity leading to the recruitment of other antioxidant compounds, such as ascorbic acid, tocopherols, carotenoids determining the decline in total antioxidant capacity. For instance, vanillic acid, epicatechin, and theobromine are only found in P suspensions. On the other hand, caffeic acid, coumaric acid, chlorogenic acid, and kaempferol show a higher concentration in Ps solutions than in M30. Since polyphenols are positively correlated with all photosynthetic and growth parameters, we hypothesize that P seedlings, irrespective of preparation procedure and infusion time of solutions, adopted active strategies to counteract the increase of polyphenols and maintain an optimal physiological status throughout the plant growth period.
Conclusions
Through a soilless cultivation approach, this study investigated the effect of different coffee solutions obtained by infusing fresh coffee powder or SCGs from mocha and pods for 7 or 30 days on Cucumis sativus seedlings. Such a cultivation method allows us to assess only the influence of SCGs disposal in the culture means, avoiding possible interferences deriving from multiple soil-plant interactions. The different coffee solutions showed proper polyphenol compositions. Applied to seeds or as rooting media, all the infusions affected seedlings’ anatomy, photosynthetic efficiency, and antioxidant content. More specifically, fresh coffee powder arrested seedling development just after one week, 7 days mocha suspension altered cotyledon structure, while 30-day mocha and pods suspensions stimulated biomass, leaf area, photosynthesis, and polyphenol concentration. Based on our data the timing of suspension must be considered before applying the coffee wastes in plant growing media to obtain only positive outcomes on plant ecological and agronomic traits.
The overall results demonstrated that the fresh coffee powder and the mocha suspension obtained after a 7 days infusion are unsuitable for cultivation. Conversely, 30 days mocha and pods suspensions are promising natural fertilizers for seedlings, indicating an alternative and ecological reuse of spent coffee grounds for increasing productivity in a large-scale soilless cultivation system.
The application of SCGs, opportunely treated, encourages the implementation of recycling policies to promote potential and innovative uses of coffee by-products in agriculture, reducing their disposal in the environment.
However, as the chemical composition of coffee wastes changes during time, the use of SCGs deserves further investigation focused on the understanding of the dynamics of released components which depends on the time of exposure to environmental factors, the type of coffee source, and the potential interaction with soil microorganisms in the case of the application directly to the soil.
Data Availability
The data supporting the findings of this study are available from the corresponding author (C.A.), upon reasonable request.
References
Liang, N., Kitts, D.D.: Antioxidant property of coffee components: assessment of methods that define mechanisms of action. Molecules 19, 19180–19208 (2014). https://doi.org/10.3390/molecules191119180
Samoggia, A., Riedel, B.: Consumers’ perceptions of Coffee health benefits and motives for coffee consumption and purchasing. Nutrients 11, 653 (2019). https://doi.org/10.3390/nu11030653
ICO, International Coffe Organization: https://www.ico.org/Market-Report-20-21-e.asp (2022) Accessed 27 Aug 2022
Stylianou, M., Agapiou, A., Omirou, M., Vyrides, I., Ioannides, I.M., Maratheftis, G., Fasoula, D.: Converting environmental risks to benefits by using spent coffee grounds (SCG) as a valuable resource. Environ. Sci. Pollut. Res. 25, 357–357 (2018)
Mussatto, S.L., Machado, E.M.S., Martins, S., Teixeira, J.A.: Production, composition, and application of coffee industrial residues. Food Bioproc. Tech. 4(5), 661–672 (2011)
Leifa, F., Pandey, A., Soccol, C.R.: Solid state cultivation-an efficient method to use toxic agro-industrial residues. J. Basic Microbiol. 40, 187–197 (2000)
Deblonde, T., Cossu-Leguille, C., Hartemann, P.: Emerging pollutants in wastewater: a review of the literature. Int. J. Hyg. Environ. Health 214(6), 442–448 (2011)
Martin, J., Camacho-Munoz, D., Santos, J.L., Aparicio, I., Alonso, E.: Occurrence of pharmaceutical compounds in wastewater and sludge from wastewater treatment plants: removal and ecotoxicological impact of wastewater discharges and sludge disposal. J. Hazard. Mater. 239–240, 40–47 (2012)
Karnjanapiboonwong A, Morse AN, Maul JD, Anderson TA (2010) Sorption of estrogens, triclosan, and caffeine in a sandy loam and a silt loam soil. J Soils Sediments 10:1300–1307.DOI https://doi.org/10.1007/s11368-010-0223-5.
