Abstract
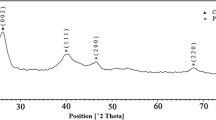
A zinc-modified octa(aminopropyl)silsesquioxane and the cyan compound Na2[Fe(CN)5NO] were prepared to create a hybrid electroactive material (ACZnN) characterized employing the spectroscopic techniques Raman spectroscopy, Diffuse Reflectance (DR), X-Ray diffraction (XRD), Scanning Electronic Microscopy (SEM), thermogravimetric analysis (TGA) and voltammetric measurements. The cyclic voltammetry technique indicated the solid ACZnN on the electrode surface as presenting two redox processes, with EIθ' = 0.21 V and EIIθ' = 0.51 V (vs Ag/AgCl(sat)) attributed to the redox pair Fe2+(CN)5NO/ Fe3+(CN)5NO in the absence and presence of Zn2+, respectively, as well as adequate stability, reliability and efficiency for practical applications. The ACZnN-modified graphite paste electrode exhibited a sensitive and catalytic oxidation response towards N-acetylcysteine and was successfully employed 1in the catalytic electro-oxidation of N-acetylcysteine employing differential pulse voltammetry techniques. The lowest limit of detection and best amperometric sensitivity obtained by differential pulse voltammetry were 0.657 µmol L−1 and 500 mA/mol L−1, respectively, from 1.0 × 10–6 to 1.0 × 10–5 M. Further analyses applying this method also demonstrated the compound’s efficiency in detecting N-acetylcysteine in synthetic urine samples.
Similar content being viewed by others
Data availability
All data generated or analyzed during this study are included in the published article.
Code availability
Not applicable.
References
Voronkov MG, Lavrent'yev VL (1982) Polyhedraloligosilsesquioxanes and their homo derivates. Top Curr Chem 102:199–236. https://doi.org/10.1007/3-540-11345-2_12
Baney RH, Itoh M, Sakakibara A, Suzuki T (1995) Silsesquioxanes. Chem Rev 95:1409–1430. https://doi.org/10.1021/cr00037a012
Provatas A, Matisons JG (1997) Silsesquioxanes: synthesis and applications. Trends Polymer Sci 10:327–332
Shi H, Yang J, You M, Li Z, He C (2020) Polyhedral Oligomeric Silsesquioxanes (POSS)-Based Hybrid Soft Gels: molecular design, material advantages, and emerging applications. Acs Materials Letters 2:296–316. https://doi.org/10.1021/acsmaterialslett.9b00491
Li G, Wang L, Ni H, Pittman CU Jr (2001) Polyhedral Oligomeric Silsesquioxane (POSS) Polymers and Copolymers: A Review. J Inorg Organomet Polym 11:123–154. https://doi.org/10.1023/A:1015287910502
Lichtenhan J, POSS-Based Polymers Lichtenhan JD, Pielichowski K, Blanco I (2019) Polymers 11(10), 1727. https://doi.org/10.3390/polym11101727
Cordes DB, Lickiss PD, Rataboul F (2010) Recent developments in the chemistry of cubic polyhedral oligosilsesquioxanes. Chem Rev 110:2081–2173. https://doi.org/10.1021/cr900201r
Blanco I, Abate L, Botino FA (2017) Mono substituted octaphenyl POSSs: The effects of substituents on thermal properties and solubility. Thermochim Acta 655:117–123. https://doi.org/10.1016/j.tca.2017.06.019
Kataoka S, Banerjee S, Kawai A, Kamimura Y, Choi JC, Kodaira KS, Endo A (2015) Layered Hybrid Perovskites with Micropores Created by Alkylammonium Functional Silsesquioxane Interlayers. J Am Chem Soc 137:4158–4163. https://doi.org/10.1021/jacs.5b00290
