Abstract
Purpose of Review
Application of ultrasound in clinical anesthesia practice extends beyond regional anesthesia. In this review, we have discussed other point-of-care applications of ultrasound in perioperative care and anesthetic management with emphasis on regional anesthesia practice.
Recent Findings
Point-of-care ultrasound (POCUS) for anesthesiologists has gained widespread interest and popularity. Recent literature has highlighted multiple perioperative POCUS applications on various organ systems and their benefits. In this section, we have analyzed the recent available data and evidence for perioperative POCUS for airway, gastric contents and trauma, its applications, benefits, and limitations in perioperative medicine.
Summary
Perioperative POCUS skill is a natural extension of ultrasound guided regional anesthesia. Utilizing POCUS in the perioperative period would accelerate the level of appropriate care, safety, and improve outcomes. More anesthesiologists embracing POCUS would enhance patient care in a timely fashion. POCUS in regional anesthesia practice, including ambulatory centers, can be valuable in avoiding delay or cancellation of surgeries and improving patient satisfaction.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Point-of-care ultrasound (POCUS) is a bedside application of ultrasound and hailed to be “the stethoscope of the twenty-first century” [1, 2]. It is widely used across multiple specialties for timely diagnosis and management of critical clinical situations. The utility and scope of POCUS continues to expand with advances in technology, portability, and affordability of ultrasound machines.
POCUS has been well established in the practice of emergency medicine and critical care [3, 4]. Recently, the importance and utility of POCUS in anesthetic practice is being widely recognized and strongly recommended [5, 6]. The expectation of competency in this skill is not limited to the practicing anesthesiologists but also for the trainees [7, 8]. The American Society of Anesthesiologists (ASA) published recommendations regarding scope of practice and training in POCUS [9••]. Several other international anesthesia societies have also adopted guidelines, recommendations for training, practice, and competency in perioperative POCUS skills [10, 11, 12•]. Regional anesthesiologists, as early adopters of ultrasound, have a unique skill set that lends itself to using POCUS effectively throughout the perioperative period [13]. Regional anesthesiologists can be champions and leaders of POCUS applications and education.
The I-AIM (Indication, Acquisition, Interpretation, Medical decision) framework described by Bahner et al. delineates POCUS to be a goal directed application compared to a comprehensive ultrasound exam [14]. We will use clinical scenarios as examples of indications and review the evidence for interpretation and decision-making in our discussion on perioperative POCUS.
Perioperative POCUS for Regional Anesthesiologists
The utility of ultrasound for peripheral nerve blocks and vascular access has been well established in the practice of regional anesthesiologists. Advantages of POCUS use in preoperative clinics and its influence on decisions regarding further testing, optimization, choice of anesthetic technique, and monitoring were reviewed by Meier et al. and found to be complementary to existing diagnostic tools and authors suggested that it can provide additional information quickly and reproducibly [15]. A multicenter study demonstrated that utilization of cardiac and pulmonary POCUS in a post anesthesia care unit (PACU) decreases the number of differential diagnoses and the duration of stay in the unit [16•].
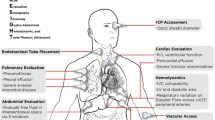
Soon, there will be an expectation for all anesthesiologists to possess some basic POCUS skills and the ASA’s diagnostic POCUS certificate program is geared towards encouraging this competency [7]. Our discussion will focus on perioperative uses of POCUS (Table 1) from a regional anesthesiologist’s perspective. In this article, we will discuss POCUS for evaluation of airway, gastric contents, the benefits and utility of cardiac, vascular, pulmonary, abdominal, and optic nerve POCUS for a regional anesthesiologist.
Airway POCUS
Airway examination is an integral part of any pre-op assessment. Airway POCUS has been described to identify the upper airway structures both in the operating room and the emergency room areas [17, 18].
Indications for airway POCUS include identification of upper airway structures that can be helpful in nerve blocks of the airway, cricothyrotomy or confirmation of endotracheal intubation—especially outside of the operating room or during cardiac compressions.
Technique: most upper airway structures can be scanned using high-frequency, linear transducer probe, while the tongue and deeper suprahyoid structures may need a curvilinear probe. The ideal position to scan the airway is to have the patient lying supine, with the head extended, and neck flexed (sniffing position), as is required for intubating a patient.
Airway POCUS for identification of structures can be performed with both transverse and longitudinal scans [19]. A linear probe placed transversely (Fig. 1) can image the hyperechoic inverted V-shaped thyroid cartilage. The cricothyroid membrane (CTM) with the air-mucosal interface located posteriorly is identified by moving the probe in the caudal direction. The transducer is slid more caudally to image an arch shaped, hypoechoic cricoid cartilage. The transducer is moved cephalad back to the CTM to complete the imaging sequence known as the TACA (thyroid cartilage–airline– cricoid cartilage–airline) technique.
The CTM can also be identified with the transducer placed longitudinally (Fig. 2) above the suprasternal notch in the sagittal plane. The hypoechoic tracheal rings resemble a “string-of-pearls.” The cricoid lies cephalad as a larger and more superficial hypoechoic structure. Further cephalad movement of the probe identifies the thyroid cartilage separated from the cricoid cartilage by the CTM.