Vieira, L.R., Soares, A.M.V.M., Freitas, R.: Caffeine as a contaminant of concern: a review on concentrations and impacts in marine coastal systems. Chemosphere 286(2), 131675 (2022). https://doi.org/10.1016/j.chemosphere.2021.131675
Korekar, G., Kumar, A., Ugale, C.: Occurrence, fate, persistence and remediation of caffeine: a review. Environ. Sci. Pollut. Res. 27, 34715–34733 (2020). https://doi.org/10.1007/s11356-019-06998-8
Mustard, J.A.: The buzz on caffeine in invertebrates: effects on behavior and molecular mechanisms. Cell. Mol. Life Sci. 71, 1375–1382 (2014). https://doi.org/10.1007/s00018-013-1497-8
Fernandes, A.S., Mello, F.V.C., Thode Filho, S., Carpes, R.M., Honório, J.G., Marques, M.R.C., Felzenszwalb, I., Ferraz, E.R.A.: Impacts of discarded coffee waste on human and environmental health. Ecotoxicol. Environ. Saf. 141, 30–36 (2017). https://doi.org/10.1016/j.ecoenv.2017.03.011
McNutt, J., He, Q.S.: Spent coffee grounds: a review on current utilization. J. Ind. J. Ind. Eng. Chem. 71, 78–88 (2019)
Kasongo, R.K., Verdoodt, A., Kanyankagote, P., Baert, G., Van Ranst, E.: Coffee waste as an alternative fertilizer with soil improving properties for sandy soils in humid tropical environments. Soil Use Manag. 27(1), 94–102 (2011)
Cruz R, Baptista P, Cunha, Pereira JA, Casal S (2012) Carotenoids of lettuce (Lactuca sativa L.) grown on soil enriched with spent coffee grounds. Molecules 17:1535–1547.
Cruz, R., Morais, S., Mendes, E., Pereira, J.A., Baptista, P., Casal, S.: Improvement of vegetables elemental quality by espresso coffee. Food Chem. 148, 294–299 (2014). https://doi.org/10.1016/j.foodchem.2013.10.059
Xu, L., Geelen, D.: Developing biostimulants from agro-food and industrial by-prosucts. Front. Plant Sci. 9, 1567 (2018). https://doi.org/10.3389/fpls.2018.01567
IFOAM, International Federation of Organic Agriculture Movements. https://www.organicseurope.bio/ Accessed 27 Aug 2022
Cervera-Mata, A., Pastoriza, S., Rufián-Henares, J.A., Párraga, J., Martín-García, J.M., Delgado, G.: Impact of spent coffee grounds as organic amendment on soil fertility and lettuce growth in two Mediterranean agricultural soils. Arc. Agro. Soil Sci. 64(6), 790–804 (2017). https://doi.org/10.1080/03650340.2017.1387651
Cervera-Mata, A., Navarro-Alarcón, M., Delgado, G., Pastoriza, S.: Spent coffee grounds improve the nutritional value in elements of lettuce (Lactuca sativa L.) and are an ecological alternative to inorganic fertilizers. Food Chem. 282, 1–8 (2019). https://doi.org/10.1016/j.foodchem.2018.12.101
Cervera-Mata, A., Navarro-Alarcón, M., Rufián-Henares, J.A., Pastoriza, S., Montilla-Gómez, J., Delgado, G.: Phytotoxicity and chelating capacity of spent coffee grounds: two contrasting faces in its use as soil organic amendment. Sci. Total Environ. 717, 137247 (2020). https://doi.org/10.1016/j.scitotenv.2020.137247
Chilosi, G., Aleandri, M.P., Luccioli, E., Stazi, S.R., Marabottini, R., Morales-Rodríguez, C., Vettraino, A.M., Vannini, A.: Suppression of soil-borne plant pathogens in growing media amended with espresso spent coffee grounds as a carrier of Trichoderma spp. SciHortic 259, 108666 (2020)
Yamane, K., Kono, M., Fukunaga, T., Iwai, K., Sekine, R., Watanabe, Y., Iijima, M.: Field evaluation of coffee grounds application for crop growth enhancement, weed control, and soil improvement. Plant Prod. Sci. 17, 93–102 (2014)
