Abstract
In this research, an industrially important enzyme tannase and product gallic acid was produced with an inexpensive novel substrate Swietenia macrophylla. Fermentation of S. macrophylla was optimized using a two-step approach: First, the traditional One variable at-a-time technique, and second, the statistical Box-Behnken design for co-production of tannase enzyme and gallic acid. This two-step method of optimization showed the highest tannase activity and gallic acid yield of 0.0497 U/mL and 225 µg/mL respectively which is a 29.5 and 49-fold increase when compared to unoptimized conditions. Further, the partially purified tannase enzyme was characterized and showed optimal tannase activity at pH 4.0 and 30 ℃, and was stable between pH 3.0–6.0 and 4−40 ℃ for 24 h and 10 h, respectively. Also, metal ions such as Ca2+, Na+, and K+ at 1 mM concentration; and organic solvents methanol, and isoamyl alcohol at 20% v/v exhibited the highest activity at optimized reaction conditions. Whereas, Mn2+, Zn2+, Mg2+, Fe2+ and Fe3+, EDTA, TritonX 100, toluene, and hexane caused the tannase inhibition at higher concentrations. In the end, the fermentative production of gallic acid was verified qualitatively through thin-layer chromatography and Fourier transform infrared spectroscopy.
Graphical Abstract

Similar content being viewed by others
Avoid common mistakes on your manuscript.
Statement of Novelty
Currently, gallic acid is produced with acid hydrolysis of synthetic substrate tannins, which results in low purity, high cost, and causes environmental pollution. Hence, in the present study an alternative cost-effective natural substrate i.e. the bark and leaves of Swietenia macrophylla was discovered for the co-production of tannase enzyme and gallic acid. The mathematical model was developed to optimize the fermentation of S. macrophylla and showed a 29.5 and 49-fold increase in tannase and gallic acid respectively. The partially purified tannase was optimized for enzyme-substrate reaction conditions for possible industrial applications. The production of the product gallic acid was validated through thin-layer chromatography and Fourier transform infrared spectroscopy.
Introduction
Semi-solid-state fermentation (semi-SSF) describes the process of producing microbial metabolites using solid support and substrate while using minimal amounts of liquid medium. The defining characteristic of solid-state fermentation (SSF) is that microbial growth in the fermentation system happens without a free-flowing aqueous phase. This method has been widely exploited to produce antibiotics, organic acids, enzymes, and other such compounds by using natural or semi-synthetic substrates [1]. The process of SSF has been extensively practiced in history, mostly in Asian countries to create processed and fermented food items such as soy sauce, miso, sake, etc. In ancient Egypt, basic SSF techniques were used to bake bread for more than 4000 years [2]. In the modern world, SSF has been applied in the fields of large-scale food processing, biorefineries, processing of fossil fuel alternatives, and other industries to minimize the use of water and optimize the economics of process biotechnology [3]. The most crucial characteristic displayed by SSF and semi-SSF is that of the resistance of microorganisms to the inhibition of enzyme production due to catabolic repression due to the fermentation system containing carbon sources such as sucrose, glucose, and other substrates [4]. Semi-SSF can be considered over SSF due to the advantage of providing moisture to the microbial culture can lead to more stable growth. However, adding excess moisture to the substrate, as in the case of submerged fermentation (SmF), can lead to lower levels of oxygen penetration, leading to growth inhibition of the microbial culture [5, 6].
The process of semi-SSF is being conducted on Swietenia macrophylla by using a strain of Bacillus gottheilii M2S2, which is aerobic, rod-shaped, Gram-positive, and motile bacteria that has optimum growth at pH 8.0 and 30 °C. The bacterial cell fatty acid profile contains major amounts of iso-C15:0, anteiso-C17:0, and anteiso-C15:0, while the phospholipids contained in the cell are phosphatidylglycerol, aminophospholipid, diphosphatidylglycerol, and phosphatidylethanolamine. The G + C content of the genomic DNA determined was 38.7 mol% as per previously conducted experiments [7]. It has been characterized via various research articles that Bacillus gottheilii M2S2 has a good yield in producing extracellular tannase enzymes [8, 9]. Hence, this strain of bacterial isolate can be used as a method of converting tannic acid present in the substrate to produce gallic acid as tannase extracted from bacterial fermentation can hydrolyze tannic acid [10].
Swietenia macrophylla, also called Big-Leaf Mahogany is a plant species generally found in major regions of South America, Central America, and certain parts of South India and Malaysia. This plant also bears fruits appropriately named “sky fruits” due to the fruits pointing towards the sky and are widely used as a natural remedy for improving blood circulation and skin conditions in folk medicine. Due to its pharmacological activity such as anti-inflammatory, antimicrobial, antidiabetic, and antioxidative behavior, it has been used in folk medicine for hundreds of years [11]. In past research, S. macrophylla has indicated the capacity of inducing mitochondrial-mediated apoptosis of HCT116 colorectal carcinoma cells via the upregulation of p53 [12]. The S. macrophylla has also been studied for its exhibition of antioxidant, antimicrobial, antimutagenic, antimalarial, antifeedant, antidiabetic, acaricidal, anti-nociceptive, antidiarrheal, hypolipidemic, and heavy metal phytoremediation activity [11]. The high levels of hydrolyzable tannins in S. macrophylla make the plant sample a good candidate for bioprocessing to extract gallic acid. The ready availability of S. macrophylla in temperate regions makes it a cost-effective alternative as the production of gallic acid from hydrolyzable tannins can be conducted by the bioconversion and biotransformation of tannic acid to gallic acid with the use of enzyme tannase [8, 10]. The action of B. gottheilii M2S2 on the plant sample is majorly due to the production of the tannase enzyme, leading to a yield of gallic acid. To the best of our knowledge, this is the first report on the bark and leaves of Swietenia macrophylla, as an alternative cost-effective natural substrate for the co-production of tannase and gallic acid.
Tannase, also referred to as tannin acyl hydrolase (EC 3.1.1.20), acts on tannins by hydrolyzing the bonds in hydrolyzable tannins and also in gallic acid–based esters. The catalysis by tannase on these substrates leads to the production of gallic acid [13]. This action breaks down tannins which are toxic secondary metabolites produced by various plants. A variety of microorganisms face growth inhibition as tannins are capable of binding to and precipitating proteins and iron, interfering in various biological processes [14]. A major use of tannase occurs in the detannification of food, removal of pollutants, production of gallic acid, or in producing leather [9, 13]. Another major impact of the tannase enzyme is in the process of preserving vitamin C in fruit juices as the hydrolysis of tannins produces gallic acid, which can act as an antioxidant, preventing the oxidation of vitamin C [15]. The area of interest for the use of tannase as a biocatalyst is to reduce the use of chemicals to conduct acid hydrolysis of tannins, which would in turn reduce the purification costs during the extraction of compounds such as gallic acid from the reaction mixture [13, 16]. This shift in using biocatalysts also helps in reducing the environmental strain and the release of pollutants into the environment as a result of using highly corrosive acids and bases. Another advantage of using biocatalysts is that they generally have high bioavailability of the products, leading to easier uptake by the human body.
The hydrolysis of tannic acid, gallic acid esters, and other tannins produces gallic acid and is the primary method of production for gallic acid. Gallic acid is a phenolic acid with two intramolecular and five intermolecular hydrogen bonds. It is a significant bioactive compound due to its well-pronounced antioxidant and anti-cancer capabilities. Derivatives of gallic acid have been used in various phytomedicines due to their varying pharmacological activities such as radical scavenging, cell signaling pathway interference, cancer cell apoptosis, and other activities. This range of the applicability of gallic acid is caused because of a fine amalgam between its pro-oxidant and antioxidant potential [17]. A major obstacle observed in the process of synthetic production of gallic acid is the use of synthetic chemicals resulting in low purity, high cost, and low yield given by acidic hydrolysis of tannic acid [18]. However, bacterial semi-SSF can be a great alternative to improve the above-mentioned parameters and also improve the bioavailability of the gallic acid produced, while also ensuring sustainable production economics and better efficiency of processing.