Jelen P, Bik M, Nocun M, Gaweda M, Dlugon E, Sitarz M (2016) Free carbon phase in SiOC glasses derived from ladder-like silsesquioxanes. J Mol Struct 1126:172–176. https://doi.org/10.1016/j.molstruc.2016.03.096
Dule M, Biswas M, Paira TK, Mandal TK (2015) Hierarchical nanostructures of tunable shapes through self-aggregation of POSS end-functional polymer and poly(ionic liquid) hybrids. Polym 77:32–41. https://doi.org/10.1016/j.polymer.2015.09.020
Yang FF, Li ZX, Xu YJ, Huang YP, Liu ZS (2018) Enhanced molecular recognition for imprinted monolithic column containing polyhedral oligomeric silsesquioxanes by dendritic effect of mesoporous molecular sieve scaffolds. Anal Bioanal Chem 410:5183–5193. https://doi.org/10.1007/s00216-018-1166-8
Li S, Wang ZY, Gao GG, Li B, Luo P, Kong YJ, Liu H, Zang SQ (2018) Smart Transformation of a Polyhedral Oligomeric Silsesquioxane Shell Controlled by Thiolate Silver(I) Nanocluster Core in Cluster@Clusters Dendrimers. Angew Chem Int 57:12775–12779. https://doi.org/10.1002/anie.201807548
Yuan G, Wang X, Wu D, Hammouda B (2016) Structural analysis of dendrimers based on polyhedral oligomeric silsesquioxane and their assemblies in solution by small-angle neutron scattering: Fits to a modified two correlation lengths model. Polym 100:119–125. https://doi.org/10.1016/j.polymer.2016.06.062
Ayandele E, Sarkar B, Alexandridis P (2012) Polyhedral oligomeric silsesquioxane (POSS)-containing polymer nanocomposites. J Nanomater 2:445–475. https://doi.org/10.3390/nano2040445
Rao YQ, Frenton J, Jenkins R, Evans J (2018) Structure and its thickness dependence of thin films of phenyl and methyl silsesquioxane polymers. J Non-Cryst Solids 502:128–135. https://doi.org/10.1016/j.jnoncrysol.2018.07.066
Zhang LP, Tang SH, Mo CE, Wang C, Huang YP (2018) Synergistic effect of liquid Crystal and polyhedral oligomeric silsesquioxane to prepare molecularly imprinted Polymer for paclitaxel delivery. Eur Polym J 98:226–236. https://doi.org/10.1016/j.eurpolymj.2017.11.021
Zapp E, Da Silva PS, Westphal E, Gallardo H, Spinelli A, Vieira IC (2014) Troponin T immunosensor based on a liquid crystal and silsesquioxane-supported gold nanoparticles. Bioconjugate Chem 25:1638–1643. https://doi.org/10.1021/bc500341a
Liu F, Zhang Y, Xu L, Zhang W (2015) Morphology-Controlled Self-Assembly of an Organic/Inorganic Hybrid Porphyrin Derivative Containing Polyhedral Oligomeric Silsesquioxane (POSS). Chem Eur J 21:5540–5547. https://doi.org/10.1002/chem.201405334
Li X, Hao J, Jiang Q, Mu J, Jiang Z (2015) Phosphorus-containing polyhedral oligomeric silsesquioxane/polyimideshybrid materials with low dielectric constant and low coefficients of thermal expansion. J Appl Polym Sci 132:42611. https://doi.org/10.1002/app.42611
Oban R, Matsukawa K, Matsumoto A (2018) Heat Resistant, and Transparent Organic-Inorganic Hybrid Materials Composed of N-Allylmaleimide Copolymer and Random-Type SH-Modified Silsesquioxane. J Polym Sci Part A: Polym Chem 56; 2294–2302. https://doi.org/10.1002/pola.29202
Wang J, Sun J, Zhou J, Jin K, Fang Q (2017) Fluorinated and Thermo-Cross-Linked Polyhedral Oligomeric Silsesquioxanes: New Organic-Inorganic Hybrid Materials for High-Performance Dielectric Application. ACS Appl Mater Interfaces 9:12782–12790. https://doi.org/10.1021/acsami.7b01415
