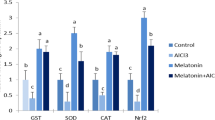
Abstract
Repeated manganese (Mn) exposure may cause increased production of reactive oxygen species (ROS), with a consequent imbalance in the glutathione (GSH) antioxidant defence system, resulting in cellular dysfunctions, and eventually cell death, particularly in the brain. d-ribose-l-cysteine (RibCys) has been demonstrated to effectively promote the synthesis of glutathione, a potent neutralizer of ROS. In the present study, we examined the effects of RibCys on glutathione levels, apoptotic and astrocytic responses, neuronal ultrastructural integrity, following Mn exposure. Wild-type rats were exposed to either saline, Mn, or/and RibCys for 2 weeks. The Mn-exposed rats received RibCys either as pre-, co-, or post-treatments. Mn caused a marked decrease in GSH levels, overexpression of GFAP and caspase-3, reflecting astrocytosis and apoptosis, and altered ultrastructural integrities of the neuronal nuclei, mitochondria, and myelin sheath of the striatum and motor cortex respectively, while all interventions with RibCys minimized and prevented the neurotoxic events. Our study demonstrates that RibCys effectively attenuates the neurotoxic effects of Mn and may be useful as a therapeutic strategy against neurological consequences of Mn overexposure.










Similar content being viewed by others
Data Availability
The data and material are available.
Change history
05 September 2023
A Correction to this paper has been published: https://doi.org/10.1007/s12640-023-00665-0
References
Adair JC, Knoefel JE, Morgan N (2001) Controlled trial of N-acetylcysteine for patients with probable Alzheimer’s disease. Neurology 57:1515–1517
Aggoun-Zouaoui D, Margalli I, Borrega F, Represa A, Plotkine M, Ben-Ari Y, Charriaut-Marlangue C (1998) Ultrastructural morphology of neuronal death following reversible focal ischemia in the rat. Apoptosis 3:133–141
Allen J, Bradley RD (2011) Effects of oral glutathione supplementation on systemic oxidative stress biomarkers in human volunteers. J Altern Complement Med 17:827–833
Almeida RG, Lyons DA (2017) On myelinated axon plasticity and neuronal circuit formation and function. J Neurosci 37:10023–10034. https://doi.org/10.1523/jneurosci.3185-16.2017
Aschner M, Erikson KM, Hernández EH, Tjalkens R (2009) Manganese and its role in Parkinson’s disease: from transport to neuropathology. NeuroMol Med 11:252–266
Babbar M, Sheikh MS (2013) Metabolic stress and disorders related to alterations in mitochondrial fission or fusion. Mol Cell Pharmacol 5:109
Bouabid S, Tinakoua A, Lakhdar-Ghazal N, Benazzouz A (2016) Manganese neurotoxicity: behavioral disorders associated with dysfunctions in the basal ganglia and neurochemical transmission. J Neurochem 136:677–691
Bowman AB, Kwakye GF, Hernández EH, Aschner M (2011) Role of manganese in neurodegenerative diseases. J Trace Elem Med Biol 25:191–203
Bray TM, Taylor CG (1994) Enhancement of tissue glutathione for antioxidant and immune functions in malnutrition. Biochem Pharmacol 47:2113–2123
Chen P et al (2019) Iron and manganese-related CNS toxicity: mechanisms, diagnosis and treatment. Expert Rev Neurother 19:243–260
Chiu K, Lau WM, Lau HT, So K-F, Chang RC-C (2007) Micro-dissection of rat brain for RNA or protein extraction from specific brain region. JoVE:e269. https://doi.org/10.3791/269
Chtourou Y, Fetoui H, Garoui EM, Boudawara T, Zeghal N (2012) Improvement of cerebellum redox states and cholinergic functions contribute to the beneficial effects of silymarin against manganese-induced neurotoxicity. Neurochem Res 37:469–479
Chtourou Y et al (2010) Silymarin, a natural antioxidant, protects cerebral cortex against manganese-induced neurotoxicity in adult rats. Biometals 23:985–996