Materials and Methods
Materials
Rhodanine, gallic acid, methyl gallate, methanol, Bovine serum albumin (BSA), sodium dodecyl sulfate (SDS), tri-ethanolamine, potassium hydroxide, Coomassie Blue dye, ferric chloride, and citrate buffer solution were used as reagents in this study and all procured from Hi-media Laboratories Pvt. Ltd., Mumbai-India and Sigma Aldrich Co. LLC, Mumbai-India.
Microorganism and Cultural Conditions
A previously isolated strain of Bacillus gottheilii M2S2 was used for this study due to its well-researched capabilities of tannase production. This strain was isolated from tannery effluent soil [9]. Identification of the strain was done by 16 S rRNA sequence analysis, where the nucleotide sequence was further submitted to Gene Bank (Accession number: KU866380). A sample of the isolated strain was deposited in the Microbial Type Culture Collection Centre- Chandigarh, India (MTCC 12,554). Cultures of Bacillus gottheilii M2S2 were maintained on nutrient agar plates at 4 ℃.
Inoculum Preparation
The preparation of the inoculum was done before semi-SSF. 250 mL Erlenmeyer flasks with 50 mL of nutrient broth were inoculated with 2 mL of an 18 h old Bacillus gottheilii M2S2 culture. The flasks were then incubated in an incubator at 32 ℃ and 180 rpm for 18 h. The inoculum was then further used to inoculate the substrate and perform Semi-SSF. A load of viable cell count in the inoculum was found to be 4 × 1012 cells/mL using the colony count technique [9].
Substrate Preparation
Swietenia macrophylla was chosen as the substrate after conducting a literature review on the composition of bioactive compounds of multiple plant samples due to having a high concentration of phenolic compounds such as free gallic acid, tannins, and flavonoids [11]. Equal-weight bark and leaf samples were retrieved from End Point, Manipal, Karnataka, India (13°22′ 11″ N, 74°47′6″ E). The samples were then dried for 48 h under sunlight at 30 ℃ ± 3 ℃ and atmospheric pressure at 1008 hPa ± 3 hPa. The samples were pulverized to minute particles, sieved, and further preserved for analysis of bioactive compounds.
Qualitative Test for Tannin Content
The presence of tannins in the substrate was checked by mixing 0.5 g of pulverized plant sample in 20 mL of distilled water in a test tube and boiling for 10 min. The mixture was filtered using a Whatmann filter. The filtrate was taken and 3–4 drops of 0.1% FeCl3 were added to be allowed to sit till a color change was observed. The color change of the filtrate to a blue–black/brownish–green hue indicates the presence of hydrolyzable tannins in the substrate [19].
Semi-Solid State Fermentation of Swietenia macrophylla
Semi-solid state fermentation (semi-SSF) was conducted on the plant sample by measuring 1 g of plant substrate, adding modified Czapek–Dox media with the composition (g/L) [20]: FeSO4·7H2O, 0.01; NaNO3, 3; MgSO4·7H2O, 0.5; KCl, 0.5; K2HPO4, 1 and then autoclaved at standard conditions. Post autoclaving, the flasks were cooled to room temperature and inoculated with 1 mL of inoculum per 1 g of a solid substrate in a laminar airflow chamber and further incubated in an incubator at 32 ℃ for 48 h.
Tannase Enzyme Production Optimization via One Variable at-a-time (OVAT) Analysis
Six variables were selected to optimize the production of tannase using the OVAT analysis technique in shake flasks. The variables were the amount of substrate (1–5 g), amount of moistening media (Czapek–Dox media) (2–10 mL), carbon sources (sucrose, dextrose, fructose, maltose, lactose, glucose), inducers (1% tannic acid, 1% methyl gallate), inoculum volume (2–10 mL) and fermentation time (8–72 h). The use of OVAT analysis entailed changing one variable while keeping the other variables constant and repeating the studies upon finding the optimum value for that particular variable. OVAT was chosen as it is a convenient method of designing media composition for initial studies and the optimization of process factors [21]. All studies were conducted in duplicate.
Optimization of Fermentation Media Using Box-Behnken Design
Optimization of media conditions was done with the use of the Box-Behnken design (BBD) of experiments for the most optimum tannase activity and gallic acid yield. Three parameters were chosen for the conducting BBD and were designed using MINITAB 17 Software (Trial Version). Parameters chosen for the optimization were the amount of substrate (1–5 g); the amount of moistening media (2–10 mL) and inoculum volume (2–10 mL). BBD was conducted at 3 levels (− 1, 0, + 1) and 15 trials were designed. The moistening media was enriched with 1% tannic acid. Table 1 represents the coded values of parameters used for BBD. Table 2 represents the experimental design matrix of BBD design for the optimization of media. All the experiments were conducted in duplicates and the average values of tannase activity and gallic acid yield are represented in Table 2.
Validation of the Mathematical Model
The data of tannase enzyme activity acquired from BBD was analyzed by analysis of variance (ANOVA) using MINITAB 17 software (Trial version). This analysis identified statistically significant variables and interactions between variables. Further, multiple regression analysis determined the coefficients of regression for linear, quadratic, and linear interactions of variables. The coefficients were then modeled to fit a polynomial equation (Eq. 1).
Where Y pertains to the tannase enzyme production (predicted response); x refers to independent variables of the model; β0 represents the intercept; βi refers to the model’s linear coefficients; βii refers to the quadratic coefficients for the factor i; βij represents the interaction coefficients relating to factors i and j. F-values and R2 were determined via ANOVA and used to determine the validity of the polynomial equation. A semi-SSF was conducted at optimal conditions to validate the statistical model. Through the response optimizer in the model programmer, the response optimized values were obtained [22].
Extraction of Crude Tannase
The fermented matter was extracted after semi-SSF and was suspended with 30 mL of citrate buffer (0.05 M citrate buffer at pH 5.0). Incubation was done at 180 rpm at 32 ℃ for 1 h. Post incubation, centrifugation was carried out for 15 min at 4 ℃ and 10,000 g to retrieve the crude tannase enzyme. Further, the cell-free supernatant was analyzed for gallic acid, protein, tannic acid, and tannase activity by various biochemical assays [9].
Analytical Methods
Gallic Acid Estimation
Gallic acid content in the crude extract of fermentation matter was estimated as described elsewhere [23]. Here, the gallic acid produced during fermentation reacts with methanolic rhodanine to form an intermediate. The intermediate complex reacts with KOH to form a pinkish-red complex, which can be spectrophotometrically analyzed. This method is capable of sensing gallic acid concentrations as low as 5 nmol, with a precision of 1.7% relative standard deviation. To conduct the assay, 0.25 mL of the crude enzyme was mixed with 0.25 mL of citrate buffer and incubated at room temperature for 5 min. 0.3 mL of 0.667% methanolic rhodanine was added to the mix and let incubate for 5 min. Post incubation, 0.2 mL of 0.5 N KOH was added to the mix and incubated for 5 min at room temperature. Distilled water was added and the volume was brought up to 5 mL and incubated at room temperature for 10 min. The absorbance was spectrophotometrically analyzed at 520 nm.
Tannase Activity Estimation
The modified tannase activity estimation assay [23] works on the principle of the hydrolysis of tannic acid in the reaction mixture yielding gallic acid from the substrate. Measurement of tannase activity is conducted by considering one unit of tannase enzyme to be the formation of one micromole gallic acid formed per min, which can be analyzed spectrophotometrically at 520 nm. The assay was conducted on the crude sample by mixing 0.25 mL each of crude enzyme and tannic acid and allowed to incubate for 5 min. 0.3 mL of 0.667% methanolic rhodanine was added to the mix and let incubate for 5 min. Further, 0.2 mL of 0.5 N KOH was mixed into the reaction mixture and incubated for 5 min. The volume of the reaction mixture was brought up to 5 mL by adding distilled water. The mix was then incubated for 10 min and then the absorbance was analyzed spectrophotometrically.
Protein Content
Bradford’s method [24] is a very widely used protein estimation assay. This assay works on the principle of binding protein molecules to Coomassie Blue dye under acidic conditions, causing a change of color from brown to blue [25]. The assay was conducted by taking 0.25 mL of crude tannase enzyme and adding 0.75 mL of distilled water. 3mL of Bradford’s reagent was added to the mix, vortexed, and then let incubate for 5 min in a dark environment. The reaction mixture was then analyzed in a spectrophotometer at 595 nm.