A high-frequency linear probe in the paramedian sagittal plane (Fig. 3) enables identification of the hyoid bone, thyroid cartilage, and thyrohyoid membrane connecting the two. It is this view that is utilized when anesthetizing the airway. Bilateral injection of local anesthetic through the thyrohyoid membrane reliably blocks the internal branch of the superior laryngeal nerve. Transtracheal injection of local anesthetic for lower airway blockade can also be performed through the cricothyroid membrane identified with ultrasound imaging as described above.
Clinical scenario: A colleague requests help with airway nerve blocks for a planned awake fiberoptic intubation. Patient is a morbidly obese man with a short, large neck with a known difficult airway On examination, it was difficult to identify the landmarks on the anterior neck due to the body habitus. AUS helped in identifying and marking hyoid bone, thyroid cartilage, cricoid cartilage, tracheal rings, thyrohyoid membrane, and cricothyroid membrane on the surface of the neck. The superior laryngeal nerve and translaryngeal block were performed with AUS guidance. Subsequently, an awake fiberoptic intubation was performed successfully |
Airway POCUS has been reported helpful in prediction of difficult airway, confirmation of endotracheal intubation, prediction of double lumen tube (DLT) size for thoracic surgery, and cricothyrotomy [19, 20•, 21,22,23,24,25]. Ultrasound guidance significantly improves the success and safety of cricothyrotomy, especially in obese individuals and difficult airways. Regional blocks of upper airway with the guidance of airway ultrasound have been shown to facilitate awake fiberoptic intubation [26, 27]. Several airway ultrasound measurements and their ratios (distance to epiglottis, hyoid, hyomental distance ratio, and ratio of preepiglottic to epiglottic-vocal cord distance) have been described in predicting a difficult laryngoscopy. Recent reviews and meta-analyses of these studies suggest the current evidence is inconclusive [28, 29•]. While airway ultrasound may not be the first-line choice for securing the airway, it is an invaluable tool for patients with difficult anatomy.
Gastric POCUS
Aspiration of gastric contents is a dreaded complication and the leading cause of death from airway related anesthesia events [30, 31]. Patients who experience aspiration are at high risk for hypoxia, pneumonitis, acute respiratory distress syndrome, all of which can lead to shock and multisystem organ failure [32, 33].
A full stomach can be easily identified in patients who are clearly not nil per os (npo) on interview, such as emergency cases. In patients with severe reflux disease, gastric dysmotility, autonomic dysfunction, hiatal hernia, and/or a history of bariatric surgery that require more critical thinking by the anesthesiologist, gastric ultrasound can be helpful. Albeit some of these pathologies can make gastric ultrasound technically difficult. A study done in the urgent surgical population clearly showed that anesthesiologists’ clinical judgment is unreliable compared to gastric ultrasound in evaluation of full stomach status [34].
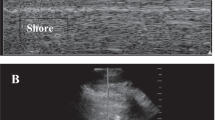
Technique: gastric POCUS is performed in the supine and right lateral decubitus (RLD) positions to image and measure the cross-sectional area of the gastric antrum. In the right lateral decubitus position, gastric contents move toward the antrum and any air present will move towards the fundus, aiding in image optimization. Preferably, a low-frequency curvilinear probe is placed in the sagittal plane between the umbilicus and xiphoid process with the indicator pointing cranially. The liver should be in view anteriorly with the aorta, superior mesenteric artery, and pancreas lying posterior to the antrum (Fig. 4). An empty stomach will appear flat or as a “bull’s eye sign.” As the stomach becomes distended with food or liquid, it is possible to measure the cross-sectional area of the antrum and approximate its volume. Volume (ml) = 27 + (14.6 × CSA − (1.28 × Age) [35].
Clinical scenario: A 54-year-old woman with a past medical history of type 2 diabetes mellitus, hypertension, and hyperlipidemia presented for a left total knee arthroplasty. The anesthetic plan included a peripheral nerve block, spinal anesthesia, and sedation. On interview, the patient reported that for the last two months she has been taking medication (semaglutide) for weight loss A gastric ultrasound was performed to assess the gastric contents which showed a significant volume of solid contents. After discussion with the patient, a decision was made to intubate the patient to keep the airway protected during the surgery |
Recently, there has been an increased concern and wide discussion regarding management of patients taking glucagon-like-peptide-1 (GLP-1) agonists. Patients commonly take GLP-1 agonists for diabetes management and/or weight loss. One of the chief mechanisms of action for these medications is to delay gastric emptying which in theory increases the risk of aspiration among surgical patients, which has to this point only been described in case reports [36, 37]. Recently, the ASA released consensus-based guidance on the perioperative management of these medications [38]. They recommend holding daily medications for at least one day, and weekly dosing medications for at least one week, irrespective of medication indication. On the day of the procedure, they recommend proceeding based on symptoms and clinical history and using gastric ultrasound to guide the clinical management. A recent editorial suggested holding long acting GLP-1 agonists for about three half-lives before an elective procedure [39•]. There were no evidence-based recommendations or guidelines at the time of writing this review due to limited data. A gastric POCUS on the morning of surgery for these patients would enable the anesthesiologist to make an informed decision regarding safe management of the airway.