Seino M, Wang W, Lofgreen JE, Puzzo DP, Manabe T, Ozin GA (2011) Low-k Periodic Mesoporous Organosilica with Air Walls: POSS-PMO. J Am Chem Soc 133:18082–18085. https://doi.org/10.1021/ja2080136
Handke M, Kowalewska A (2011) Siloxane, and silsesquioxane molecules—Precursors for silicate materials. Spectrochim Acta, Part A 79:749–757. https://doi.org/10.1016/j.saa.2010.08.049
Guo Y, Mylonakis A, Zhang Z, Lelkes PI, Levon K (2007) Oligoaniline-contained electroactive silsesquioxane precursor for synthesizing novel siliceous materials. Macromol 40:2721–2729. https://doi.org/10.1021/ma0622985
Magossi MS, Maraldi VA, Dias Filho NL, Do Carmo DR (2018) Silica Gel Functionalized with 4-Amino-5-(4pyridyl)-4H-1,2,4-triazole-3-thiol and their Use as a Copper Sorbent and Electromediator for Voltammetric Detection of Ascorbic Acid. Electroanal 30:1–9. https://doi.org/10.1002/elan.201800361
Da Silveira TFS, Fernandes DS, Barbosa PFP, Do Carmo DR (2017) Preparation and use of a Grafted Silica with Imidazole Groups for Cadmium Sorption and Subsequent Voltammetric Detection of Ascorbic Acid. Silicon 10:635–643. https://doi.org/10.1007/s12633-016-9506-9
Vieira EG, Soares IV, Pires G, Ramos RAV, Do Carmo DR, Dias Filho NL (2015) Study on determination and removal of metallic ions from aqueous and alcoholic solutions using a new POSS adsorbent. Chem Eng J 264:77–88. https://doi.org/10.1016/j.cej.2014.11.050
Quadrelli EA, Basset JM (2010) On silsesquioxanes’ accuracy as molecular models for silica-grafted complexes in heterogeneous catalysis. Coord Chem Rev 254:707–728. https://doi.org/10.1016/j.ccr.2009.09.031
Vieira EG, Silva RO, Do Carmo DR, Furlani Junior E, Dias Filho NL (2017) Synthesis and comparison of the activities of a catalyst supported on two silicate materials. Mater Chem Phys 191:197–205. https://doi.org/10.1016/j.matchemphys.2017.01.045
Chojnowski J, Fortuniak W, Rosciszewski P, Werel W, Lukasiak J, Kamisz W, Halasa R (2006) Polysilsesquioxanes and oligosilsesquioxanes substituted by alkylammonium salts as antibacterial biocides. J Inorg Organomet Polymer Mater 16:219–230. https://doi.org/10.1007/s10904-006-9048-5
Ro HW, Park ES, Soles DYCL, Yoon DY (2010) Structure−Property Relationships for Methylsilsesquioxanes. Chem Mater 22:1330–1339. https://doi.org/10.1021/cm901771y
Da Silveira TFS, Silvestrini DR, Bicalho UO, Do Carmo DR (2013) Voltammetric Study of a Cubic Silsesquioxane Organically Modified with Imidazole and their Subsequent Reaction with Cadmium and Hexacyanoferrate (III). Int J Electrochem Sci 8:872–886
Do Carmo DR, Silvestrine DR, Barud HS, Dias Filho NL, Bicalho UO, Soares LA (2014) A Silsesquioxane Organically Modified with 4-Amino-5-(4-pyridyl)-4 H-1,2,4-triazole-3-thiol: Thermal Behavior and It’s Electrochemical Detection of Sulfhydryl Compounds. J Nanomat 2014:1–11. https://doi.org/10.1155/2014/695954
Li K, Liu Y, Pu KY, Feng SS, Zhan R, Liu B (2011) Polyhedral Oligomeric Silsesquioxanes-Containing Conjugated Polymer Loaded PLGA Nanoparticles with Trastuzumab (Herceptin) Functionalization for HER2-Positive Cancer Cell Detection Adv. Funct Mater 21:287–294. https://doi.org/10.1002/adfm.201001435
Ghanbari H, Cousins BG, Seifalian AM (2011) A Nanocage for Nanomedicine: Polyhedral Oligomeric Silsesquioxane (POSS) Macromol. Rapid Commun 32:1032–1046. https://doi.org/10.1002/marc.201100126