Hardgrove, S.J., Livesley, S.J.: Applying spent coffee grounds directly to urban agriculture soils greatly reduces plant growth. Urban Urban Green 18, 1–8 (2016). https://doi.org/10.1016/j.ufug.2016.02.015
Al-Charchafchi, F., Al-Quadan, F.: Effect of chlorogenic acid on germination and seedling growth, and on the enzymes activity extracted from Artemisia herbaalba ASSO. Part I: germination and seedling growth. Dirasat. Pure Sci. 33(2), 168–175 (2006)
Alam, S.M., Ala, S.A., Ansari, R., Khan, M.A.: Influence of leaf extract of bermuda grass (Cynodondactylon L.) on the germination and seedling growth of wheat. Int. J. Wheat Breed Genet. 92, 1–4 (2001)
Muscolo, A., Panuccio, M.R., Sidari, M.: The effect of phenols on respiratory enzymes in seed germination respiratory enzyme activities during germination of Pinus laricio seeds treated with phenols extracted from different forest soils. Plant Growth Regul. 35, 31–35 (2001)
Gomes T, Pereira JA, Ramalhosa E, Casal S, Baptista P. Effect of fresh and composted spent coffee grounds on lettuce growth, photosynthetic and mineral composition. In: 7th Congreso Ibérico de Agroingenieria y Ciencias Horticolas; SECH e SEAgIng:Madrid, Spain, (2013)
Ronga, D., Pane, C., Zaccardelli, M., Pecchioni, N.: Use of spent coffee ground compost in peat-based growing media for the production of basil and tomato potting plants. Commun. Soil Sci. Plant Anal. (2016). https://doi.org/10.1080/00103624.2015.1122803
Cruz, R., Mendes, E., Torrinha, A., Morais, S., Pereira, J.A., Baptista, P., Casal, S.: Revalorization of spent coffee residues by a direct agronomic approach. Food Res. Int. 73, 190–196 (2015)
Motta, C.M., Cerciello, R., De Bonis, S., Mazzella, V., Cirino, P., Panzuto, R., Ciaravolo, M., Simoniello, P., Toscanesi, M., Trifuoggi, M., Avallone, B.: Potential toxicity of improperly discarded exhausted photovoltaic cells. Environ. Pollut. 216, 786–792 (2016). https://doi.org/10.1016/j.envpol.2016.06.048. (Epub 2016 Jul 1. PMID: 27376992)
Motta, C.M., Simoniello, P., Arena, C., Capriello, T., Panzuto, R., Vitale, E., Agnisola, C., Tizzano, M., Avallone, B., Ferrandino, I.: Effects of four food dyes on development of three model species, Cucumis sativus, Artemia salina and Danio rerio: Assessment of potential risk for the environment. Environ. Pollut. 253, 1126–1135 (2019). https://doi.org/10.1016/j.envpol.2019.06.018
Avallone, B., Arena, C., Simoniello, P., Di Lorenzo, M., Vitale, E., Capriello, T., Ferrandino, I., Raggio, A., Sasso, M., Napolitano, G., De Bonis, S., Fogliano, C., Agnisola, C., Motta, C.M.: Comparative toxicity of Vegan Red, E124, and E120 food dyes on three rapidly proliferating model systems. Environments 9(7), 89 (2022). https://doi.org/10.3390/environments9070089
Napolitano, G., Motta, C.M., Agnisola, C., Venditti, P., Fasciolo, G., Ferrandino, I., Capriello, T., Vitale, E., Costanzo, G., Avallone, B., Simoniello, P., Arena, C.: Commercial red food dyes preparations modulate the oxidative state in three model organisms (Cucumis sativus, Artemia salina, and Danio rerio). Environments 9(5), 63 (2022). https://doi.org/10.3390/environments9050063
Noman A, Ali Q, Maqsood J, Iqbal N, Javed MT, Rasool N, Naseem J(2018). Deciphering physio-biochemical, yield, and nutritional quality attributes of water-stressed radish (Raphanus sativus L.) plants grown from Zn-Lys primed seeds. Chemosphere 195:175–189.