Gastric POCUS provides both qualitative and quantitative assessments of the gastric content [40, 41]. Kruisselbrink et al. showed that gastric ultrasound assessment can be dependable with a sensitivity of 1.0 and specificity of 0.975 [42]. Objective evidence of absence/presence, quality, and quantity of gastric contents enables regional anesthesiologists to formulate a safe anesthetic plan. Choices may vary from definitively securing the airway with an endotracheal tube, keeping the patient awake, or sedating a patient with an unsecured airway. The gastric POCUS has been proven to be a useful tool to provide diagnostic information in real time to help guide these decisions. In a prospective case series of thirty-eight elective surgical patients, gastric ultrasound was reported to have influenced a change in anesthetic management in 71% of the cases who did not follow fasting instructions [43].
POCUS for Deep Vein Thrombosis
Regional anesthesiologists are familiar with identifying vascular structures on ultrasound as landmarks for peripheral nerve blocks and for vascular access. This enables them to be at an advantage for learning and performing deep vein thrombosis (DVT) ultrasound.
Trauma, immobilization, and surgery are well recognized risk factors for DVT. DVT is an acute condition that requires timely diagnosis and treatment to avoid life-threatening complications like pulmonary embolism. Radiology services to diagnose DVT by doing contrast venography or duplex ultrasound might not be available at all centers and learning POCUS for DVT is a valuable tool for practicing regional anesthesiologists. It is gaining widespread use and has proved to be both time-saving and cost-effective [44, 45, 46•]. Quicker diagnosis in turn can expedite treatment and alter surgical strategies that could be lifesaving.
Clinical scenario: A 37-year-old healthy male reported to a surgicenter for repair of left ankle fracture sustained three days back. The nurse admitting the patient reported an unusually high heart rate (around 110/mt) and other vitals essentially within normal limits. Patient denied any shortness of breath or other unusual symptoms. He also alluded to limited activity and mobility due to the fracture. No significant swelling or tenderness of either lower extremity was noted other than around the fractured ankle An ultrasound examination of lower extremities revealed a small echogenic shadow and non-compressibility of the left greater saphenous vein. The patient was subsequently sent to a hospital for further management that included anticoagulation and surgery was postponed to a later date |
Technique: Patient is positioned supine and preferably in slight reverse Trendelenburg position. The lower extremity is placed in a frog leg position. A high-frequency linear probe is used to obtain a cross-sectional view of the veins (Fig. 5). The goal is to visualize a segment of the venous lumen and compress to occlude the lumen (Figs. 6, 7, 8, and 9). There are two different techniques (2-point and 3-point) described and both include the proximal femoral and popliteal vein scanning. Scan of the proximal femoral segment should include a few centimeters above and below the junction of the great saphenous vein with the femoral vein. The popliteal segment should be scanned until it trifurcates into anterior tibial, posterior tibial, and peroneal veins. The addition to the 3-point scan is mid-femoral segment that includes bifurcation of common femoral to superficial and deep femoral veins.
Multiple studies have shown that POCUS has high sensitivity and specificity for diagnosis of DVT [47, 48]. POCUS for DVT involves identification of echogenic thrombus in the lumen of the deep vein or the inability to compress the vein completely. A meta-analysis showed both 2-point and 3-point techniques are reliable and had no significant differences in sensitivity or specificity [49].
Focused Cardiac Ultrasound (FOCUS)/ Focus Assessed Transthoracic Echocardiography (FATE)
FOCUS differs from a complete transthoracic echocardiogram in that it provides quick diagnosis with limited views and without quantitative measurements [50]. It is one of the most utilized POCUS modalities but one that requires more training and practice [51••]. Initially used in critical care units, its use has expanded to emergency rooms, wards, and perioperative settings. Guidelines and recommendations for use of cardiac ultrasound in critically ill patients can be applied during perioperative management as well [52].
In the preoperative phase, FOCUS can complement physical exam and assessment tools in diagnosis of cardiac diseases. In addition to a rapid assessment of global function, it is helpful in diagnosis of specific structural or functional cardiac pathologies previously undiagnosed [53, 54]. POCUS enabled medical students or residents to diagnose cardiac conditions better than experienced physicians without ultrasound [55, 56]. Incorporating POCUS in preoperative evaluation in the clinic or holding area is expected to become more common. In the practice of regional anesthesia, this would help in changing or modifying anesthetic plan, technique, and/or monitoring.
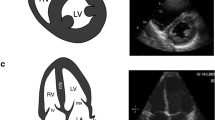
Technique: FOCUS requires a low-frequency cardiac phased array probe. The four basic views of FOCUS are parasternal long axis (PLAX) (Fig. 10), parasternal short axis (PSAX) (Fig. 11), apical four chamber (A4C) (Fig. 12), and subcostal four chamber (SC4C) (Fig. 13). Although basic views of FOCUS can be obtained in supine position, turning the patient left lateral and abducting the left arm can help in optimizing the images [57]. FOCUS during the intraoperative period could be limited by the surgical field (abdominal or thoracic) and inability to change the patient's position to optimize the image.