Chtourou Y, Garoui EM, Boudawara T, Zeghal N (2014) Protective role of silymarin against manganese-induced nephrotoxicity and oxidative stress in rat. Environ Toxicol 29:1147–1154
Chtourou Y, Trabelsi K, Fetoui H, Mkannez G, Kallel H, Zeghal N (2011) Manganese induces oxidative stress, redox state unbalance and disrupts membrane bound ATPases on murine neuroblastoma cells in vitro: protective role of silymarin. Neurochem Res 36:1546–1557
da Silva Santos V et al (2014) Anthocyanin-rich açaí (Euterpe oleracea Mart.) extract attenuates manganese-induced oxidative stress in rat primary astrocyte cultures. J Toxic Environ Health A 77:390–404
Dahmardeh N, Shabani M, Basiri M, Kalantaripour TP, Asadi-Shekaari M (2019) Functional antagonism of sphingosine-1-phosphate receptor 1 prevents harmaline-induced ultrastructural alterations and caspase-3 mediated apoptosis. Malays J Med Sci 26:28
de Water E et al (2018) Prenatal manganese exposure and intrinsic functional connectivity of emotional brain areas in children. Neurotoxicology 64:85–93
Demongeot J, Glade N, Hansen O, Moreira A (2007) An open issue: the inner mitochondrial membrane (IMM) as a free boundary problem. Biochimie 89:1049–1057
dos Santos APM, Milatovic D, Au C, Yin Z, Batoreu MCC, Aschner M (2010) Rat brain endothelial cells are a target of manganese toxicity. Brain Res 1326:152–161
Ellman GL (1959) Tissue sulfhydryl groups. Arch Biochem Biophys 82:70–77
Emokpae O, Ben-Azu B, Ajayi AM, Umukoro S (2020) d-Ribose-l-cysteine attenuates lipopolysaccharide-induced memory deficits through inhibition of oxidative stress, release of proinflammatory cytokines, and nuclear factor-kappa B expression in mice. Naunyn Schmiedeberg's Arch Pharmacol 1–17
Filley CM, Fields RD (2016) White matter and cognition: making the connection. J Neurophysiol 116:2093–2104. https://doi.org/10.1152/jn.00221.2016
Franco R, Cidlowski J (2009) Apoptosis and glutathione: beyond an antioxidant. Cell Death Differ 16:1303–1314
García GB, Quiroga AD, Stürtz N, Martinez AI, Biancardi ME (2004) Morphological alterations of central nervous system (CNS) myelin in vanadium (V)-exposed adult rats. Drug Chem Toxicol 27:281–293
Gomes LC, Di Benedetto G, Scorrano L (2011) During autophagy mitochondria elongate, are spared from degradation and sustain cell viability. Nature Cell Biol 13:589–598
Gould EA et al (2018) Mild myelin disruption elicits early alteration in behavior and proliferation in the subventricular zone. Elife 7:e34783. https://doi.org/10.7554/eLife.34783
Guilarte TR (2013) Manganese neurotoxicity: new perspectives from behavioral, neuroimaging, and neuropathological studies in humans and non-human primates. Front Aging Neurosci 5:23
Harischandra DS, Ghaisas S, Zenitsky G, Jin H, Kanthasamy A, Anantharam V, Kanthasamy A (2019) Manganese-induced neurotoxicity: new insights into protein misfolding, mitochondrial impairment and neuroinflammation. Front Neurosci 13:654
Ijomone OM, Okori SO, Ijomone OK, Ebokaiwe AP (2018) Sub-acute nickel exposure impairs behavior, alters neuronal microarchitecture, and induces oxidative stress in rats’ brain. Drug Chem Toxicol 41:377–384
Jurkowska H, Uchacz T, Roberts J, Wróbel M (2011) Potential therapeutic advantage of ribose-cysteine in the inhibition of astrocytoma cell proliferation. Amino Acids 41:131–139
Kader T, Porteous CM, Williams MJ, Gieseg SP, McCormick SP (2014) Ribose-cysteine increases glutathione-based antioxidant status and reduces LDL in human lipoprotein (a) mice. Atherosclerosis 237:725–733
Kaplowitz N, Aw TY, Ookhtens M (1985) The regulation of hepatic glutathione. Annu Rev Pharmacol Toxicol 25:715–744