Arena, C., Santorufo, L., Cataletto, P.R., Memoli, V., Scudiero, R., Maisto, G.: Eco-physiological and antioxidant responses of Holm Oak (Quercus ilex L.) leaves to cd and Pb. Water Air Soil Pollut. 228, 459 (2017). https://doi.org/10.1007/s11270-017-3638-4
Vitale E, Vitale L, Costanzo G, Velikova V, Tsonev T, Simoniello P, De Micco V, Arena C. (2021) Light spectral composition influences structural and eco-physiological traits of Solanum lycopersicum L. cv. ‘Microtom’ in response to High-LET Ionizing Radiation. Plants 10(8):1752. Doi: https://doi.org/10.3390/plants10081752.
Vitale E, Izzo LG, Amitrano C, Velikova V, Tsonev T, Simoniello P, De Micco V, Arena C. (2022) Light quality modulates photosynthesis and antioxidant Properties of B. vulgaris L. plants from seeds irradiated with high-energy heavy ions: Implications for cultivation in space. Plants 11(14):1816. Doi: https://doi.org/10.3390/plants11141816.
Genty B, Briantais JM, Baker NR (1989) The relationship between the quantum yield of photosynthetic electron transport and quenching of chlorophyll fluorescence. Biochim Biophysi Acta, 990:87–92.
Maxwell, K., Johnson, G.N.: Chlorophyll fluorescence-a practical guide. J. Exp. Bot. 345, 659–668 (2000)
Bilger W, Björkman O (1990). Role of the xanthophyll cycle in photoprotection elucidated by measurements of light-induced absorbance changes, fluorescence and photosynthesis in leaves of Hedera canariensis. PhotosynthRes 25:173–185.
Lichtenthaler, H.K.: Chlorophylls and carotenoids, the pigments of photosynthetic biomembranes. Methods Enzymol. 148, 350–382 (1987). https://doi.org/10.1016/0076-6879(87)48036-1
George, B., Kaur, C., Khurdiya, D.S., Kapoor, H.C.: Antioxidants in tomato (Lycopersium esculentum) as a function of genotype. Food Chem. 84, 45–51 (2004)
Tanti, A.J., Bhattacharyya, P.N., Sandilya, S.P., Dutta, P.: Allelopathic potential of caffeine as growthand germination inhibitor to popular tea weed, Borreria hispida L. Curr. Life Sci. 2(4), 114–117 (2016). https://doi.org/10.5281/zenodo.163671
Khan, M.A., Ungar, I.A.: Inhibition of germination in Atriplex triangularis seeds by application of phenols reversal of inhibition by growth regulators. Bot. Gaz. 147, 148–151 (1986)
Li, Z.H., Wang, Q., Ruan, X., Pan, C.D., Jiang, D.A.: Phenolics and plant allelopathy. Molecules 15, 8933–8952 (2010). https://doi.org/10.3390/molecules15128933
Batish, D.R., Singh, H.P., Kaur, M., Kohli, R.K., Yadav, S.S.: Caffeine affects adventitious rooting and causes biochemical changes in the hypocotyl cuttings of mung bean (Phaseolus aureus Roxb.). Acta Physiol. Plant 30, 401–405 (2008)
Silva, R.M., Brigatti, J.G., Santos, V.H., Mecina, G.F., Silva, L.P.: Allelopathic effect of the peel of coffee fruit. SciHortic. 158, 39–44 (2013). https://doi.org/10.1016/j.scientia.2013.04.028
Politycka, B.: Free and glucosylated phenolics, phenol 13-glucosyltransferase activityand membrane permeability in cucumber roots affected by derivatives of cinnamic and benzoic acids. Acta Physiol. Plant 19(3), 311–317 (1997)
Yu, J.Q., Ye, S.F., Zhang, M.F., Hu, W.H.: Effects of root exudates and aqueous rootextracts of cucumber (Cucumis sativus) and allelochemicals, on photosynthesis andantioxidant enzymes in cucumber. Biochem. SystEcol. 31, 129–139 (2003)
Andriana, Y., Quy, T.N., Xuan, T.D.: Phenolic acids as plant growth inhibitors from Tridax procumbens L. IOP Conf. Ser: Earth Environ. Sci. 250, 012024 (2019). https://doi.org/10.1088/1755-1315/250/1/012024
Alkhatib R, Alkhatib B, Al-Eitan L, Abdo N, Tadros M, Bsoul E (2018) Physio-anatomical responses of tobacco under caffeine stress. Photosynthetica 56(4):1140–1146. DOI: https://doi.org/10.1007/s11099-018-0798-4.