Clinical scenario: A 83-year-old lady was brought in for urgent surgery after a hip fracture. Her medical history included hypertension, hyperlipidemia, and glaucoma but a murmur was heard on physical examination in the holding area A focused cardiac ultrasound showed a calcified aortic valve with stenosis and hypertrophic left ventricle. The anesthesia team now aware of the patient’s cardiac status managed with general anesthesia for the surgery |
A myriad of cardiac conditions can be detected with FOCUS (Table 2), and it provides the ability to narrow the causes of refractory hypotension or hemodynamic instability during the perioperative period [58•, 59••]. FOCUS can be helpful in prompt diagnosis and management of hemodynamic instability resulting from adverse effects or complications of regional anesthesia such as local anesthetic systemic toxicity (LAST) [60]. It is particularly useful when there are dynamic or rapid changes in fluid status or cardiac function during the perioperative period.
Though the basic views can help with a rough estimate of volume status, subcostal long axis view (SCIVC) of inferior vena cava (IVC) (Fig. 14), measuring diameter and respiratory variation has been studied for assessing fluid status and responsiveness. The IVC diameter less than 2 cm and respiratory collapsibility more than 50% has been shown to be fluid responsive [59••]. The IVC diameter and collapsibility estimated with POCUS could predict hypotension on induction [61,62,63]. Other groups have shown this not to be very reliable [64, 65] and a meta-analysis concluded that the usefulness and reliability of these measures remains to be established [66]. These IVC indices probably are more valuable when combined with assessment from other cardiac views and correlating with the patient’s clinical history and present status.
The subcostal long axis view combined with subcostal four chamber and lung views have been used to provide information to describe seven different phenotypes in septic shock [67]. The authors have described a rapid assessment and classification that would identify different causes of shock (hypovolemic, distributive, and left or right or biventricular dysfunctions). They also describe how this assessment guides decisions on therapeutic interventions. This supports our belief that one should utilize multiple views for diagnosis and management rather than relying on one view or window.
Cardiac arrest is another critical and emergent event where FOCUS is helpful. FOCUS aids in diagnosis of the reversible causes of cardiac arrest, assessment of the quality of resuscitative efforts, monitoring responsiveness to the treatments, and prognosis of the patient [68]. Bughrara et al. showed that by incorporating subcostal only view in the advanced life support protocol (EASy-ALS), residents could consistently obtain valuable images during this critical period [69].
A prospective study showed that using POCUS for acute hypotension and hypoxia in a post anesthesia care unit (PACU) helps to narrow diagnosis and decreases PACU length of stay [16•]. It is not uncommon for a regional anesthesiologist to encounter hypotension and hypoxia in a patient recovering after a spinal anesthetic or brachial plexus block. POCUS could decrease time and resources needed to diagnose and manage these common situations in the postoperative period.
Focused Assessment with Sonography in Trauma (FAST)
The FAST exam is routinely utilized by the emergency medicine and surgical teams in quick screening of trauma patients to detect free fluid in the abdomen. In a detailed review, the basics of the FAST exam and its utility for regional anesthesiologists were described by Manson et al. [70]. A basic FAST exam includes views of the abdomen in the right upper quadrant, left upper quadrant, and pelvic cavity. An extended FAST (e-FAST) to evaluate pneumothorax, hemothorax, and pericardial effusion has also been described [71]. Various views for e-FAST, corresponding probe positions and common indications, are listed in Table 3. FAST can avoid delays in diagnosing free fluid in the abdominal or thoracic cavity, and in making timely decisions for patients who need surgical interventions.
Clinical scenario: A 43-year-old patient who was involved in a motor vehicle accident is brought to the operating room for repair of right tibial and fibular fractures. He had a negative FAST exam in the emergency room and was fluid resuscitated. On examination in the holding area of the operating room, the patient was hypotensive which did not respond to moderate fluid boluses and routine pressors A repeat FAST exam revealed presence of subdiaphragmatic free fluid in the left upper quadrant of the abdominal cavity. A laparotomy revealed a rupture in the spleen which was subsequently managed before proceeding with repair of right lower extremity fractures |
It is not uncommon for trauma patients to undergo changes in hemodynamic stability after an unremarkable initial assessment, so a careful eye should be kept for patients under observation/become unstable suddenly despite adequate resuscitation. FAST exam in the perioperative period can help with identifying causes for such changes and subsequently their management as well. Case reports have demonstrated that even in elective surgeries, the FAST exam enables quick diagnosis and management of life-threatening perioperative complications like extravasation of fluids [72, 73]. Post operative hypotension should be evaluated with FAST examination to rule out intrabdominal blood collection. Repeated examinations and assessments with FAST examinations after any treatments or interventions are beneficial.
Technique: The FAST exam has four components: subcostal cardiac, right upper quadrant (RUQ), left upper quadrant (LUQ), and pelvic views. Traditionally, the patient is placed in a supine position, and the views can be obtained in any order with most providers sticking to a pattern that is expeditious and complete. Either the curvilinear or phased array probes can be used.
To obtain the cardiac view the probe is placed in the subxiphoid or subcostal space just to the right of the xiphoid process and directed towards the patient’s left shoulder, indicator pointing left to visualize the pericardium and a four-chamber view of the heart (Fig. 15). The depth on the screen is usually 20–25 cm but can be changed as per the body habitus. Anterior to the heart will typically be a small section of the liver which abuts the right ventricle, with the left ventricle being the deepest structure visualized. From this view, the operator can assess pericardial fluid, global cardiac function, and many cardiac pathologies.