Ke T, Sidoryk-Wegrzynowicz M, Pajarillo E, Rizor A, Soares FAA, Lee E, Aschner M (2019) Role of astrocytes in manganese neurotoxicity revisited. Neurochem Res 1–11
Kondadi AK, Anand R, Reichert AS (2019) Functional interplay between cristae biogenesis, mitochondrial dynamics and mitochondrial DNA integrity. Int J Mol Sci 20:4311
Lee S, Liu W, Dickson D, Brosnan C, Berman J (1993) Cytokine production by human fetal microglia and astrocytes. Differential induction by lipopolysaccharide and IL-1 beta. J Immunol 150:2659–2667
Lin MT, Beal MF (2006) Mitochondrial dysfunction and oxidative stress in neurodegenerative diseases. Nature 443:787–795
Lowry O, Rosebrough N, Farr A, Randall R (1951) Protein measurement with the Folin phenol reagent. J Biol Chem 193:265
Lu SC (2013) Glutathione synthesis. Biochim Biophys Acta Gen Subj 1830:3143–3153
Martins AC, Krum BN, Queirós L, Tinkov AA, Skalny AV, Bowman AB, Aschner M (2020) Manganese in the diet: bioaccessibility, adequate intake, and neurotoxicological effects. J Agric Food Chem 68:12893–12903
Maulik M, Mitra S, Sweeney M, Lu B, Taylor BE, Bult-Ito A (2019) Complex interaction of dietary fat and Alaskan bog blueberry supplementation influences manganese mediated neurotoxicity and behavioral impairments. J Funct Foods 53:306–317
N’guessan BB et al (2018) In vitro antioxidant potential and effect of a glutathione-enhancer dietary supplement on selected rat liver cytochrome P450 enzyme activity. Evid Based Complement Alternat Med 2018
National Research Council (2011) Guide for the care and use of laboratory animals, Eighth Edition. Washington, DC: NAP. https://doi.org/10.17226/12910
Pajevic S, Basser PJ, Fields RD (2014) Role of myelin plasticity in oscillations and synchrony of neuronal activity. Neuroscience 276:135–147. https://doi.org/10.1016/j.neuroscience.2013.11.007
Parmalee NL, Aschner M (2016) Manganese and aging. Neurotoxicology 56:262–268
Peres TV, Aschner M (2017) Nutritional, genetic, and molecular aspects of manganese intoxication. In: Molecular, Genetic, and Nutritional Aspects of Major and Trace Minerals. Elsevier, pp 367–376
Peres TV, Schettinger MRC, Chen P, Carvalho F, Avila DS, Bowman AB, Aschner M (2016a) Manganese-induced neurotoxicity: a review of its behavioral consequences and neuroprotective strategies. BMC Pharmacol Toxicol 17:57
Peres TV, Schettinger MRC, Chen P, Carvalho F, Avila DS, Bowman AB, Aschner M (2016b) Manganese-induced neurotoxicity: a review of its behavioral consequences and neuroprotective strategies. BMC Pharmacol Toxicol 17:1–20
Pfalzer AC, Bowman AB (2017) Relationships between essential manganese biology and manganese toxicity in neurological disease. Curr Environ Health Rep 4:223–228
Racette BA, Nielsen SS, Criswell SR, Sheppard L, Seixas N, Warden MN, Checkoway H (2017) Dose-dependent progression of parkinsonism in manganese-exposed welders. Neurology 88:344–351
Roberts JC, Charyulu RL, Zera RT, Nagasawa HT (1992) Protection against acetaminophen hepatotoxicity by ribose-cysteine (RibCys). Pharmacol Toxicol 70:281–285
Roberts JC, Phaneuf HL, Szakacs JG, Zera RT, Lamb JG, Franklin MR (1998) Differential chemoprotection against acetaminophen-induced hepatotoxicity by latentiated L-cysteines. Chem Res Toxicol 11:1274–1282
Santos D, Batoreu MC, Almeida I, Ramos R, Sidoryk-Wegrzynowicz M, Aschner M, Dos Santos AM (2012a) Manganese alters rat brain amino acids levels. Biol Trace Elem Res 150:337–341
Santos D, Milatovic D, Andrade V, Batoreu MC, Aschner M, Dos Santos AM (2012b) The inhibitory effect of manganese on acetylcholinesterase activity enhances oxidative stress and neuroinflammation in the rat brain. Toxicology 292:90–98