Tannic acid Estimation Assay
Tannic acid content was determined as described by Hagerman and Butler [26], which is a rapid and reproducible method where the bovine serum albumin (BSA) precipitates the tannic acid and can be pelleted out upon centrifugation, which can be further analyzed by spectrophotometrically. In brief, 1 mL of the crude enzyme was vortexed with 2 mL of BSA (1.0 mg/mL) and incubated for 15 min. The reaction mixture was subjected to centrifugation for 15 min at 4 ℃ and 5000 g. The supernatant was then discarded, and 4 mL of SDS-triethanolamine solution was added to the pellet for dissolution. 1 mL of FeCl3 was added to the reaction mixture and immediately vortexed and let incubate for 15 min. Analysis of the reaction mixture was done spectrophotometrically at 510 nm.
Partial Purification of Tannase Enzyme
Ammonium Sulfate Precipitation
The crude tannase enzyme was concentrated for partial purification by adding ammonium sulfate ((NH4)2SO4) salt to the enzyme, dissolving and then subjecting it to dialysis. Adding salt to a protein mix increases the solubility of globular proteins due to salting-in but at higher concentrations, salting-out occurs and precipitation occurs [27]. The ammonium sulfate salt (20–80% w/v) was added to the crude enzyme at 4 ℃ with the use of a magnetic stirrer. The extract after each cut was then centrifuged, precipitated, and analyzed further. The workflow of the protein precipitation with the use of ammonium sulfate was conducted as described in the standard protocol [28].
Dialysis
Dialysis was followed after ammonium sulfate salt precipitation to further purify the enzyme tannase. Dialysis membranes are generally treated before use to remove any impurities on the membrane or to decrease protein absorption in the membrane [29]. Dialysis was conducted on the 60% extract from the ammonium sulfate protein purification experiment and also on a pooled sample of all ammonium sulfate extracts. After conducting dialysis, the samples were analyzed by using Bradford’s assay and tannase activity estimation assay. The dialysis membrane (10 kDa) was prepared for purification by boiling the membrane in distilled water for 10 min. and then boiling in 2% sodium bicarbonate for 10 min to activate the membrane, post which the membrane was again kept in boiling distilled water for 10 min. Extracted ammonium sulfate cuts were then loaded into the membrane, clipped, and then suspended with citrate buffer in a beaker. The suspension was stirred constantly for 24 h by placing the beaker on a magnetic stirrer at 4 ℃. The buffer was changed every 2 h [30] and the analysis was done by Bradford’s assay and tannase activity estimation assay as per standard protocol.
Characterization of Tannase Enzyme
Characterization of the enzyme is an integral process in determining the process conditions. The study conducted on the enzyme by testing it against various parameters helps in understanding the effects of additives and reactor conditions, helping us in optimizing the process for maximum enzymatic activity and yield of product. To characterize the tannase enzyme, the following parameters were considered and tested: pH, pH stability, temperature, thermal stability, additives, metal ions, and organic solvents.
The effect of pH on tannase were studied with different buffer systems: 0.05 M HCl/KCl buffer (pH 1.0–2.0), 0.05 M citrate buffer (pH 3.0–6.0), 0.05 M phosphate buffer (pH 7.0–8.0) and 0.05 M Tris/HCl buffer (pH 9.0) at the standard reaction conditions as described elsewhere [23]. Whereas the stability of the tannase enzyme at various pH (1.0–9.0) was studied by incubating with their respective buffers at 30 ℃ and 4 ℃ for 24 h. After the incubation, the residual tannase activities were analyzed [31, 32].
The effect of temperature on tannase activity was studied at different ranges from 4 to 90 ℃ at optimum pH as described elsewhere [23]. Whereas the thermal stability of the tannase was also studied by incubating the enzyme at various temperatures for 10 h. Then, the residual tannase activity was estimated at standard reaction conditions [31, 32].
Next, the effect of additives such as sodium dodecyl sulfate (SDS), Tween60, Ethylenediaminetetraacetic acid (EDTA), β-mercaptoethanol, and TritonX 100 at 1%(w/v) was studied by incubating with tannase enzyme for 30 min at optimum pH and temperature. Then, the residual activity was analyzed at standard reaction conditions.
Further, the effect of metallic salts on the tannase enzyme was studied as described elsewhere [31, 32]. Metallic salts such as MnCl2, KCl, ZnCl2, MgCl2, CaCl2, NaCl, FeCl2, and FeCl3 were prepared individually in a citrate buffer of pH 4.0 at three different concentrations of 1 mM, 5 mM, and 10 mM. To study the impact of metal ions on the tannase enzyme, 0.5 mL of each salt solution was mixed with 0.5 mL of enzyme and incubated at 30 ℃ for 30 min. Then, the individual tannase-metal salt mixture was analyzed for the residual tannase activities.
Finally, the effect of organic solvents on the tannase enzyme was studied. The organic solvents used were methanol, hexane, toluene, butanol, and isoamyl alcohol of three different concentrations i.e. 20%(v/v), 40%(v/v), and 60%(v/v) solutions (prepared in citrate buffer). A 0.5 mL of the respective organic solvent was mixed with 0.5 mL of tannase enzyme and incubated for 30 min at 30 ℃. After incubation, residual tannase activity was estimated as per the standard reaction protocol [31, 32].
Reaction Kinetics of Tannase Enzyme
The determination of the reaction kinetics of an enzyme is essential in studying the kinetic parameters of the enzymatic reaction. Using a Lineweaver–Burk double reciprocal plot [33], tannase enzyme activity was analyzed by conducting a tannase activity estimation assay and plotting in a double reciprocal graph. Tannic acid at varying concentrations (0.2–2 mM) was used as the substrate for the reaction at 30 ℃ and pH 4.0. The Michaelis–Menten constant (Km, mM) and the maximum rate (Vm, µmol/mL min) were determined according to the given equation (Eq. 3):
Characterization of Gallic Acid Production
Thin-Layer Chromatography (TLC)
Thin-layer chromatography describes the process where chromatography is employed to separate non-volatile mixtures. It just generally conducted on inert sheets with a coating of an adsorbent material such as silica acting as the stationary phase. The sample is loaded on this phase along with a standard to compare. A mobile phase solvent is then prepared, the composition of which is based on the targeted separation for a given quantitative or qualitative test (Ethyl acetate: Butanol: Formic acid (5:4:1)) [34]. The mobile phase travels up the plates by capillary action and the movement of the solvent results in the separation of components of the samples at varying rates due to the analytes traveling up the plates at varying rates. After the completion of the chromatography run, the analytes are visualized with the use of a spraying reagent and then the retention factor (Rr) is computed by dividing the travel of the analyte on the plate by the travel of the mobile phase. The formula is given as (Eq. 2):
For this study, silica plates were marked and coated with 100% methanol. The plates were then activated at 110 ℃ in a hot air oven for 5 min [34, 35]. The protocol mentioned in [34] was modified to conducted TLC on a pure gallic acid standard and the gallic acid obtained after enzymatic hydrolysis of tannins. The dialysis sample was then spotted on the plate along with a standard gallic acid (1.0 mg/mL) and run with the use of a mobile phase composed of ethyl acetate, butanol and formic acid in the ratio of 5:4:1. The plate was then air-dried and sprayed with the spraying agent (1% FeCl3) and again air-dried. Post-air-drying, the plates were photographed and the Rf values were calculated.
Fourier Transform Infrared (FTIR) Spectroscopy
FTIR method of spectroscopy focuses on obtaining an infrared absorption spectrum of a sample, collecting high-resolution spectral data over a given spectral range. They analyze samples by probing the vibrational characteristics of amino acids and other co-factors. These factors can be analyzed via FTIR as these properties are very sensitive to structural changes. In the case of plant extracts, FTIR is used to probe the molecular vibrational properties of proteins, co-factors, and amino acids as they are hypersensitive to conformational changes. IR signatures of these molecules can be visualized for each specific residue of the extract and can be used to identify the specific compound [36]. For the analysis of the gallic acid in the fermented matter, the probe and plate were cleaned with trichloromethane and a few drops of the gallic acid extract and placed on the plate. The probe was then placed on the gallic acid extract and FTIR was conducted to find the IR signatures. Further, different wavenumbers can be used to identify structures in the sample and classify the compounds present in the sample [37].