The RUQ view is captured by placing the ultrasound probe in the right mid axillary line at the level of the 9–10th rib or subcostally, with the indicator pointing towards the patient’s head. Ideally this orientation will show the liver and right kidney (Fig. 16). The operator may have to slide the probe caudad, cephalad, or posterior to visualize both structures. The hepatorenal space (Morrison’s pouch) is then centered to identify any fluid collection. Sweep the probe cranially to image the free edge of the liver. Slight rotation of the probe can help in elimination of rib shadows. Additionally, part of the right hemithorax can be visualized to identify pathology. The probe can be moved cephalad along the ribs to extend the FAST exam (e-FAST) and assess the lungs and pleura. Identification of the diaphragm is the key landmark to differentiate abdominal and thoracic pathologies.
For the LUQ view, the transducer is oriented in the sagittal plane along the left posterior axillary line at about the level of the 8th rib (Fig. 17). Ideally, this will reveal the spleen and left kidney, but oftentimes a full stomach can obscure the view. The probe may need to be slid posterior, caudad, and cephalad to identify the spleen which is smaller than the liver and less easily visualized. Once both the kidney and spleen are identified, the probe is fanned to identify any fluid collection in the splenorenal recess and the subdiaphragmatic space.
In order to obtain a view of the pelvis, the transducer is placed in the midline, just superior to the pubic symphysis, in the axial plane (Fig. 18). The presence of urine in the bladder will improve image quality. Sweeping the probe caudad and cephalad will allow examination of the bladder and rectovesical pouch.
FAST has been shown to have high specificity but varied or limited sensitivity [74, 75]. The limitation of the FAST is that a negative test cannot exclude intra-abdominal free fluid, but serial exams can improve the sensitivity, thus also changing the eventual management of the patient.
Bladder Ultrasound
POCUS assessment of the urinary bladder is an essential skill for anesthesiologists. Transabdominal scanning of the bladder is often used as a surrogate to guide pelvic FAST examinations. Bladder ultrasound can be used to assess bladder volume, obstruction, bladder pathologies like calculi, masses, and foley catheter placement.
Bladder volume assessment with POCUS is a quick and easy process at the bedside. The width and depth are measured in transverse view while the height is measured in sagittal view (Fig. 18). A simple formula to estimate bladder volume = width (cm) × depth (cm) × height (cm) × coefficient. The coefficient value depends on the shape of the bladder but is commonly taken as 0.6–0.7.
Clinical scenario: A PACU nurse calls your attention to a 68-year-old woman who recently arrived after her total knee arthroplasty surgery. She was concerned that the patient remained hypertensive, tachycardic (BP: 168/102 mm Hg, HR: 114/mt, EKG: sinus tachycardia) and uncomfortable despite not having recovered from the spinal anesthetic, with block height at T11 to ice. Her surgery was uneventful, and the patient received 1.5 l of IV fluids intraoperatively Bedside ultrasound of the urinary bladder showed a volume of about 700 ml. The bladder was drained with a single pass urinary catheter which provided immediate relief and resolution of cardiovascular symptoms |
Post-op bladder distension is a common cause for pain and high blood pressure among certain surgical patients in the post anesthesia care unit (PACU). Postoperative urinary retention (POUR) can be easily diagnosed by estimating the bladder volume and appropriate steps can be taken for the management [76, 77]. One of the important discharge criteria for ambulatory patients is their ability to void urine and not having high post void residual bladder volume especially after neuraxial blocks. Systematic and effective use of bladder scan can help determine if urinary catheterization is necessary, confirm proper placement of catheter, prevent unnecessary bladder interventions, and reduce urinary tract infection [78, 79•].
Lung Ultrasound (LUS)
Bedside lung ultrasound has been an established practice in critical care medicine and emergency departments. More recently, several articles have highlighted its uses and advantages in the perioperative period [6, 57, 80•, 81]. The enhanced role and utility of LUS in assessment, monitoring, and prognosis of critically ill patients during the pandemic cannot be overstated [82, 83].
Regional anesthesiologists can utilize LUS for assessment of pleura, alveolar interstitium, and diaphragm. LUS evaluation can help in narrowing diagnosis including pneumothorax (PTX), pulmonary edema, pleural effusion, or decreased diaphragmatic function [84]. There are several recommendations and protocols for comprehensive exam and interpretation of LUS [85,86,87] but perioperative application would require some modifications.
Technique: A high-frequency linear probe can be used for superficial structures like pleura. A curvilinear, low-frequency probe enables a complete LUS exam including the parenchyma, costo-phrenic angles, and diaphragm. Ideal patient position and area of scan varies depending on the clinical question and can be limited by clinical conditions (respiratory distress or surgical position). Most perioperative LUS are performed in supine position.
Pleura is identified as a bright hyperechoic line in between and deeper to rib shadows (Fig. 19). A normal pleura is shimmery and shows characteristic sliding. The presence of sliding in M-mode is described as a “seashore sign” and the absence of sliding as a “barcode sign.” Absence of sliding and “barcode sign” is suggestive of PTX and identification of “lung point” can be definitive for PTX.