Schrantz N, Blanchard DA, Mitenne F, Auffredou M-T, Vazquez A, Leca G (1999) Manganese induces apoptosis of human B cells: caspase-dependent cell death blocked by bcl-2. Cell Death Differ 6:445–453
Schulz JB, Weller M, Moskowitz MA (1999) Caspases as treatment targets in stroke and neurodegenerative diseases. Ann Neurol 45:421–429
Scorrano L, Ashiya M, Buttle K, Weiler S, Oakes SA, Mannella CA, Korsmeyer SJ (2002) A distinct pathway remodels mitochondrial cristae and mobilizes cytochrome c during apoptosis. Dev Cell 2:55–67
Sofroniew MV, Vinters HV (2010) Astrocytes: biology and pathology. Acta Neuropathol 119:7–35
Spurr AR (1969) A low-viscosity epoxy resin embedding medium for electron microscopy. J Ultrastruct Res 26:31–43
Tarale P, Chakrabarti T, Sivanesan S, Naoghare P, Bafana A, Krishnamurthi K (2016) Potential role of epigenetic mechanism in manganese induced neurotoxicity. Biomed Research Int 2016
Tjalkens RB, Popichak KA, Kirkley KA (2017) Inflammatory activation of microglia and astrocytes in manganese neurotoxicity: Neurotoxicity of Metals. Adv Neurobiol 159–181
Vale J, Proudfoot A (1995) Paracetamol (acetaminophen) poisoning. Lancet 346:547–552
Villalobos V, Hernández-Fonseca JP, Bonilla E, Medina-Leendertz S, Mora M, Mosquera J (2015) Ultrastructural changes of caudate nucleus in mice chronically treated with manganese. Ultrastruct Pathol 39:217–225
Volman V, Ng LJ (2013) Computer modeling of mild axonal injury: implications for axonal signal transmission. Neural Comput 25:2646–2681
Winter AN et al (2017) A cystine-rich whey supplement (Immunocal®) provides neuroprotection from diverse oxidative stress-inducing agents in vitro by preserving cellular glutathione. Oxidative Med Cell Longev 2017
Xie F, Liang P, Fu H, Zhang JC, Chen J (2014) Effects of normal aging on myelin sheath ultrastructures in the somatic sensorimotor system of rats. Mol Med Rep 10:459–466. https://doi.org/10.3892/mmr.2014.2228
Zick M, Rabl R, Reichert AS (2009) Cristae formation—linking ultrastructure and function of mitochondria. Biochim Biophy Acta Mol Cell Res 1793:5–19
Acknowledgements
GTA acknowledges the support of the International Society of Neurochemistry (ISN) CAEN 1A grant and the Company of Biologists DMMJ Traveling Fellowship.
Funding
OMI was supported by the International Brain Research Organization (IBRO) Return Fellowship 2018 and Young IBRO Connecting Regions Award 2019. MA is supported by the National Institute of Health (NIH), USA grants; NIEHS R01 10563 and NIEHS R01 07331.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Ethical Approval
All experimental protocols were in strict accordance with the guidelines for animal research, as detailed in the Guide for the Care and Use of Laboratory Animals (National Research Council 2011), and approved by the Institutional Ethical Review Committee (UERC/ASN/2019/1741), University of Ilorin, Nigeria.
Consent to Participate
Not applicable.
Conflict of Interest
d-ribose-l-cysteine was a gift to OM Ijomone from Max International, USA. However, Max International had no input into the conception, design, and execution of the research, as well as no input into the decision to publish. Authors declare no other known or perceived conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Akingbade, G.T., Ijomone, O.M., Imam, A. et al. d-Ribose-l-Cysteine Improves Glutathione Levels, Neuronal and Mitochondrial Ultrastructural Damage, Caspase-3 and GFAP Expressions Following Manganese-Induced Neurotoxicity. Neurotox Res 39, 1846–1858 (2021). https://doi.org/10.1007/s12640-021-00404-3
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12640-021-00404-3