Zhou Z, Lu ZR (2014) Dendritic Nanoglobules with Polyhedral Oligomeric Silsesquioxane Core and Their Biomedical Applications. Nanomedicine 9:2387–2401. https://doi.org/10.2217/nnm.14.133
Feher FJ, Wyndham KD, Soulivong D, Nguyen F (1999) Syntheses of highly functionalized cube-octameric polyhedral oligosilsesquioxanes (R8Si8O12). J Chem Soc, Dalton Trans, 1491-1497. https://doi.org/10.1039/a807302c
Foroughi MM, Beitollahi H, Tajik S, Akbari A, Hosseinzadeh R (2014) Electrochemical determination of N-acetylcysteine and folic acid in pharmaceutical and biological samples using a modified carbon nanotube paste electrode. Int J Electrochem Sci 9:8407–8421
Da Silva IS, Araújo MFA, Ferreira HA, Varela Junior JJG, Tanaka SMCN, Tanaka AA, Angnes L (2011) Quantification of Nacetylcysteinein pharmaceuticals using cobalto phthalocyanine modified grafite electrodes. Talanta 83:1701–1706. https://doi.org/10.1016/j.talanta.2010.11.070
Wang X, Chi D, Song D, Su G, Li L, Shao L (2012) Quantification of glutathione in plasma samples by HPLC using 4-fluoro-7-nitrobenzofurazan as a fluorescent labeling reagent. J Chromatogr Sci 50:119–122. https://doi.org/10.1093/chromsci/bmr039
Zheng X, Duan C, Shen J, Duan X (2015) Determination of reduced glutathione by spectrophotometry coupled with anti-interference compensation. Anal Methods 7:5006–5011. https://doi.org/10.1039/c5ay00825e
Tao Y, Zhang X, Wang J, Wang X, Yang N (2012) Simultaneous determination of cysteine, ascorbic acid and uric acid by capillary electrophoresis with electrochemiluminescence. J Electroanal Chem 674:65–70. https://doi.org/10.1016/j.jelechem.2012.03.009
Du JX, Li YH, Lu JR (2001) Investigation on the chemiluminescence reaction of luminol–H2O2–S2−/R–SH system. Anal Chim Acta 448:79–83. https://doi.org/10.1016/S0003-2670(01)01242-9
Ogwu V, Cohen G (1998) A simple colorimetric method for the simultaneous determination of N-acetylcysteine and cysteine. Free Radical Biol Med 25:362–364. https://doi.org/10.1016/s0891-5849(98)00024-0
AL-Ghannam S, EL-Brashy A, AL-Farhan B (2022) Fluorimetric: determination of some thiol compounds in their dosage forms. II Farmao 57; 625-629. https://doi.org/10.1016/S0014-827X(02)01223-5
De Sa AC, Paim LL, Bicalho UO, Do Carmo DR (2011) Determination of N-acetylcysteine by cyclic voltammetry using modified carbon paste electrode with copper nitroprusside adsorbed on the 3–aminopropylsilica. Int J Electrochem Sci (6):3754–3767
Xiao C, Chen J, Liu B, Chu X, Wu L, Yao S (2011) Sensitive and selective electrochemical sensing of L-cysteine based on a caterpillar-like manganese dioxide– carbon nanocompositew. Phys Chem Chem Phys 13:1568–1574. https://doi.org/10.1039/c0cp00980f
Liu X, Lv H, Sun Q, Zhong Y, Zhao J, Fu J, Lin M, Wang J (2012) Differential pulse voltammetric determination of L-cysteine after cyclic voltammetry in presence of catechol with glassy carbon electrode. Anal Lett 45:2246–2256. https://doi.org/10.1080/00032719.2012.686133
Do Carmo DR, da Silva RM, Stradiotto NR (2003) Electrocatalytic and Voltammetric Determination of Sulfhydryl Compounds Through Iron Nitroprusside Modified Graphite Paste Electrode. J Braz Chem Soc 14:616–620