Parenchymal or interstitial LUS (Fig. 20) primarily involves identification of B-lines. They are reverberation artifacts that look like comet tails from pleura all the way down to the lower end of the screen (Fig. 21). Presence of B-lines can be normal and confirms absence of PTX. The presence of more than 3 B-lines on the screen is suggestive of interstitial edema. Pleural effusion or hemothorax can be identified as an anechoic (dark) area in the costophrenic angles or dependent part of the thoracic cavity. Fluid in the pleural space can also enable the spine to be visualized above the diaphragm, described as ‘spine sign’ [88••].
Clinical Sscenario: A 68-year-old male with history of hypertension, dyslipidemia, coronary artery disease, and mild emphysema underwent a reverse shoulder arthroplasty under general anesthesia. Preoperative Interscalene block was performed for post-op analgesia. Postoperatively in the PACU, the patient complained of shortness of breath and oxygen saturation was 88%. Differential diagnoses included hemi-diaphragmatic palsy and pneumothorax A bedside ultrasound exam ruled out pneumothorax and confirmed decreased contraction of diaphragm on the side of the interscalene block. The patient was reassured, given nasal oxygen, and monitored until symptoms improved |
Ultrasound for assessment of diaphragmatic function is very helpful for regional anesthesiologists, as brachial plexus blocks at the level of trunks and cords give rise to a high incidence of phrenic nerve palsy. Diaphragm can be visualized through the hepatic and splenic shadows [89] or along the anterior axillary line described as “ABCDE method,” which looks at the zone of apposition [90]. M-mode can be used to measure the excursion or contraction of the diaphragm.
It is inherently evident that LUS is time saving, non-invasive, and cost effective when compared to chest X-ray (CXR) or computed tomography (CT). In addition, LUS was shown to be an equal or better alternative to CXR or CT in diagnosing pleural effusion, consolidation and interstitial syndrome [91]. A systematic review and meta-analysis concluded that LUS has better accuracy and sensitivity than CXR in diagnosing PTX [92, 93]. Similarly, LUS had better diagnostic accuracy than CXR for pulmonary edema in heart failure patients [94] and pneumonia [95].
The expanded role of LUS in thoracic anesthesia includes predicting the size of double lumen tube (DLT) and confirmation of one lung ventilation and can help in reducing the complications of DLT [96].
Ocular Ultrasound
Ultrasound examination of the eye is a non-invasive procedure, avoids the risk of ionizing radiation (scans to assess for eye trauma/intracranial pressure (ICP)), infection or hemorrhage and has a very steep learning curve. The eye is an ideal organ for ultrasound examination with fluid-filled structures that are clearly demarcated and can be a valuable addition to a regional anesthesiologist’s armamentarium for POCUS.
Ocular ultrasound can be used to diagnose/exclude foreign bodies, lens dislocation, retinal detachment, vitreous hemorrhage, raised intracranial pressure (ICP), and other injuries that may cause loss of vision. Optic nerve sheath diameter (ONSD) is increasingly being used in critical care and emergency departments to assess for raised ICP. POCUS for ONSD and the technique will be discussed here.
Optic nerve has four parts—intraocular (optic nerve head), intraorbital, intraosseous, and intracranial. Embryologically, it is an outpouching of the forebrain and is covered by all three meningeal layers. Approximately 3.0 mm posterior to the globe, there is a bulbous portion of the optic nerve which seems to be most sensitive to changes in ICP. The upper limit of optic nerve sheath diameter (ONSD) corresponding to a normal Intracranial pressure has been subject of various studies and systematic reviews and is approximately 5.0 mm in adults.
Clinical scenario: A 82-year-old lady who lived alone was brought to the emergency department at a small general hospital after an unwitnessed fall. On examination, she was a bit dazed and had a Glasgow coma scale of 14/15. She had superficial wounds on the left side of the face, some abrasions/bruises over the left arm and left neck of femur fracture. A senior orthopedic resident examined her, and she was scheduled for surgery under spinal anesthesia the following day after routine investigations. She was seen by the perioperative hip fracture team anesthesiologist who did optic ultrasound as part of the assessment (was not convinced with the vague history given by the patient). Optic nerve sheath diameter (ONSD) measurements were 4.0 mm on right and 6.5 mm on left. She had an urgent MRI scan, which showed chronic subdural hematoma. Neurosurgery opined for conservative management She underwent surgery the following day under general anesthesia and peripheral nerve blocks rather than a spinal anesthesia. The surgery was uneventful, and she was discharged to rehabilitation home on day 5 |
Technique: Linear high-frequency probe with a smaller footprint is preferable. Ultrasound should be set to ‘small parts’ to keep the acoustic output as low as possible to reduce injury to lens and retina. A barrier dressing on the patient’s closed eye upon which a copious amount of gel is applied. The probe is placed transversely on the eye (a sagittal view is also used) with care taken to not press too hard on the ocular structures. Operator’s hand can rest on the cheek/nasal bridge/forehead as appropriate. Small movements in all four planes (caudad/cephalad and temporal/nasal) are utilized to visualize the areas of interest in the same plane–anterior chamber (AC), lens, posterior chamber (PC), and the optic nerve sheath.