Results and Discussion
Qualitative Test of Tannin Content
The semi-solid-state fermentation of plant substrates allows us to extract bioactive compounds that have essentially been used for centuries in ayurvedic medicine, food preservation and processing, and other purposes. With advancements in technology and the use of sophisticated equipment, we can exploit plant samples to release compounds that can be used for medical treatment, replacing synthetic compounds in cosmetics, health maintenance, and other purposes [11, 12]. The qualitative test for tannin content indicated the presence of tannins in the plant substrate. The substrate exhibited a color change of a brownish-green hue after being reacted with 3–4 drops of FeCl3 (Fig. 1). The presence and concentration of tannins in the substrate may vary based on the soil composition, mean atmospheric temperature, moisture content of the soil, and other environmental conditions in different regions.
Tannase Enzyme Production Optimization via One Variable at-a-time (OVAT) Analysis
Impact of the Amount of Substrate
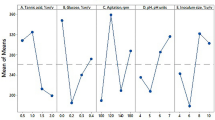
The yield of tannase and gallic was expressed to be maximum at 1 g of the substrate after 24 h of fermentation, with 0.0017 ± 3.333E-04 U/mL and 5.3636 ± 0.2682 µg/mL respectively (Fig. 2a). This phenomenon can be interpreted as an increase in substrate quantity that could lead to intermediate hydrolysates inhibiting the action of the tannase enzyme either competitively or non-competitively. Further, the increase in bed height of the substrate can lead to inadequate diffusion of liquid in the substrate, leading to lesser hydrolysis of the substrate [38].
Experimental results of one variable at-a-time analysis optimize the co-production of tannase enzyme and gallic acid with Swietenia macrophylla under semi-SSF: Impact of the amount of substrate (a); Impact of moistening media (b); Impact of carbon sources (c); Impact of inducers (d); Impact of inoculum volume (e); Impact of fermentation time (f and g)
Impact of Moistening Media
The tannase activity and gallic acid yield were expressed when levels of moistening media were varied and reached a peak value at 6 mL with 0.0018 ± 1.543E-04 U/mL and 8.0909 ± 0.4046 µg/mL respectively (Fig. 2b). The presence of moisture in the initial stage of fermentation is integral to the growth of B. gottheilii M2S2 and the yield of the enzyme as cell development and synthesis require a certain amount of substrate moisture. It was observed that the tannase activity and subsequently, the gallic acid content increased gradually peaked at the optimum level of 6 mL, later decreased with an increase in moisture content. However, excess water can lead to a lack of oxygen penetration in the system and lead to growth inhibition, lower accessibility of nutrients, and lower enzyme yield [5, 6].
Impact of Carbon Sources
The addition of synthetic carbon sources such as sucrose, dextrose, fructose, glucose, maltose, and galactose to the fermentation media exhibited a net negative effect on the activity of the tannase enzyme and yield of gallic acid when compared with the control (Fig. 2c). This can be explained by the inference that the carbon sources such as sucrose are inhibiting the binding of substrate to the enzyme either competitively or non-competitively, leading to lower production of tannic acid [4]. As per previously conducted research, the addition of carbon sources caused inhibition of tannase enzyme production in Bacillus licheniformis [39, 40] and Lactobacillus sp. ASR-S1[41]. It is possible that the addition of carbon sources to the substrate may have caused osmotic stress and result in lower tannase yield as previously reported by [42].
Impact of Inducers
The inducers methyl gallate and tannic acid were added into the fermentation media individually at 1%(w/v). After fermentation, the media with 1% tannic acid showed an increase in tannase activity and gallic acid yield of 0.02 ± 0.0052 U/mL and 27.3636 ± 1.369 µg/mL respectively (Fig. 2d). In comparison to the control, both inducers helped in improving the yield of tannase enzyme and gallic acid during fermentation. Among them, the addition of 1% (w/v) tannic acid increased the yield of tannase enzyme and gallic acid by 11.765 and 5.102-fold respectively. Previously conducted research indicated the maximum tannase production in the range of 0.6%(w/v) to 1.5%(w/v) of tannic acid [39,40,41]. Whereas the addition of tannic acid above 1%(w/v) caused inhibition of tannase production, a result of substrate toxicity as described elsewhere [43].
Impact of Inoculum Volume
An increase in the inoculum volume resulted in increased tannase and gallic acid yield and showed their maximum at 6 mL/g (i.e. 24 × 1012 cells/g) with 0.023 ± 0.0011 U/mL and 49.818 ± 2.491 µg/mL respectively (Fig. 2e). This can be attributed to the microbe having easy access to carry out hydrolytic action throughout the substrate. Lower inoculum volume may not be adequate for efficiently yielding tannase and providing high-growth kinetics. Increasing the inoculum concentration causes rapid synthesis of the enzyme due to a higher proliferation of biomass [44, 45].
Impact of Fermentation time
At the end of the OVAT analysis, the kinetics of tannase, gallic acid, tannic acid, and protein were studied through semi-SSF of Swietenia macrophylla at the above-discussed optimized conditions (Fig. 2f and g). The maximum tannase activity recorded was 0.0258 ± 3.729E-04 U/mL at 48 h of incubation, indicating a 15.31-fold increase from the unoptimized conditions. The maximum gallic acid yield observed was 70.545 ± 3.3273 µg/mL (i.e. 2116 µg/g of the substrate) which is a 13.15-fold increase from unoptimized conditions (Fig. 2f), whereas the initial gallic acid content in the substrate was very negligible 4.141 ± 0.561 µg/mL (i.e.120.141 µg/g of the substrate). The study also showed the maximum yield of total protein at 48 h, reported 1.3603 ± 0.02497 mg/mL protein (Fig. 2 g). The initial tannic acid present in the crude substrate Swietenia macrophylla was 12.6 ± 0.211 mg/mL; and at 48 h of fermentation time the 60% tannic acid was utilized i.e. 7.26 ± 0.240 mg/mL (Fig. 2 g). The reported specific activity of the tannase as shown in Fig. 2f and g indicates an exponential growth phase occurs from 16 to 48 h for Bacillus gottheilii, which also corresponds to the exponential increase in tannase activity and gallic acid yield. The variation of the optimized time for fermentation occurs due to the differences in the strain of microorganisms, environmental conditions, nutrient content of media, and other factors [46]. Arshad et al., [47], reported the optimum incubation time of 96 h for A.oryzae (FCBP-PTF-1202), indicating that Bacillus gottheilii is a better alternative due to shorter incubation time, leading to faster production of gallic acid. It is also observed that the increase in protein content corresponds to increased tannase activity, indicating the higher production of tannase enzyme till 48 h and thereby decreasing due to depletion of nutrients. The increased yield of the tannase enzyme has also resulted in a gradual decline of tannin content in the plant substrate due to the hydrolysis of tannins into gallic acid through bacterial semi-SSF as depicted in Fig. 2g.
Optimization of Media Using Box-Behnken Design
To optimize the extraction of tannase enzyme and free gallic acid in the system, the BBD of response surface methodology was employed. The results of the BBD experiments yielded maximum tannase activity of 0.0497 ± 0.0025 U/mL and a maximum gallic acid yield of 224.818 ± 11.241 µg/mL at run 3 (Table 2). The process conditions of run order 3 were: 1 g of the substrate; 10 mL of moistening media; and 6 mL of inoculum volume. The lowest value for tannase activity and gallic yield were observed in run 16 and run 11 respectively, with the values as 0.0013 ± 0.0001 U/mL and 48.091 ± 0.00013 µg/mL. The linear terms for the amount of substrate (A), and moistening media (B); the quadratic term for the amount of substrate (A2); and the two-way interaction of the amount of substrate and moistening media (AB) were determined to be significant due to 95% confidence (P-value ≤ 0.05). As determined by ANOVA, the linear model can be observed to be highly significant due to achieving an F-value of 189.64 and a P-value of 0.000 (Table 3). The regression equation obtained was as follows (Eq. 4):
Where, A = Amount of substrate (g), B = Moistening media (mL) and C = Inoculum volume (mL).
