Abstract
Purpose
There is growing evidence to suggest a deficiency in anesthesiologists’ diagnosis of airway pathology and subsequent airway management planning, and conventional instruments have not shown increases in safety. Virtual endoscopy (VE) is a tool that can detail intraluminal anatomical “fly-through” information in a format visually similar to the flexible endoscopic views familiar to anesthesiologists. We aimed to determine the effect of VE on diagnostic accuracy and airway management strategies when compared with conventional tools.
Methods
Clinical scenarios, along with computerized tomography (CT) imaging, were presented to 20 anesthesiologists, and structured questions were asked regarding diagnosis of airway pathology and airway management strategy. Virtual endoscopy videos were then provided and the questions were repeated. Following the CT and VE presentations, the anesthesiologists’ responses involving diagnostic accuracy and airway management strategy were compared between the CT and VE techniques. Answers relating to the utility of VE were also sought.
Results
Diagnostic accuracy was 54.1% with CT alone and increased to 67.7% when VE was added (P = 0.007). In 48% of cases, the addition of VE to clinical history and CT led to changes in airway management strategy (P < 0.001), and 90.6% of these changes were deemed more cautious (P < 0.001).
Conclusion
Virtual endoscopy improves the accuracy in diagnosis of airway pathology when compared with CT alone. Furthermore, it leads to more conservative and potentially safer airway management strategies in patients with head and neck pathology.
Résumé
Objectif
Des données probantes s’accumulent suggérant des lacunes dans le diagnostic des pathologies des voies aériennes par les anesthésiologistes et dans leur planification subséquente de prise en charge des voies aériennes, et les instruments traditionnels n’ont pas montré d’amélioration de la sécurité. L’endoscopie virtuelle (EV) est un outil qui peut détailler les informations anatomiques intracaluminaires « au passage » dans un format semblable, d’un point de vue visuel, aux vues obtenues à l’aide d’un dispositif endoscopique flexible, lesquelles sont bien connues des anesthésiologistes. Notre objectif était de déterminer l’effet de l’EV sur la précision diagnostique et les stratégies de prise en charge des voies aériennes par rapport aux outils traditionnels.
Méthode
Des cas cliniques accompagnés d’images tomodensitométriques ont été présentés à 20 anesthésiologistes, et on leur a posé des questions structurées concernant le diagnostic de la pathologie des voies aériennes et la stratégie de prise en charge qu’ils proposaient. Des vidéos d’endoscopie virtuelle leur ont ensuite été présentées et les questions répétées. Après la présentation des images tomodensitométriques et d’EV, les réponses des anesthésiologistes touchant à la précision diagnostique et à la stratégie de prise en charge des voies aériennes ont été comparées entre les techniques de tomodensitométrie et d’EV. Les réponses liées à l’utilité de l’EV ont également été recherchées.
Résultats
La précision diagnostique était de 54,1 % avec les images tomodensitométriques seules et a augmenté à 67,7 % lorsqu’on a ajouté les images d’EV (P = 0,007). Dans 48 % des cas, l’ajout de l’EV aux antécédents cliniques et aux images tomodensitométriques a entraîné des modifications de la stratégie de prise en charge des voies aériennes (P < 0,001), et 90,6 % de ces modifications ont été jugées plus prudentes (P < 0,001).
Conclusion
L’endoscopie virtuelle améliore la précision du diagnostic d’une pathologie des voies aériennes par rapport à une imagerie tomodensitométrique seule. En outre, cette modalité suscite des stratégies de prise en charge des voies aériennes plus conservatrices et potentiellement plus sécuritaires chez les patients souffrant de pathologies de la tête et du cou.