Once the image is captured, measurements are done with electronic calipers (Figs. 22 and 23). If there are discrepancies in the size seen while scanning, averaging out ONSD measurements is suggested. An overall clinical context is needed, and serial scans can be done for assessments over time. Color Doppler can visualize the retinal artery and may help as a reference point.
Various systematic reviews and meta-analyses have shown that ONSD of more than 5.0 mm in adults have a concurrent ICP value above 20 mm Hg. The sensitivity of this value is about 0.9 and specificity of approximately 0.86 across the data from mixed pathologies in patients. In essence, an ONSD of less than 5.0 mm with high sensitivity and low negative likelihood ratio may rule out increased ICP. However, with ONSD more than 5.0 mm with high specificity and positive likelihood ratio may indicate raised intracranial pressure and require other confirmatory diagnostic tests [97].
A systematic review by Propst et al. showed sensitivity and specificity of over 0.9 for a variety of conditions with ocular ultrasound including retinal detachment, vitreous hemorrhage, lens dislocation, intraocular foreign body, and globe rupture [98•]. The utility and feasibility of using ONSD and ophthalmic artery Doppler in pregnant patients to detect preeclampsia has been explored recently and is promising [99].
Conclusion
Along with the advances in ultrasound technology, newer applications of POCUS continue to evolve as well [79•]. The value of POCUS in the practice of anesthesiology has been widely recognized [80•, 88••, 100••]. The regional anesthesiologist is uniquely positioned to be leaders in the use of POCUS in the perioperative period. The technical skillset to perform regional anesthesia and familiarity with the ultrasound easily translates to POCUS skills. We reviewed the major elements of POCUS and how it can be used in various clinical situations relevant to the regional anesthesiologists’ daily clinical experiences. Perioperative POCUS, when utilized in a standardized, goal-directed method improves patient safety by providing timely diagnosis and intervention of clinical problems in the perioperative period. It is our strong belief that incorporating POCUS applications in regional anesthesia practice increases the level of perioperative care and improves patient outcomes.
References
Papers of particular interest, published recently, have been highlighted as: • Of importance •• Of major importance
Moore CL, Copel JA. Point-of-care ultrasonography. N Engl J Med. 2011;364(8):749–57.
Gillman LM, Kirkpatrick AW. Portable bedside ultrasound: the visual stethoscope of the 21st century. Scand J Trauma Resusc Emerg Med. 2012;9(20):18.
Emergency ultrasound guidelines. Ann Emerg Med. 2009;53(4):550–70.
Fagley RE, Haney MF, Beraud AS, Comfere T, Kohl BA, Merkel MJ, et al. Critical care basic ultrasound learning goals for american anesthesiology critical care trainees: recommendations from an expert group. Anesth Analg. 2015;120(5):1041–53.
Mahmood F, Matyal R, Skubas N, Montealegre-Gallegos M, Swaminathan M, Denault A, et al. Perioperative ultrasound training in anesthesiology: a call to action. Anesth Analg. 2016;122(6):1794–804.
Ramsingh D, Bronshteyn YS, Haskins S, Zimmerman J. Perioperative point-of-care ultrasound. Anesthesiology. 2020;132(4):908–16.
Diagnostic POCUS Certificate Program [Internet]. [cited 2023 Jun 27]. Available from: https://www.asahq.org/education-and-career/educational-and-cme-offerings/pocus
OSCE_Content_Outline_2022–02. Available from: https://www.theaba.org/wp-content/uploads/2022/12/OSCE_Content_Outline.pdf
•• Bronshteyn YS, Anderson TA, Badakhsh O, Boublik J, Brady MBW, Charnin JE, et al. Diagnostic point-of-care ultrasound: recommendations from an expert panel. J Cardiothorac Vasc Anesth. 2022 Jan 1;36(1):22–9. (American Society of Anesthesiologists’ ad hoc committee recommendations on applications, scope, training, safe and ethical use of perioperative POCUS.)
Meineri M, Arellano R, Bryson G, Arzola C, Chen R, Collins P, et al. Canadian recommendations for training and performance in basic perioperative point-of-care ultrasound: recommendations from a consensus of Canadian anesthesiology academic centres. Can J Anaesth J Can Anesth. 2021;68(3):376–86.
Mongodi S, Bonomi F, Vaschetto R, Robba C, Salve G, Volta CA, et al. Point-of-care ultrasound training for residents in anaesthesia and critical care: results of a national survey comparing residents and training program directors’ perspectives. BMC Med Educ. 2022;22(1):647.
• Jarman RD, Colclough A, McDermott C, Bøtker M, Knudsen L, Harris T, et al. EFSUMB clinical practice guidelines for point-of-care ultrasound: part one (common heart and pulmonary applications) short version. Ultraschall Med - Eur J Ultrasound. 2022;13:36–49 (Detailed analysis and recommendations for POCUS from European federation of societies for Ultrasound in Medicine and Biology).
Haskins SC, Boublik J, Wu CL. Point-of-care ultrasound for the regional anesthesiologist and pain specialist: a series introduction. Reg Anesth Pain Med. 2017;42(3):281–2.
Bahner DP, Hughes D, Royall NA. I-AIM: a novel model for teaching and performing focused sonography. J Ultrasound Med Off J Am Inst Ultrasound Med. 2012;31(2):295–300.