The interaction effect of the parameters was studied further through 3D-surface plots as depicted in Fig. 3, in which a positive effect for the interaction between the amount of substrate (A) and moistening media (B) on the yield of tannase enzyme as the levels converged towards the center point (0). Whereas the interactions between the amount of substrate (A) and inoculum volume (C); and moistening media (B) and inoculum volume (C) showed a net negative effect on the yield of tannase enzyme, possibly due to varying combinations of B and C at different levels, even if B is individually significant.
Validation of the Mathematical Model
The solution for the mathematical regression model was obtained by using the response optimizer in MINITAB 17 (Trial version). This was used to optimize the design model for maximum tannase enzyme yield, where the optimum values for the parameters were: the amount of substrate = 1.0 g; moistening media = 10.0 mL; inoculum volume = 2.0 mL. All obtained values were contained within the experimental range and the predicted enzyme activity at optimized conditions was 0.0529 U/mL (Table 4). The validation of the mathematical regression model was carried out under optimized conditions of the fermentation process in duplicates. The experimental tannase activity obtained at above mentioned optimized conditions was 0.0497 ± 0.0025 U/mL. The experimental and predicted activities show a good correlation, indicating that the model is viable (Fig. 4). The optimization resulted in a 29.5-fold increase in tannase activity when compared to the unoptimized condition. Whereas, the gallic acid yield under optimized conditions was observed to be 224.818 ± 11.241 µg/mL, which is a 42-fold increase in comparison with unoptimized conditions (Table 4). To determine the goodness of fit of the model, the determination coefficient (R2) was calculated to be 0.9938. The R2 value observed was 99.38% and the predicted R2 value was 90.13%, indicating good validity of experimental data and reasonable agreement with the tannase production experiment model.
Partial Purification of Tannase Enzyme
Ammonium Sulfate Precipitation
Ammonium sulfate salt was added from 0 to 80% w/v and the maximum yield was observed at 60% w/v, quantified by measuring protein content and tannase activity. The results observed at 60% w/v for yield and purification fold were 21.3% and 0.8527, while total activity was 286.52 µM/min and total protein content was 17.704 mg/mL. It can be inferred that salting-in of the ammonium sulfate salt occurred till 60% w/v of the salt and salting-out occurred at concentrations above that (Table 5 and Supplementary Fig. 1).
Dialysis
Dialysis is a process that occurs between two fluids where the solute of interest travels across a semipermeable membrane due to a concentration gradient which is by Fick’s 1st law of diffusion [48]. Dialysis is generally used in the purification of proteins by using a dialysis membrane of a pore size that can be used to trap the protein of interest in the membrane. The optimum 60% ammonium salt precipitation of crude tannase was dialyzed and showed a 6.1% increase in the yield of protein and thereby the purification fold was increased to 1.266 (Table 5).
Characterization of Tannase Enzyme
Effect of pH on Tannase Activity
Enzymes work at an optimal pH, above or below which the activity exponentially decreases, a primary reason for which is protein denaturation leading to loss of activity [49]. In this study, the maximum tannase activity of 0.0758 ± 0.0038 U/mL was observed at pH 4.0 and both temperatures 4 ℃ and 30 ℃ (Fig. 5a). Whereas the tannase enzyme was stable between the pH range of 3.0–6.0 for 24 h while retaining the residual activity of 99.6% (Fig. 5a). The higher activity at a lower temperature can be explained by the reason that at 4 ℃, the enzyme structure is the most well preserved from being denatured, while storage at higher temperatures can lead to rapid and eventual denaturation of enzymatic activity [50]. In comparison with previously conducted research, the tannase procured from B. gottheilii had an optimum activity at a slightly acidic pH of 4.0 and stable from pH 3.0–6.0, which is in contrast to the results obtained from the fungal strain Penicillium montanense URM 6486, which showed maximum activity at pH 9.0 [51]. Saeed et al. [52] depicted the optimum pH for highest gallic acid yield at pH 5.0 while using Aspergillus niger FCPB-SF-0002 on various fruit peels.
Effect of Temperature on Tannase Activity
In this study, the optimal tannase activity of 0.0761 ± 0.0038 U/mL was shown at 30 ℃ and pH 4.0 (Fig. 5b). The increase in temperature increases the enzymatic activity to the optimum temperature of the enzyme [53], after which the protein starts denaturing and losing the enzymatic activity [49]. This ambient optimum temperature of 30 ℃ may allow us to process the tannase enzyme from the tannin-rich plant residues, leading to lower operational costs. In comparison to other relevant research, the optimum temperature for tannase activity is concurrent at 30 ℃. However, the results of Jiménez et al. [54] show the stability of tannase activity from Lactobacillus plantarum strains was maintained between 20 and 30 ℃; while the tannase in this research showed stability over a range of 4–40 ℃. As per Saeed et al. [55], the optimum temperature for incubation was observed to be 30 °C, which is in agreeance with the results of this study. Tannase obtained from Fusobacterium nuclear sub. polymorphum showed the highest activity at 55 ℃; which requires external heating to the fermentation vessel to provide the necessary process conditions, further increasing the cost [56].
The enzyme tannase was stable between the range of 4–40 ℃ for 10 h with residual activity of 98%, indicating that the enzyme can be stored between this range of temperatures and have a negligible loss in activities (Fig. 2b).
Effect of Additives on Tannase Activity
Additives can act as either an inhibitor or promoters in the action of the enzyme. Various compounds such as β-mercaptoethanol act as reducing agents, inactivating RNases and other enzymes, leading to loss of activity [57]. In this study, 1%(w/v) of SDS, Tween60, EDTA, β-mercaptoethanol, and TritonX 100 were tested for their effect on tannase activity. Among them, SDS and Tween60 resulted in increased tannase activity, with 9.22% and 20.2% respectively (Fig. 5c). In contrast, EDTA, β-mercaptoethanol, and TritonX 100 showed decreased enzymatic activity, resulting in a 25.76%, 100%, and 31.94% decrease in activity respectively (Fig. 5c), indicating that the additives can be used in tandem with metal ions and organic solvents to further study the effect on the fermentation kinetics for the optimization of bioprocessing and biotransformation of tannic acid to gallic acid.
Effect of Metal ions on Tannase Activity
The study yielded the results where metal ions at 1 mM concentration increased the activity of tannase activity in comparison to the control (Fig. 5d). Metal ions of Mn2+, K+, Ca2+, and Na+ exhibited increased activity at 1 mM, with an increase of 2.92%, 29.44%, 28.17%, and 68.02% respectively. At 1 mM concentration, Zn2+, Mg2+, Fe2+ and Fe3+ showed a decrease in activity by 72.33%, 14.72%, 24.37% and 41.75% respectively. At 5 mM concentration, only K+, Ca+ and Na+ ions showed an increase in activity by 25.13%, 16.5% and 53.05% respectively. Whereas at 10 mM, an increase in activity was observed only for K+ and Na+ ions, with an increase of 21.81% and 46.73% respectively (Fig. 5d). Metal ions can aid in the catalytic activity of an enzyme by providing metal-protein interactions such as enzyme-bridge, metal- and ligand- complexes by acting as electron acceptors and donors, regulators, or Lewis’ acid molecules [58]. Integral active-site amino acid chains in enzymes can act as electrostatic catalysts and stabilize intermediates of a catalyzed reaction or act as general acid-base catalysts that contribute to the transfer of protons [59]. As the concentration of metallic salts is increased, salting out of protein is more rapid, which can lead to a loss in enzymatic activity.
Effect of Organic Solvents on Tannase Activity
The effect of organic solvents was studied by preparing 20%, 40%, and 60%(v/v) solutions of methanol, hexane, toluene, butanol, and isoamyl alcohol in citrate buffer. Among them, 20%(v/v), methanol, toluene, butanol, and isoamyl alcohol produced an increase in tannase activity by 7.494%, 3.81%, 4.755%, and 46.694% respectively. Hexane caused a decrease of 14.326% in enzymatic activity at 20%(v/v). At 40% and 60% concentrations, all organic solvents caused a decrease in tannase activity (Fig. 5e). Researchers have reported that the less polar solvents show higher enzymatic activity due to hydration of protein, resulting in the onset of activity due to the protein reaching total mobility [60, 61]. Certain side reactions such as racemization, decomposition, hydrolysis, and polymerization take place in water-based systems, limiting the catalysis of reactions by enzymes and also hindering the synthesis of enzymes [62]. Organic solvents can curb this problem and disrupt cell membranes to release intracellular enzymes and reduce possible contamination.