Similar content being viewed by others
In 2011, the Fourth National Audit Project (NAP4),1 a review of all major complications of airway management in the United Kingdom, was published. Ever since, an introspective assessment of how to increase the safety of elective and emergent airway management has developed across the specialty. A number of tools have since been created, adapted, or adopted with this aim in mind, including a host of new supraglottic airway devices, endotracheal tubes,2 videolaryngoscopes, and pharmacological agents.3-5 Nevertheless, one of the key findings of the NAP4 report was the poor assessment and subsequent planning of cases that ultimately led to significant airway morbidity.1 Additionally, patients with head, neck, and tracheal disease were far more likely to experience difficulties in the management of their airway. Yet, to date, there has been little advancement in improving airway assessment.6 Clinical examination has been shown to be both inaccurate and insufficient, particularly in patients with head, neck, and tracheal disease.7
Multi-plane computerized tomography (CT) scanning is performed for a large proportion of patients with head and neck disease. This technique provides detailed information on anatomical deformity, lesion location, and tumour involvement of secondary tissues or organs.8 These static CT images are presented in a two-dimensional format and do not allow dynamic intraluminal assessment and informed airway planning. In order to overcome this difficulty, flexible nasendoscopy may be performed to provide a dynamic assessment of supraglottic disease and a more complete understanding of intraluminal anatomy. This procedure will support, and may indeed influence, a well-planned airway management strategy.9,10 There are limitations to this technique, however, including a requirement for specialized equipment, appropriately trained personnel, patient compliance, tolerance, and, potentially, sedation. It also fails to provide any subglottic luminal information.
Virtual endoscopy (VE) is a valuable tool that bridges the gap in airway assessment by providing a noninvasive, anatomically accurate representation of intraluminal geography of the airway, including supraglottic, glottic, and subglottic structures. The OsiriX viewer (Pixmeo Sari, Bernex, Switzerland), a multidimensional image navigation and display tool, has been available for several years. Though the technology is not new,11,12 its application in anesthetic practice is novel. By reconstructing diagnostic CT images, a three-dimensional (3D) “fly-through” video of endoscopic anatomy is created, allowing tailored comprehensive airway management strategies in advance of any direct patient intervention. Nevertheless, there is yet no evidence that VE influences airway assessment and planning in either expert or non-expert hands.
The primary objective of this study was to determine the effect of VE on diagnostic accuracy and airway management. We hypothesized that the use of VE by anesthesiologists would improve preoperative diagnostic accuracy in patients with head and neck pathology and lead to modification of the airway management plan when used in addition to CT imaging.
Methods
Institutional research and development board approval was obtained for this prospective cohort study conducted in a specialist centre, and therefore, the study was exempted from formal ethical approval. All data were handled in accordance with national guidance and the principles of the “Declaration of Helsinki”,13 and the “STARD 2015” (Standards for Reporting Diagnostic Accuracy) statement was followed.14 Informed consent was obtained from all subjects recruited.
Subjects
Any staff anesthesiologist at Guy’s Hospital, London, UK with regular operating lists at our tertiary centre was eligible for inclusion. A convenience series of staff were recruited who either did or did not routinely anesthetize for head and neck operating lists. Data were collected regarding experience with head and neck surgical patients. Airway group (AG) subjects consisted of staff anesthesiologists who regularly performed at least one head and neck operating list per week. Non-airway group (NG) subjects included staff who did not anesthetize for a head and neck operating list on a regular basis. Exclusion criteria included withdrawing or withholding consent or failure to answer all questions. The sample size was determined by the number of anesthesiologists at our centre who were available throughout the study period. Data collection ran from February-July 2015.