Ahmad M, Pan C, Zhu J (2010) Electrochemical determination of L-cysteine by an elbow shaped, Sb-doped ZnO nanowire-modified electrode. J Mater Chem 20:7169–7174. https://doi.org/10.1039/C0JM01055C
Song-Jiang M, Sheng-Lian L, Hai-Hui Z, Ya-Fei K, Xiao-Hui N (2008) Electrocatalytic oxidation behavior of L-cysteine at Pt microparticles modified nanofibrous polyaniline film electrode. J Cent South Univ Technol 15:170–175. https://doi.org/10.1007/s11771-008-0033-8
Xiao C, Chen J, Liu B, Chu X, Wu L, Yao S (2011) Sensitive and selective electrochemical sensing of L-cysteine based on a caterpillar-like manganese dioxide–carbon nanocompositew. Phys Chem Chem Phys 13:1568–1574. https://doi.org/10.1039/C0CP00980F
Karimi-Maleh H, Keyvanfard M, Alizad K, Khosravi V, Asnaashariisfahani M (2012) Electrocatalytic determination of glutathione using multiwall carbon nanotubes paste electrode as a sensor and isoprenaline as a mediator. Int J Electrochem Sci 7:6816–6830
Safavi A, Maleki N, Farjami E, Mahyari FG (2009) Simultaneous electrochemical determination of glutathione and glutathione disulfide at a nanoscale copper hydroxide composite carbono ionic liquid electrode. Analytical Chemistr 81:7538–7543. https://doi.org/10.1021/ac900501j
Magossi MS, Magossi MS, Dias Filho NL, Do Carmo DR (2021) Isoniazid-sensing Behavior of a Hybrid Silsesquioxane and Cobalt Pentacyanonitrosylferrate-based Nanocomposite. Electroanalysis 33:1–10. https://doi.org/10.1002/elan.202100119
Brooks T, Keevil CW (1997) A simple artificial urine for the growth of urinary pathogens. Lett Appl Microbiol 24:203–206. https://doi.org/10.1046/j.1472-765x.1997.00378.x
Osiry H, Cano A, Reguera L, Lemus-Santana AA, Reguera E (2015) Mercury (I) nitroprusside: a 2d structure supported on homometallic interactions. J Solid State Chem 221:79–84. https://doi.org/10.1016/j.jssc.2014.09.021
Rodríguez-Hernández J, Reguera L, Lemus-Santana AA, Reguera E (2015) Silver nitroprusside: atypical coordination within the metal nitroprussides series. Inorg Chim Acta 428:51–56. https://doi.org/10.1016/j.ica.2014.12.023
Benavente A, De Morán JA, Piro OE, Castellano EE, Aymonino PJ (1997) Crystal and anion structure, TGA, DTA, and infrared and Raman spectra of managanese(II) nitroprusside dihydrate, Mn [Fe(CN)5NO]·2H2O. J Chem Crystallogr 27:343–352. https://doi.org/10.1007/bf02576566
Reguera E, Marín E, Calderón A, Rodríguez-Hernández J (2007) Photo-induced charge transfer in Prussian blue analogues as detected by photoacoustic spectroscopy. Spectrochim Acta Part A Mol Biomol Spectrosc 68:191–197. https://doi.org/10.1016/j.saa.2006.11.013
Do Carmo DR, Fernandes DS, Cumba LR, Magossi MS, Dos Santos VS (2016) Solvent mixture effect in the zinc hexacyanoferrate (III) nanoparticles: synthesis, characterization and voltammetric application. Mater Res Bull 84:370–377. https://doi.org/10.1016/j.materresbull.2016.08.034
Do Carmo DR, Guinesi LS, Dias Filho NL, Stradiotto NR (2004) Thermolysis of octa (hydridodimethylsiloxyl) octasilsesquioxane in pyridine media and subsequent toluidine blue O adsorption. Appl Surf Sci 235:449–459. https://doi.org/10.1016/j.apsusc.2004.02.061
Do Carmo DR, Paim LL, Dias Filho NL, Stradiotto NR (2007) Preparation, characterization and application of a nanostructured composite: octakis(cyanopropyldimethylsiloxy)octasilsesquioxane. Appl Surf Sci 253:3683–3689. https://doi.org/10.1016/j.apsusc.2006.07.080