Rey, D., Pautou, M.P., Meyran, J.C.: Histopathological effects of tannic acid on the midgut epithelium of some aquatic Diptera larvae. J. InvertebrPathol. 3(2), 173–81 (1999). https://doi.org/10.1006/jipa.1998.4810
Bahrami, M.N., Mikani, A., Moharramipour, S.: Effect of caffeic acid on feeding, α-amylase and protease activities and allatostatin. A content of egyptian cotton leafworm, Spodoptera littoralis (Lepidoptera: Noctuidae). J. Pest Sci. 43(2), 73–78 (2018)
Muzaffar, S., Ali, B., Wani, N.A.: Effect of catechol, gallic acid and purogallic acid on the germination, seedling growth and the level of endogenous phenolics in cucumber (Cucumis sativus L.). Int. J. Life Sc Bt& Pharm. Res. 1(3), 50–55 (2012)
Patterson, D.: Effects of allelopathic chemicals on growth and physiological responses of soybean (Glycine max). Source: Weed Sci. 29(1), 53–59 (1981)
KılıçgünH, A.D.: Correlation between antioxidant effect mechanisms andpolyphenol content of Rosa canina. Pharmacogn. Mag. 6(23), 238–41 (2010). https://doi.org/10.4103/0973-1296.66943
Babich, H., Schuck, A.G., Weisburg, J.H., Zuckerbraun, H.L.: Research Strategies in the study of the pro-oxidant nature of polyphenol nutraceuticals. J. Toxicol. 2011, 467305 (2011). https://doi.org/10.1155/2011/467305
Morikawa, C.K.: A new green approach to Fenton’s chemistry using tea dregs and coffee grounds as raw material. Green Process Synth. 3, 117–125 (2014). https://doi.org/10.1515/gps-2013-0113
Azam, S., Hadi, N., Khan, N.U., Hadi, S.M.: Antioxidant and prooxidant properties of caffeine, theobromine and xanthine. Med. Sci. Monit. 9(9), 325–30 (2003)
Funding
Open access funding provided by Università degli Studi di Napoli Federico II within the CRUI-CARE Agreement. The authors declare that no funds, grants, or other support were received during the preparation of this manuscript.
Author information
Authors and Affiliations
Contributions
Conceptualization: CA, CMM, EV; formal analysis and investigation: EV, CMM, BA, CF, GB, MS, CF; data curation and visualization: EV, CA; supervision: CA, CMM, RP; Resources: CA, CMM, AA; Writing-original draft preparation: EV, CA; Writing-Review & editing: All the authors. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Competing interest
The authors have no financial or non-financial interests to disclose.
Ethical Approval
Not applicable.
Consent to Participate
Not applicable.
Consent to Publish
Not applicable.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Vitale, E., Motta, C.M., Avallone, B. et al. Sustainable Reuse of Expresso Coffee By-products as a Natural Fertilizer to Improve Growth and Photosynthesis in Cucumber (Cucumis sativus L.) Plants. Waste Biomass Valor 15, 543–559 (2024). https://doi.org/10.1007/s12649-023-02143-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12649-023-02143-2