Clinical scenarios
Ten anonymized clinical scenarios were prepared, each comprising a brief clinical history, preoperative helical CT imaging of airway pathology (axial, coronal, and sagittal views), and VE videos. The clinical context of the scenarios involved anonymized information of patients presenting with supraglottic, glottic, or subglottic pathology, and they were specifically selected to match the distribution of pathology reported in NAP4 (Table 1).1 The helical CT images were routinely performed during the diagnostic workup of patients undergoing head and neck surgery. The VE videos were generated by the anesthesiologist study investigators, and no external assistance was required. The Digital Imaging and Communications in Medicine (DICOM) data of the helical CT images were obtained from the institutional Patient Archive and Communication System (PACS), Centricity Enterprise Web 2006 (GE Medical Systems, Chicago, IL, USA), fully anonymized, and exported to an external disk. The DICOM data files were then imported to OsiriX Viewer v5.5 32-Bit (Pixmeo Sari, Bernex, Switzerland), and the VE videos were reconstructed from these images using the software. The “3D endoscopy” software function was utilized, and the 3D flight path was constructed by advancing and rotating a virtual camera to simulate endoscopic views. Key stages of advancement through a reconstruction comprising axial, coronal, and sagittal views of the airway were recorded as individual frames. The series of frame images were interpolated at a frame count of 200 using a spline method to produce a smoother transition between frames. The video files were then saved in the .mov format at a resolution of 1228 × 657 ppi. No training on interpretation and analysis of VE videos was provided to any recruited subjects.
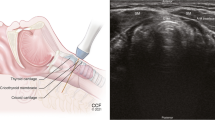
Data collection
All subjects were independently presented with each clinical history and series of CT images. None of the anesthesiologists were involved in the care of the patients presented in the clinical scenarios, and the decisions were not communicated to the responsible anesthesiologist. Nasendoscopy images are not routinely available in our institution and were therefore not utilized within this study. In the first stage of the study, a set of specific questions was asked (Appendix) regarding the diagnosis of the underlying airway pathology, the planned airway management, and the value of CT imaging in planning the airway management. The second stage of the study—i.e., when the clinical scenarios were again presented, took place following a time interval of no less than one week in order to minimize recall bias. This time the scenarios were presented in a different order from the first stage and, on this occasion, VE videos were shown in addition to the clinical history and CT images (Fig. 1; Video as Electronic Supplementary Material). The questions on diagnosis of airway pathology and airway management were then repeated. Finally, generic questions were asked relating to each subject’s use of CT imaging of the head and neck as well as which medium they deemed more useful for formulating airway plans (i.e., dynamic VE videos or CT images).
The subjects were blinded to radiological reports, all of which were formally reported by a radiologist. These radiological reports were identified as the reference standard of diagnostic accuracy. Answers relating to the accuracy of the pathological diagnosis were objectively marked against the original radiological report produced by a radiologist. Marking was limited to having the anesthesiologists define the pathology of interest. One mark was given for correctly identifying the pathology or the absence of any pathology. In one of the ten scenarios, the patient had more than one pathology; therefore, two marks were given for this scenario, one mark for each relevant abnormality. Therefore, at each stage of the study, each individual anesthesiologist could achieve a maximum score of 11 for the ten scenarios. There were no marks attributed to answers relating to airway management plans. We also aimed to establish whether the addition of VE videos influenced individual airway management plans vs reviewing the CT images alone. Answers in this section were categorized into groups described as “more cautious”, “less cautious”, or “no change”. This change in cautiousness was related to the difference in management strategy described by anesthesiologists without VE videos vs with VE videos. Cautiousness was defined based on a change of any one or more of the following parameters: induction technique, intubation technique, laryngoscopy technique, tracheal tube chosen, performance of tracheostomy, presence of a surgeon or presence of an experienced colleague (Table 2). A management strategy that was deemed more cautious overall was one where the majority of changes were more cautious. A management strategy that was deemed less cautious overall was one where the majority of changes were less cautious. If there was a change in technique that did not meet any of the parameters defined in Table 2, then this was judged to represent no change in cautiousness. Examples of this might include a change in type of tracheal tube used (reinforced vs polyvinyl chloride tube), a change in the route of intubation (nasal vs oral), or a change in the drugs used during anesthesia (total intravenous anesthesia vs volatile anesthesia).