Reaction Kinetics of Tannase Enzyme
The reaction kinetics of the tannase enzyme was studied by conducting a tannase activity estimation assay on various concentrations of substrate (0.2–2.0 mM tannic acid dissolved in 0.05 M citrate buffer). The graphical analysis was conducted by using a Lineweaver–Burk double reciprocal plot resulting in a maximum reaction rate (Vmax) of 0.0232 µmol/mL min and a substrate saturation constant (Km) of 3.81 mM, with the R2 being 0.9584 (Supplementary Fig. 2). A low Km value indicates the high specificity of the produced tannase enzyme towards the substrate tannic acid. Comparably, the Km and Vmax values for the tannase enzyme produced using Aspergillus niger and Streptomyces sp. AL1L with gallotannin and 0.1% tannic acid as substrates respectively were 6.611mM and 12.406 µmol/mL [63] and 1.4 mM and 15.6 µmol/mL [64].
Characterization of Gallic acid Production
Thin-Layer Chromatography (TLC)
The bacterial fermentative production of gallic acid from Swietenia macrophylla was validated qualitatively by thin-layer chromatography and was compared against a standard gallic acid solution (1 mg/mL). The retention factor of the standard and product gallic acid were 0.356 and 0.323 respectively, indicating the production of a higher concentration of gallic acid through semi-SSF of tannin-rich substrate Swietenia macrophylla (Supplementary Tables 1 and Fig. 6).
Qualitative analysis of: a substrate tannic acid before fermentation; b product gallic acid without bacterial fermentation of substrate S. macrophylla; and c product gallic acid produced with bacterial fermentation of substrate S. macrophylla through semi-SSF using Thin-layer chromatography. S, Commercial tannic acid/gallic acid; T/T1/T2, tannic acid/gallic acid
Fourier Transform Infrared (FTIR) Spectroscopy
The gallic acid production through semi-SSF from substrate Swietenia macrophylla was analyzed by FTIR and showed vibrational frequency bands at wavenumbers 3278.43, 2899.11, 2832.09, 2160.43, 2073.44, 2049.20, 2013.55, 1638.50, 1487.34, 795.72, 737.25, 586.10, 523.35, 501.96, 474.87, 466.31, 439.22, 433.51 and 410.70 cm−1 (Fig. 7). These frequency bands indicate C–H stretching, O–H stretching, aromatic C–C, C–O stretching, conjugated C–O, and para–O–H presence in the sample [65]. Flavonoids such as flavone, fisein, myricetin, quercetin, morin, and hesperetin generally represent C = O bands between 1615 and 1655 cm−1, while they exhibit –C–OH stretching vibrations between 1110 and 1175 cm−1 [66, 67]. The strong peak at 3278.43 cm−1 corresponds to –OH group stretching, indicating that our product contains significant levels of phenolic compounds i.e. gallic acid (Fig. 7). The frequency bands between the IR range of 2160.43–2013.55 cm−1 indicate the presence of amino acids corresponding to the presence of the tannase enzyme and other proteins in the sample (Fig. 7). The lack of a peak at wavenumber 2260 cm−1 represents an absence of cyanide groups in the optimized sample, indicating a nontoxic behavior. The lack of strong bands between 1260 and 1130 cm−1 indicates the absence of secondary alcohols and ester compounds. The presence of a strong frequency band at wavenumber 1638.50 cm−1 indicates the presence of amides in the sample.
Conclusion
The co-production of tannase enzyme and gallic acid from Swietenia macrophylla through semi-solid state fermentation is an economically viable and sustainable process. The optimization studies conducted in this research have indicated the maximum co-production of tannase enzyme and gallic acid of 0.04973 U/mL and 224.818 µg/mL which is a 29.5 and 49-fold increase respectively when compared to unoptimized conditions. Considering that the partially purified tannase was thermally stable up to 40 ℃ over a range of pH 3.0 to 6.0, the semi-SSF can be considered a sustainable and inexpensive method of producing tannase enzyme and microbially hydrolyzing tannins from the inexpensive source S. macrophylla to produce gallic acid and tannase.
-
1.
Qualitative analysis of tannic acid content in the substrate S. macrophylla; Left: Control; and Right: substrate.
-
2.
Experimental results of one variable at-a-time analysis optimize the co-production of tannase enzyme and gallic acid with S. macrophylla under semi-SSF: Impact of the amount of substrate (a); Impact of moistening media (b); Impact of carbon sources (c); Impact of inducers (d); Impact of inoculum volume (e); Impact of fermentation time (f and g).
-
3.
Interaction plots among the significant parameters for enhanced co-production of tannase enzyme and gallic acid with S. macrophylla under semi-SSF: the amount of substrate × moistening media (a); amount of substrate × inoculum volume (b); moistening media × inoculum volume (c).
-
4.
Normal probability plot of optimized tannase enzyme using Box-Behnken design with R2 0.9938; and predicted R2 0.9013.
-
5.
Results of characterization of enzyme tannase produced from S. macrophylla through Semi-SSF: (a) Effect of pH and stability; (b) Effect of temperature and stability; (c) Effect of additives; (d) Effect of metal ions on the enzyme; and (e) Effect of organic solvents.
-
6.
Qualitative analysis of: (a) substrate tannic acid before fermentation; (b) product gallic acid without bacterial fermentation of substrate S. macrophylla; and (c) product gallic acid produced with bacterial fermentation of substrate S. macrophylla through semi-SSF using Thin-layer chromatography. S, Commercial tannic acid/gallic acid; and T/T1/T2, tannic acid/gallic acid.
-
7.
FTIR spectra of the product gallic acid produced with bacterial fermentation of substrate S. macrophylla through Semi-SSF.
Data Availability
The datasets used and/or analyzed during the current study are available from the corresponding author upon reasonable request.