Pournaghi-Azar MH, Nahalparvari H (2005) Preparation and characterization of electrochemical and electrocatalytic behavior of a zinc pentacyanonitrosylferrate film-modified glassy carbon electrode. J Electroanal Chem 583:307–317. https://doi.org/10.1016/j.jelechem.2005.06.016
Magossi MS, Fernandes DS, Do Carmo DR (2019) Synthesis of a novel hybrid nanocomposite based on copper pentacyanonitrosylferrate and octa(aminopropyl)silsesquioxane and its behavior on l-cysteine electrooxidation. Solid State Sci 95:105931. https://doi.org/10.1016/j.solidstatesciences.2019.105931
Lovrić M, Scholz F (1999) A model for the coupled transport of ions and electrons in redox conductive microcrystals. J Solid State Electrochem 3:172–175. https://doi.org/10.1007/s100080050144
Lovrić M, Scholz F (1997) A model for the propagation of a redox reaction through microcrystals. J SolidState Electrochem 1:108–113. https://doi.org/10.1007/s100080050030
Jayasri D, Narayanan SS (2006) Electrocatalytic oxidation and amperometric determination of BHA at graphite–wax composite electrode with silver hexacyanoferrate as electrocatalyst. Sensors And Actuators B: Chemical 119:135–142. https://doi.org/10.1016/j.snb.2005.11.064
Engel D, Grabner EW (1985) Copper Hexacyanoferrate-Modified Glassy Carbon: a novel type of potassium-selective electrode. Ber Bunsenges Phys Chem 89:982–986. https://doi.org/10.1002/bbpc.19850890911
Baldwin RP, Ravichandran K, Johnson RK (1984) A cyclic voltammetry experiment for the instrumental analysis laboratory. J Chem Educ 61:820–823. https://doi.org/10.1021/ed061p820
Bard AJ, Faulkner LR (2001) Electrochemical Methods Fundamentals and Applications, 2ª. John Wiley & Sons, INC, New York
Zhou M, Ding J, Guo LP, Shang QK (2007) Electrochemical Behavior of l-Cysteine and Its Detection at Ordered Mesoporous Carbon-Modified Glassy Carbon Electrode. Anal Chem 79:5328–5335. https://doi.org/10.1021/ac0703707
Ralph TR, Hitchman ML, Millington JP, Walsh FC (1994) The electrochemistry of l-cystine and l-cysteine. J Electroanal Chem 375:1–15. https://doi.org/10.1016/0022-0728(94)03407-9
Do Carmo DR, Silva RM, Stradiotto NR (2005) Electrochemical Behaviour of Copper Nitroprusside Generated in situ Onto the Graphite Paste Electrode Surface, and its Application in the Determination of N-Acethylcysteine. Portugaliae Electrochimica Acta. 23:457–470. https://doi.org/10.4152/pea.200504457
Da Silva IS, Araújo MFA, Ferreira HA, Varela JJG, Tanaka SMCN, Tanaka AA, Angnes L (2011) Quantification of N-acetylcysteine in pharmaceuticals using cobalt phthalocyanine modified graphite electrodes. Talanta 83:1701–1706. https://doi.org/10.1016/j.talanta.2010.11.070
Song Y, He Z, Zhu H, Hou H, Wang L (2011) Electrochemical and electrocatalytic properties of cobalt nanoparticles deposited on graphene modified glassy carbon electrode: application to some amino acids detection. Electrochim Acta 58:757–763. https://doi.org/10.1016/j.electacta.2011.10.033
Dos Santos VS, Maraldi VA, Bonfim KS, Souza TR, Nakamura APR, Magossi MS, Fernandes DS, Do Carmo DR (2017) Voltammetric Behavior of Zinc Hexacyanoferrate (III) Nanoparticles and Their Application in the Detection of N-Acetylcysteine. Int J Electrochem Sci 12:7142–7153. https://doi.org/10.20964/2017.08.06