Statistical analysis
Data were collected using standardized data capture forms (Appendix) and compiled into a Microsoft Excel 2016 (Microsoft Corp, Redmond, WA, USA) spreadsheet. Data analysis was conducted using R version 3.1.2 (CRAN, Vienna, Austria). Normality testing of continuous variables was conducted using a Shapiro-Wilk test. Normally distributed data were reported as mean (standard deviation [SD]) and assessed for differences in means with a paired samples Student’s t test, while non-normally distributed data were analyzed using the Wilcoxon signed-rank test. Categorical data were assessed using McNemar’s test, and generic answers were reported descriptively. A P < 0.05 was considered significant.
Results
Thirty-five subjects were eligible for inclusion; however, 13 were excluded as they were unavailable to complete the study. A further two subjects were excluded as they were study investigators and therefore not blinded to the pathology of the case scenarios. Twenty subjects were therefore recruited, ten AG subjects and ten NG subjects. All twenty subjects completed each stage of the study with no dropouts (Fig. 2).
Diagnostic accuracy
The maximum number of marks available at each stage was 220, as 20 participants could achieve a maximum of 11 marks. Following their review of the clinical scenario and CT images, the subjects achieved 119/220 (54.1%) marks—61/110 (55.5%) marks in AG subjects and 58/110 (52.7%) marks in NG subjects. After reviewing the VE videos in conjunction with the CT images and clinical scenarios, the diagnostic accuracy improved to 149/220 (67.7%) marks (P = 0.017)—80/110 (72.7%) marks in the AG cohort and 69/110 (62.7%) marks in the NG cohort. Overall, for each of the clinical scenarios presented, the mean (SD) number of subjects correct per case after CT alone was 11.9 (5.9), increasing to 14.9 (5.5) with the addition of VE (Table 3).
Changes in airway management planning
A maximum of 200 changes to airway management planning were possible as 20 participants could change the plan in each of the ten scenarios. The addition of VE to CT imaging with clinical scenarios led to changes in airway management planning in 96/200 (48%) cases (P = 0.005), including 72 (72%) AG changes and 81 (81%) NG changes. Of the 96 modifications to airway management plans, 87 (90.6%) were more cautious (P = 0.005), four (4.2%) were less cautious (P = 0.10), and five (5.2%) had no change in cautiousness (P = 0.10) (Table 4).
Generic answers
Twelve of the 20 subjects stated that they review CT images of the head and neck when presented with a patient undergoing airway surgery. After participating in the study, 75% (15/20) of subjects, 60% of AG and 90% of NG subjects, stated that they found VE more useful than CT images alone. All (20/20) subjects agreed that reviewing the VE videos helped with the airway assessment in all scenarios. All subjects stated that they would use VE if it were readily available as part of their airway assessment for patients with head and neck pathology or those with a suspected difficult airway.
Discussion
This study presents a novel tool to aid in airway assessment, pathological diagnosis, and airway management planning. Anesthesiologists improve their diagnostic accuracy when they review VE videos in addition to CT images of patients with head and neck pathology. Our study results have shown that anesthesiologists made changes in airway management planning after reviewing VE videos (as well as CT images) vs reviewing CT images alone. In most cases, these changes were deemed more cautious and were independent of the level of experience in complex airway management.
Although there is an increasing evidence base for the use of virtual airway navigational tools for improvement of technical skills and physician training,15-17 there is little reported on the clinical value for such use. Virtual endoscopy facilitates an anatomically accurate reproduction of endoscopic findings within the airway in a format that is easily interpreted by the anesthesiologist.18 Virtual visualization of the airway using preexisting CT images aids in planning an airway strategy. The added advantage of this technology is that any anesthesiologist who undertakes a short period of self-training can reconstruct a VE of the patient’s airway as part of a comprehensive airway assessment.18 Previous studies and case reports have shown the use of VE to aid in the airway assessment of patients with head and neck pathology,19,20 but this novel study evaluates the influence of VE videos not only on diagnostic accuracy but also on airway management planning.