References
Pandey, A.: Solid-state fermentation. Biochem. Eng. J. 13, 81–84 (2003). https://doi.org/10.1016/S1369-703X(02)00121-3
Krishna, C.: Solid-state fermentation systems-an overview. Crit. Rev. Biotechnol. 25, 1–30 (2005). https://doi.org/10.1080/07388550590925383
Manan, M.A., Webb, C.: Design aspects of solid state fermentation as applied to microbial bioprocessing. J. Appl. Biotechnol. Bioeng. 4(1), 511–532 (2017). https://doi.org/10.15406/jabb.2017.04.00094
Viniegra-González, G., Favela-Torres, E.: Why solid-state fermentation seems to be resistant to catabolite repression? Food Technol. Biotechnol. 44(3), 397–406 (2006)
Chilakamarry, C.R., Sakinah, A.M., Zularisam, A.W., Sirohi, R., Khilji, I.A., Ahmad, N., Pandey, A.: Advances in solid-state fermentation for bioconversion of agricultural wastes to value-added products: opportunities and challenges. Bioresour. Technol. 343, 126065 (2022). https://doi.org/10.1016/j.biortech.2021.126065
Singh nee’ Nigam, P., Pandey, A.: A.: Solid-state fermentation technology for bioconversion of biomass and agricultural residues. In: Singh nee’ Nigam, P., Pandey, A. (eds.) Biotechnology for agro-industrial residues utilisation. Springer Netherlands, Dordrecht (2009). https://doi.org/10.1007/978-1-4020-9942-7_10
Seiler, H., Wenning, M., Schmidt, V., Scherer, S.: Bacillus gottheilii sp. nov., isolated from a pharmaceutical manufacturing site. Int. J. Syst. Evol. Microbiol. (2012). https://doi.org/10.1099/ijs.0.036277-0
Unban, K., Kodchasee, P., Shetty, K., Khanongnuch, C.: Tannin-tolerant and extracellular tannase producing Bacillus isolated from traditional fermented tea leaves and their probiotic functional properties. Foods (2020). https://doi.org/10.3390/foods9040490
Selvaraj, S., Murty, V.: Process optimization for tannase production by Bacillus gottheilii M2S2 on inert polyurethane foam support. Biocatal. Agric. Biotechnol. (2016). https://doi.org/10.1016/j.bcab.2016.05.004
Kanpiengjai, A., Khanongnuch, C., Lumyong, S., Haltrich, D., Nguyen, T.-H., Kittibunchakul, S.: Co-production of gallic acid and a novel cell-associated tannase by a pigment-producing yeast, Sporidiobolus ruineniae A45.2. Microb. Cell Factories 19(1), 95 (2020). https://doi.org/10.1186/s12934-020-01353-w
Moghadamtousi, S.Z., Goh, B.H., Chan, C.K., Shabab, T., Kadir, H.A.: Biological activities and phytochemicals of Swietenia macrophylla King. Molecules. 18(9), 10465–10483 (2013). https://doi.org/10.3390/molecules180910465
Goh, B.H., Chan, C.K., Kamarudin, M.N.A., Kadir, A.: Swietenia macrophylla King induces mitochondrial-mediated apoptosis through p53 upregulation in HCT116 colorectal carcinoma cells. J. Ethnopharmacol. 153(2), 375–385 (2014). https://doi.org/10.1016/j.jep.2014.02.036
Cabezudo, I., Galetto, C.S., Romanini, D., Furlán, R.L.E., Meini, M.R.: Production of gallic acid and relevant enzymes by Aspergillus niger and Aspergillus oryzae in solid-state fermentation of soybean hull and grape pomace. Biomass Convers. Biorefinery. (2022). https://doi.org/10.1007/s13399-022-03435-8
Chung, K.T., Wong, T.Y., Wei, C.I., Huang, Y.-W., Lin, Y.: Tannins and human health: a review. Crit. Rev. Food Sci. Nutr. 38(6), 421–464 (1998). https://doi.org/10.1080/10408699891274273
Srivastava, A., Kar, R.: Characterization and application of tannase produced by Aspergillus Niger ITCC 6514.07 on pomegranate rind. Braz. J. Microbiol. 40(4), 782–789 (2009). https://doi.org/10.1590/S1517-83822009000400008
Werner, I., Bacher, A., Eisenreich, W.: Retrobiosynthetic NMR studies with 13 C-labeled glucose. Formation of gallic acid in plants and fungi. J. Biol. Chem. 272(41), 25474–25482 (1997). https://doi.org/10.1074/jbc.272.41.25474
Badhani, B., Sharma, N., Kakkar, R.: Gallic acid: a versatile antioxidant with promising therapeutic and industrial applications. RSC Adv. 5(35), 27540–27557 (2015). https://doi.org/10.1039/C5RA01911G
Bajpai, B., Patil, S.: A new approach to microbial production of gallic acid. Braz. J. Microbiol. 39, 708–711 (2008). https://doi.org/10.1590/S1517-838220080004000021
Evans, W.C.: In: Traiadal, B. (ed.) Trease and Evans Phamacognosy, 16th edn. Elsevier, London (1989)
Aguilar-Zárate, P., Cruz, M.A., Montañez, J., Rodríguez-Herrera, R., Wong-Paz, J.E., Belmares, R.E., Aguilar, C.N.: Gallic acid production under anaerobic submerged fermentation by two bacilli strains. Microb. Cell. Factories. 14(1), 209 (2015). https://doi.org/10.1186/s12934-015-0386-2
Venkatachalam, M., Shum-Chéong-Sing, A., Caro, Y., Dufossé, L., Fouillaud, M.: OVAT analysis and response surface methodology based on nutrient sources for optimization of pigment production in the marine-derived fungus Talaromyces albobiverticillius 30548 submerged fermentation. Mar. Drugs. 19(5), 248 (2021). https://doi.org/10.3390/md19050248
Selvaraj, S., Murty, V.: Semi-solid state fermentation: a promising method for production and optimization of tannase from Bacillus gottheilii M2S2. Res. J. Biotechnol. 12, 4 (2017)
Sharma, S., Bhat, T.K., Dawra, R.K.: A spectrophotometric method for assay of tannase using rhodanine. Anal. Biochem. 279(1), 85–89 (2000). https://doi.org/10.1006/abio.1999.4405
Bradford, M.M.: A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 72(1–2), 248–254 (1976). https://doi.org/10.1006/abio.1976.9999
He, F.: Bradford protein assay. Bio-protocol (2011). https://doi.org/10.21769/BioProtoc.45
Hagerman, A.E., Butler, L.G.: Protein precipitation method for the quantitative determination of tannins. J. Agric. Food Chem. 26(4), 809–812 (1978)
Green, A.A., Hughes, W.L.: Protein fractionation on the basis of solubility in aqueous solutions of salts and organic solvents. In: Methods in enzymology, pp. 67–90. Academic Press, Cambridge (1955)
Wingfield, P.T.: A-3F: protein precipitation using ammonium sulfate. Curr. Protoc. Protein Sci. (2016). https://doi.org/10.1002/0471140864.psa03fs84
Richmond, V.L., St Denis, R., Cohen, E.: Treatment of dialysis membranes for simultaneous dialysis and concentration. Anal. Biochem. 145(2), 343–350 (1985). https://doi.org/10.1016/0003-2697(85)90372-0
Purwanto, M.G.M.: The role and efficiency of ammonium sulphate precipitation in purification process of papain crude extract. Procedia Chem. 18, 127–131 (2016). https://doi.org/10.1016/j.proche.2016.01.020
Selvaraj, S., Vytla, R.M.: Solid state fermentation of Bacillus gottheilii M2S2 in laboratory-scale packed bed reactor for tannase production. Prep. Biochem. Biotechnol. 48(9), 799–807 (2018). https://doi.org/10.1080/10826068.2018.1509086
Chaitanyakumar, A., Anbalagan, M.: Expression, purification and immobilization of tannase from Staphylococcus lugdunensis MTCC 3614. AMB Exp. 6(1), 89 (2016). https://doi.org/10.1186/s13568-016-0261-5
Lineweaver, H., Burk, D.: The determination of enzyme dissociation constants. J. Am. Chem. Soc. 56(3), 658–666 (1934). https://doi.org/10.1021/ja01318a036
Banu, H., Nagarajan, N.: TLC and HPTLC fingerprinting of leaf extracts of Widelia chinensis (Osbeck) Merrill. J. Pharmacog. Phytochem. 2(6), 29–33 (2014)
Wagner, H., Bladt, S.: Plant Drug Analysis, 2nd edn. Springer, New York, Heidelberg (1996)
Berthomieu, C., Hienerwadel, R.: Fourier transform infrared (FTIR) spectroscopy. Photosynth. Res. 101(2), 157–170 (2009). https://doi.org/10.1007/s11120-009-9439-x
Singh, R., Mendhulkar, V.: FTIR studies and spectrophotometric analysis of natural antioxidants, polyphenols and flavonoids in Abutilon indicum (Linn) sweet leaf extract. J. Chem. Pharm. Res. 7(6), 205–211 (2015)
Kar, B., Banerjee, R., Bhattacharyya, B.C.: Microbial production of gallic acid by modified solid state fermentation. J. Ind. Microbiol. Biotechnol. 23(3), 173–177 (1999). https://doi.org/10.1038/sj.jim.2900713