Do Carmo DR, Silvestrini DR, Barud HS, Dias Filho NL, Bicalho UO, Soares LA (2014) A Silsesquioxane Organically Modified with 4-Amino-5-(4-pyridyl)-4H-1,2,4-triazole-3-thiol: thermal behavior and its electrochemical detection of sulfhydryl compounds. J Nanomater 2014:1–11. https://doi.org/10.1155/2014/695954
Pipi ARF, Do Carmo DR (2011) Voltammetric studies of titanium (IV) phosphate modified with copper hexacyanoferrate and electroanalytical determination of N-acetylcysteine. J Appl Electrochem 41:787–793. https://doi.org/10.1007/s10800-011-0296-x
Zhang J, Chang Y, Dong C (2015) Electrocatalytic oxidation and sensitive determination of N-acetyl-L-cysteine at cyclodextrin-carbon nanotubes modified glassy carbon electrode. Surf Eng Appl Electrochem 51:111–117. https://doi.org/10.3103/s1068375515020155
Raoof JB, Ojani R, Amiri-Aref M, Chekin F (2010) Catechol as an electrochemical indicator for voltammetric determination of N-acetyl-l-cysteine in aqueous media at the surface of carbon paste electrode. J Appl Electrochem 40:1357–1363. https://doi.org/10.1007/s10800-010-0093-y
Suarez WT, Marcolino LH, Fatibello-Filho O (2006) Voltammetric determination of N-acetylcysteine using a carbon paste electrode modified with copper(II) hexacyanoferrate(III). Microchem J 82:163–167. https://doi.org/10.1016/j.microc.2006.01.007
Tabeshnia M, Rashvandavei M, Amini R, Pashaee F (2010) Electrocatalytic oxidation of some amino acids on a cobalt hydroxide nanoparticles modified glassy carbon electrode. J Electroanal Chem 647:181–186. https://doi.org/10.1016/j.jelechem.2010.06.004
Acknowledgements
Mariana de Souza Magossi would like to express her gratitude to Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES) for the granted scholarship granted/ and The authors would like to acknowledge the Instituto Federal Goiano and the CNPq for the financial support
Funding
This work was supported by the Fundação de Amparo à Pesquisa do Estado de São Paulo (FAPESP – Process 2018/24576–2).
Author information
Authors and Affiliations
Contributions
Mariana de Souza Magossi: designed and performed the electrochemical experiments and verified the analytical methods; Murilo Santos Peixoto, Abner Santos Baroni Sales, Alexsandro dos Santos Felipe and Newton Luiz Dias Filho: performed several spectroscopic and analytical experiments. Devaney Ribeiro do Carmo: conceived of the idea, performed several spectroscopic experiments, and wrote the manuscript in consultation with the other authors. All authors participated in the discussion, contributing to the final manuscript.
Corresponding author
Ethics declarations
Not applicable.
Consent to participate
Not applicable.
Consent for Publication
Not applicable.
Conflicts of interest/Competing interests
The authors declare that they have no known competing financial interests or personal relationships.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
do Carmo, D.R., Peixoto, M.S., dos Santos Felipe, A. et al. Synthesis of a New Zn2+/Fe3+ Octa(aminopropyl)silsesquioxane Complex and Its Voltammetric Behavior Towards N-acetylcysteine. Silicon 15, 683–697 (2023). https://doi.org/10.1007/s12633-022-02030-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12633-022-02030-w