The currently available bedside airway assessment tools have been shown to have variable specificity and sensitivity for predicting difficult intubations.7,21,22 Thus, nasendoscopy and CT imaging have been used to delineate the extent of disease pathology when planning airway management in patients with head and neck pathology. Approximately 40% of all cases reported in the NAP4 study were associated with a disease process in the head, neck, or trachea, and 70% of these reports were associated with airway obstruction.1 Nearly a third of cases reported in NAP4 were considered poorly managed, with inadequate airway assessment and planning identified as a common contributing factor. A key recommendation of the NAP4 report was that anesthesiologists attending this patient population should be familiar with airway imaging and should use it when possible for planning airway strategy.1 Preoperative assessment should determine the nature, degree, location, and implications of airway narrowing or distortion, and our study suggests that the addition of VE to CT imaging can improve the accuracy of airway assessment and help formulate a safer airway management plan.
It is imperative to establish the level of airway obstruction,1 particularly in determining whether the pathology is located at the tongue base or at the perilaryngeal or subglottic level and whether the laryngeal inlet is affected. In our study, nine of our ten cases had such pathology; however, the accuracy in interpreting CT imaging was only 55.5% and 52.7% in the AG and NG cohorts, respectively. By adding VE to the diagnostic arsenal, we have shown a significant increase in diagnostic accuracy for supraglottic, glottic, and subglottic lesions, regardless of experience, suggesting that VE is an effective diagnostic tool. This improved accuracy could be attributed to the format of the VE videos, which replicates the more familiar endoscopic views seen by anesthesiologists. This has been shown previously by the broad applicability of VE as a diagnostic tool in a number of medical specialties, such as radiology and thoracic surgery, for lesions in the head and neck, trachea, and bronchial tree.23-32
Nørskov et al. found that, in almost half of the patients with anticipated difficult tracheal intubation, the anesthesiologist planned to attempt direct laryngoscopy for intubation, even when difficult intubation and difficult face mask ventilation were anticipated.7 We have shown that there was a change in the airway management plan in half of the cases when VE videos were reviewed, with the vast majority changing to a more cautious plan (90.6%). This finding implies that anesthesiologists receive more interpretable information with the addition of VE videos in airway assessment vs CT images alone. Furthermore, this information influences airway management planning, which appears to be in a potentially safer, more cautious manner. The isolated use of CT imaging to aid airway management planning may have led to inadequate strategies that could have contributed to failure. Nevertheless, it is important to point out that VE is not a predictor of a difficult intubation, but that it may lead to a safer airway management strategy.
Our study has several limitations. First, all data were collected from a single centre; thus, the broad applicability of this data may be subject to discussion. Nevertheless, by choosing anesthesiologists from a range of clinical backgrounds, we have maximized the generalizability of these results. Virtual endoscopy is based on static CT images of supine patients rather than on a dynamic assessment. However, as CT scanning is the standard of assessment in many institutions, we have shown that VE may be an invaluable supplement. While nasendoscopic images taken during surgical assessment of patients might be available to anesthesiologists at some institutions, they are not available in ours. Thus, the availability of such images might have provided additional information without the need for VE. Additionally, the preparation of VE videos requires access to the software and expertise in video production. In our experience with both training and production, anesthesiologists with no prior experience with VE can produce videos in approximately 20 min, and more advanced users can produce videos in five minutes. Finally, our study methodology did not involve patient intervention, only management planning, leaving an important avenue for future investigation.
To reaffirm the utility of this novel technique and determine its impact on patient outcomes, further studies should be randomized to assess diagnostic accuracy and its effect on airway management strategies in patients.
In conclusion, we have shown that the use of VE is efficacious in improving the diagnostic accuracy of anesthesiologists when compared with the use of conventional tools, and this can lead to more conservative and potentially safer airway management strategies in patients with head and neck pathology.
References
Cook TM, Woodall N, Frerk C, Fourth National Audit Project. Major complications of airway management in the UK: results of the Fourth National Audit Project of the Royal College of Anaesthetists and The Difficult Airway Society. Part 1: Anaesthesia. Br J Anaesth 2011; 106: 617-31.