Mondal, K.C., Banerjee, D., Banerjee, R., Pati, B.R.: Production and characterization of tannase from Bacillus cereus KBR9. J. Gen. Appl. Microbiol. 47(5), 263–267 (2001). https://doi.org/10.2323/jgam.47.263
Belur, P.D., Mugeraya, G.: Microbial production of tannase: state of the art. Res. J. Microbiol. 6, 25–40 (2011). https://doi.org/10.3923/jm.2011.25.40
Abdulhameed, S., Augur, C., Swati, C., Pandey, A.: Tannase production by Lactobacillus sp. ASR-S1 under solid-state fermentation. Process Biochem. 41(3), 575–580 (2006). https://doi.org/10.1016/j.procbio.2005.05.011
Singh, S., Kaur, A., Gupta, A.: Tannase production through solid-state fermentation of Shorea robusta deoiled seed cake: an industrial biomass using aspergillus flavus TF-8 for potential application in gallic acid synthesis. Biomass Convers. Biorefinery (2021). https://doi.org/10.1007/s13399-021-01634-3
Raghuwanshi, S., Dutt, K., Gupta, P., Misra, S., Saxena, R.K.: Bacillus sphaericus: the highest bacterial tannase producer with potential for gallic acid synthesis. J. Biosci. Bioeng. 111(6), 635–640 (2011). https://doi.org/10.1016/j.jbiosc.2011.02.008
Kashyap, P., Abdulhameed, S., Pandey, A., Szakacs, G., Soccol, C.: Extra-cellular L-glutaminase production by Zygosaccharomyces rouxii under solid-state fermentation. Process Biochem. 38(3), 307–312 (2002). https://doi.org/10.1016/S0032-9592(02)00060-2
Singh, J., Kaur, P.: Optimization of process parameters for cellulase production from Bacillus sp. JS14 in solid substrate fermentation using response surface methodology. Braz. Arch. Biol. Technol. 55, 505–512 (2012). https://doi.org/10.1590/S1516-89132012000400004
Kumar, S., Sharma, H.K., Sarkar, B.C.: Effect of substrate and fermentation conditions on pectinase and cellulase production by Aspergillus niger NCIM 548 in submerged (SmF) and solid state fermentation (SSF). Food Sci. Biotechnol. 20(5), 1289 (2011). https://doi.org/10.1007/s10068-011-0178-3
Arshad, R., Mohyuddin, A., Saeed, S., Hassan, A.U.: Optimized production of tannase and gallic acid from fruit seeds by solid state fermentation. Trop. J. Pharm. Res. 18, 911–918 (2019)
Yamashita, A.C., Sakurai, K.: Dialysis membranes — physicochemical structures and features. In: Suzuki, H. (ed.) Updates in Hemodialysis. IntechOpen, Rijeka (2015)
Bennett, T.P., Frieden, E.: Modern Topics in Biochemistry: Structure and Functions of Biological Molecules, pp. 43–45. Macmillan, London (1969)
Pfeiffer, J.: Enzymes, the Physics and Chemistry of Life, pp. 171–173. Simon and Schuster, NY (1954)
de Lima, J.S., Cruz, R., Fonseca, J.C., de Medeiros, E.V., Maciel, M., de Moreira, H.C., Motta, K.A.: Production, characterization of tannase from penicillium montanense URM 6286 under SSF using agro-industrial wastes, and application in the clarification of grape juice (Vitis vinifera L.). Sci. World J. (2014). https://doi.org/10.1155/2014/182025
Saeed, S., Aslam, S., Mehmood, T., Naseer, R., Nawaz, S., Mujahid, H., Firyal, S., Anjum, A.A., Sultan, A.: Production of gallic acid under solid-state fermentation by utilizing Waste from Food Processing Industries. Waste Biomass Valoriz. 12(1), 155–163 (2021). https://doi.org/10.1007/s12649-020-00980-z
Peterson, M.E., Daniel, R.M., Danson, M.J., Eisenthal, R.: The dependence of enzyme activity on temperature: determination and validation of parameters. Biochem. J. 402(2), 331–337 (2007). https://doi.org/10.1042/BJ20061143
Jiménez, N., Esteban-Torres, M., Mancheño, J.M., Rivas, B., de las, Muñoz, R.: Tannin degradation by a novel tannase enzyme present in some Lactobacillus plantarum strains. Appl. Environ. Microbiol. 80(10), 2991–2997 (2014). https://doi.org/10.1128/AEM.00324-14
Saeed, S., Bibi, I., Mehmood, T., Naseer, R., Bilal, M.: Valorization of locally available waste plant leaves for production of tannase and gallic acid by solid-state fermentation. Biomass Convers. Biorefinery. 12(9), 3809–3816 (2022). https://doi.org/10.1007/s13399-020-00989-3
Rivas, B., Lavín, J.L., Barriales, D., Abecia, L., Mancheño, J.M., Aransay, A.M., Muñoz, R., Anguita, J., Rodríguez, H.: Identification of a highly active tannase enzyme from the oral pathogen Fusobacterium nucleatum subsp. polymorphum. Microb. Cell. Factories. 17(1), 1–10 (2018). https://doi.org/10.1186/s12934-018-0880-4
Mommaerts, K., Sánchez, I., Betsou, F., Mathieson, W.: Replacing β-mercaptoethanol in RNA extractions. Anal. Biochem. (2015). https://doi.org/10.1016/j.ab.2015.03.027
Riordan, J.F.: The role of metals in enzyme activity. Ann. Clin. Lab. Sci. 7(2), 119–129 (1977)
Hernick, M., Fierke, C.: Mechanisms of metal-dependent hydrolases in metabolism. In: Liu, H.W., Ben, Mander, L. (eds.) Comprehensive Natural Products II Chemistry and Biology, pp. 547–581. Elsevier, Netherlands (2010)
Karplus, M., McCammon, J.A.: Dynamics of proteins: elements and function. Annu. Rev. Biochem. 52(1), 263–300 (1983). https://doi.org/10.1146/annurev.bi.52.070183.001403
Gupta, M.N.: Enzyme function in organic solvents. Eur. J. Biochem. 203(1–2), 25–32 (1992). https://doi.org/10.1111/j.1432-1033.1992.tb19823.x
Kumar, A., Dhar, K., Kanwar, S.S., Arora, P.K.: Lipase catalysis in organic solvents: advantages and applications. Biol. Proced. Online. 18(1), 2 (2016). https://doi.org/10.1186/s12575-016-0033-2
Anwar, Y.A.S., Artika, I.M., Danuri, H.: Fractionation and characterization of tannin acyl hydrolase from Aspergillus niger. Hayati J. Biosci. 16(3), 95–99 (2009). https://doi.org/10.4308/hjb.16.3.95
Roy, S., Parvin, R., Ghosh, S., Bhattacharya, S., Maity, S., Banerjee, D.: Occurrence of a novel tannase (tan B(LP)) in endophytic Streptomyces sp. AL1L from the leaf of Ailanthus excelsa Roxb. 3 Biotech. 8(1), 1–8 (2018). https://doi.org/10.1007/s13205-017-1055-4
Larosa, C., Salerno, M., de Lima, J.S., Meri, R.M., da Silva, M.F., de Carvalho, L.B., Converti, A.: Characterisation of bare and tannase-loaded calcium alginate beads by microscopic, thermogravimetric, FTIR and XRD analyses. Int. J. Biol. Macromol. 115, 900–906 (2018). https://doi.org/10.1016/j.ijbiomac.2018.04.138
Shaw, B.L., Simpson, T.H.: Chelate systems. Part III. Infrared spectra of flavanones and flavones. J. Chem. Soc. (1955). https://doi.org/10.1039/JR9550000655
Briggs, L.H., Colebrook, L.D.: Infra-red spectra of flavanones and flavones. Carbonyl and hydroxyl stretching and CH out-of-plane bending absorption. Spectrochim. Acta. 18(7), 939–957 (1962). https://doi.org/10.1016/0371-1951(62)80101-5
Acknowledgements
The authors of this study are thankful to the Manipal Institute of Technology, MAHE for providing support towards the conducting of research practices. The contribution and support provided by the faculty and staff of the Department of Biotechnology, MIT have been duly acknowledged and appreciated.
Funding
Open access funding provided by Manipal Academy of Higher Education, Manipal. Not applicable.
Author information
Authors and Affiliations
Contributions
AB contributed to the conduct of experimentations, analyzed data, and wrote the original draft. SS designed, and supervised the study and was involved in data curation and drafting of the manuscript. VRM involved in reviewing and providing resources. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no competing interests.
Ethical Approval
Not applicable.
Consent for Publication
Not applicable.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Borah, A., Selvaraj, S. & Murty, V.R. Production of Gallic Acid from Swietenia macrophylla Using Tannase from Bacillus Gottheilii M2S2 in Semi-Solid State Fermentation. Waste Biomass Valor 14, 2569–2587 (2023). https://doi.org/10.1007/s12649-022-02023-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12649-022-02023-1