Huitink JM, Koopman EM, Bouwman RA, et al. Tracheal intubation with a camera embedded in the tube tip (VivasightTM). Anaesthesia 2013; 68: 74-8.
McDonagh DL, Benedict PE, Kovac AL, et al. Efficacy, safety, and pharmacokinetics of sugammadex for the reversal of rocuronium-induced neuromuscular blockade in elderly patients. Anesthesiology 2011; 114: 318-29.
Geldner G, Niskanen M, Laurila P, et al. A randomised controlled trial comparing sugammadex and neostigmine at different depths of neuromuscular blockade in patients undergoing laparoscopic surgery. Anaesthesia 2012; 67: 991-8.
Amao R, Zornow MH, Cowan RM, Cheng DC, Morgte JB, Allard MW. Use of sugammadex in patients with a history of pulmonary disease. J Clin Anesth 2012; 24: 289-97.
Frerk C, Mitchell VS, McNarry AF, et al. Difficult Airway Society 2015 guidelines for management of unanticipated difficult intubation in adults. Br J Anaesth 2015; 115: 827-48.
Norskov AK, Rosenstock CV, Wetterslev J, Astrup G, Afshari A, Lundstrom LH. Diagnostic accuracy of anaesthesiologists’ prediction of difficult airway management in daily clinical practice: a cohort study of 188 064 patients registered in the Danish Anaesthesia Database. Anaesthesia 2015; 70: 272-81.
Forastiere A, Koch W, Trotti A, Sidransky D. Head and neck cancer. N Engl J Med 2001; 345: 1890-900.
Rosenblatt W, Ianus AI, Sukhupragarn W, Fickenscher A, Sasaki C. Preoperative endoscopic airway examination (PEAE) provides superior airway information and may reduce the use of unnecessary awake intubation. Anesth Analg 2011; 112: 602-7.
Pearce A. Recognition and management of the difficult airway. In: Gleeson M, Browning GG, Burton M, et al. (Eds). Scott-Brown’s Otorhinolaryngology: Head and Neck Surgery - 7th Edition. Edward Arnold Ltd; 2008: 473-4.
Mark Z, Bajzik G, Nagy A, Bogner P, Repa I, Strausz J. Comparison of virtual and fiberoptic bronchoscopy in the management of airway stenosis. Pathol Oncol Res 2008; 14: 313-9.
Rosset A, Spadola L, Ratib O. OsiriX: An open-source software for navigating in multidimensional DICOM images. J Digit Imaging 2004; 17: 205-16.
World Medical Association. World Medical Association Declaration of Helsinki: ethical principles for medical research involving human subjects. JAMA 2013; 310: 2191-4.
Bossuyt PM, Reitsma JB, Bruns DE, et al. STARD 2015: an updated list of essential items for reporting diagnostic accuracy studies. BMJ 2015; 351: h5527.
Boet S, Naik VN, Diemunsch PA. Virtual simulation training for fibreoptic intubation. Can J Anesth 2009; 56: 87-8.
Boet S, Bould MD, Schaeffer R, et al. Learning fibreoptic intubation with a virtual computer program transfers to ‘hands on’ improvement. Eur J Anaesthesiol 2010; 27: 31-5.
Baker PA, Weller JM, Baker MJ, et al. Evaluating the ORSIM® simulator for assessment of anaesthetists’ skills in flexible bronchoscopy: aspects of validity and reliability. Br J Anaesth 2016; 117: i87-91.
Ahmad I, Millhoff B, John M, Andi K, Oakley R. Virtual endoscopy—a new assessment tool in difficult airway management. J Clin Anesth 2015; 27: 508-13.
Byrne AT, Walshe P, McShane D, Hamilton S. Virtual laryngoscopy-preliminary experience. Eur J Radiol 2005; 56: 38-42.
Sgalambro F, Sanfilippo F, Santonocito C, Caltavuturo C, Grillo Cl. Virtual laryngoscopy and combined laryngoscopic-bronchoscopic approach for safemanagement of obstructive upper airways lesions. Br J Anaesth 2014; 113: 304-6.
Yentis SM. Predicting difficult intubation-worthwhile exercise or pointless ritual? Anaesthesia 2002; 57: 105-9.
Huitink JM, Bouwman RA. The myth of the difficult airway: airway management revisited. Anaesthesia 2015; 70: 244-9.
Finkelstein SE, Schrump DS, Nguyen DM, Hewitt SM, Kunst TF, Summers RM. Comparative evaluation of super high-resolution CT scan and virtual bronchoscopy for the detection of tracheobronchial malignancies. Chest 2003; 124: 1834-40.
Boiselle P, Reynolds KF, Ernst A. Multiplanar and three-dimensional imaging of the central airways with multidetector CT. AJR Am J Roentgenol 2002; 179: 301-8.
Men S, Ecevit MC, Topçu I, Kabakci N, Erdag TK, Sutay S. Diagnostic contribution of virtual endoscopy in diseases of the upper airways. J Digit Imaging 2007; 20: 67-71.
Walshe P, Hamilton S, McShane D, McConn Walsh R, Walsh MA, Timon C. The potential of virtual laryngoscopy in the assessment of vocal cord lesions. Clin Otolaryngol Allied Sci 2002; 27: 98-100.
Summers RM, Shaw DJ, Shelhamer JH. CT virtual bronchoscopy of simulated endobronchial lesions: effect of scanning, reconstruction, and display settings and potential pitfalls. AJR Am J Roentgenol 1998; 170: 947-50.
Summers RM, Aggarwal NR, Sneller MC, et al. CT virtual bronchoscopy of the central airways in patients with Wegener’s granulomatosis. Chest 2002; 121: 242-50.
De Wever W, Vandecaveye V, Lanciotti S, Verschakelen JA. Multidetector CT-generated virtual bronchoscopy: an illustrated review of the potential clinical indications. Eur Respir J 2004; 23: 776-82.
Bauer TL, Steiner KV. Virtual bronchoscopy: clinical applications and limitations. Surg Oncol Clin N Am 2007; 16: 323-8.
Rogalla P, Nischwitz A, Gottschalk S, Huitema A, Kaschke O, Hamm B. Virtual endoscopy of the nose and paranasal sinuses. Eur Radiol 1998; 8: 946-50.
Burke AJ, Vining DJ, McGuirt WF Jr, Postma G, Browne JD. Evaluation of airway obstruction using virtual endoscopy. Laryngoscope 2000; 110: 23-9.
Acknowledgements
We thank Jennie Maclean MBBS FRCA, Guy’s & St Thomas’ NHS Foundation Trust for assistance with data collection.
Conflicts of interest
None declared.
Editorial responsibility
This submission was handled by Dr. Philip M. Jones, Associate Editor, Canadian Journal of Anesthesia.
Author contributions
Kariem El-Boghdadly, Desire N. Onwochei, Britta Millhoff, and Imran Ahmad helped conduct the study, analyze the data, and prepare the final manuscript. Britta Millhoff and Imran Ahmad helped design the study. Britta Millhoff helped collect the data.
Funding
This study was not financially supported.
Author information
Authors and Affiliations
Corresponding author
Additional information
This article is accompanied by an editorial. Please see Can J Anesth 2017; 64: this issue.
Electronic supplementary material
Below is the link to the electronic supplementary material.
VIDEO
Virtual endoscopy video of patient with subglottic stenosis. A nasogastric tube can be seen passing posterior to the epiglottis and into the esophagus (MOV 5138 kb)
Appendix: Study data capture form
Appendix: Study data capture form

Rights and permissions
About this article
Cite this article
El-Boghdadly, K., Onwochei, D.N., Millhoff, B. et al. The effect of virtual endoscopy on diagnostic accuracy and airway management strategies in patients with head and neck pathology: a prospective cohort study. Can J Anesth/J Can Anesth 64, 1101–1110 (2017). https://doi.org/10.1007/s12630-017-0929-6
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12630-017-0929-6