Meier I, Vogt AP, Meineri M, Kaiser HA, Luedi MM, Braun M. Point-of-care ultrasound in the preoperative setting. Best Pract Res Clin Anaesthesiol. 2020;34(2):315–24.
• Ramsingh D, Singh S, Canales C, Guran E, Taylor Z, Antongiorgi Z, et al. The evaluation point-of-care ultrasound in the post-anesthesia unit-a multicenter prospective observational study. J Clin Med. 2021;10(11):2389 (This multicenter study demonstrated the utility and value of POCUS in the Post Anesthesia Care Unit).
You-Ten KE, Siddiqui N, Teoh WH, Kristensen MS. Point-of-care ultrasound (POCUS) of the upper airway. Can J Anesth Can Anesth. 2018;65(4):473–84.
Gottlieb M, Holladay D, Burns KM, Nakitende D, Bailitz J. Ultrasound for airway management: an evidence-based review for the emergency clinician. Am J Emerg Med. 2020;38(5):1007–13.
Kristensen MS, Teoh WH, Rudolph SS, Hesselfeldt R, Børglum J, Tvede MF. A randomised cross-over comparison of the transverse and longitudinal techniques for ultrasound-guided identification of the cricothyroid membrane in morbidly obese subjects. Anaesthesia. 2016;71(6):675–83.
• Lin J, Bellinger R, Shedd A, Wolfshohl J, Walker J, Healy J, et al. Point-of-care ultrasound in airway evaluation and management: a comprehensive review. Diagnostics [Internet]. 2023 May [cited 2023 Jun 1];13(9). Available from: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC10177245/ (A review article discussing techniques and various uses of airway POCUS.)
Votruba J, Zemanová P, Lambert L, Vesela MM. The Role of airway and endobronchial ultrasound in perioperative medicine. BioMed Res Int. 2015;2015:754626.
Martínez-García A, Guerrero-Orriach JL, Pino-Gálvez MA. Ultrasonography for predicting a difficult laryngoscopy. Getting closer J Clin Monit Comput. 2021;35(2):269–77.
Falcetta S, Cavallo S, Gabbanelli V, Pelaia P, Sorbello M, Zdravkovic I, et al. Evaluation of two neck ultrasound measurements as predictors of difficult direct laryngoscopy: a prospective observational study. Eur J Anaesthesiol EJA. 2018;35(8):605.
Sustić A, Miletić D, Protić A, Ivancić A, Cicvarić T. Can ultrasound be useful for predicting the size of a left double-lumen bronchial tube? Tracheal width as measured by ultrasonography versus computed tomography. J Clin Anesth. 2008;20(4):247–52.
Roldi E, Inghileri P, Dransart-Raye O, Mongodi S, Guinot PG, Mojoli F, et al. Use of tracheal ultrasound combined with clinical parameters to select left double-lumen tube size: a prospective observational study. Eur J Anaesthesiol EJA. 2019;36(3):215.
Krause M, Khatibi B, Sztain JF, Rahman P, Shapiro AB, Sandhu NS. Ultrasound-guided airway blocks using a curvilinear probe. J Clin Anesth. 2016;33:408–12.
Ambi US, Arjun BK, Masur S, Endigeri A, Hosalli V, Hulakund SY. Comparison of ultrasound and anatomical landmark-guided technique for superior laryngeal nerve block to aid awake fibre-optic intubation: a prospective randomised clinical study. Indian J Anaesth. 2017;61(6):463–8.
Carsetti A, Sorbello M, Adrario E, Donati A, Falcetta S. Airway ultrasound as predictor of difficult direct laryngoscopy: a systematic review and meta-analysis. Anesth Analg. 2022;134(4):740–50.
• Benavides-Zora D, Jaramillo MC, Townsley MM, Franco V, González S, Hoyos C, et al. Diagnostic performance of airway ultrasound for the assessment of difficult laryngoscopy: a systematic review and meta-analysis. J Cardiothorac Vasc Anesth. 2023;37(7):1101–9 (This meta-analysis compares multiple measures used in airway POCUS for predicting difficult laryngoscopy).
Shime N, Ono A, Chihara E, Tanaka Y. Current status of pulmonary aspiration associated with general anesthesia: a nationwide survey in Japan. Masui. 2005;54(10):1177–85.
Kozlow JH, Berenholtz SM, Garrett E, Dorman T, Pronovost PJ. Epidemiology and impact of aspiration pneumonia in patients undergoing surgery in Maryland, 1999–2000. Crit Care Med. 2003;31(7):1930–7.
Cook TM, Woodall N, Frerk C, Fourth National Audit Project. Major complications of airway management in the UK: results of the Fourth National Audit Project of the Royal College of Anaesthetists and the Difficult Airway Society. Part 1: anaesthesia. Br J Anaesth. 2011 May;106(5):617–31.
Lienhart A, Auroy Y, Péquignot F, Benhamou D, Warszawski J, Bovet M, et al. Survey of anesthesia-related mortality in France. Anesthesiology. 2006;105(6):1087–97.
Delamarre L, Srairi M, Bouvet L, Conil JM, Fourcade O, Minville V. Anaesthesiologists’ clinical judgment accuracy regarding preoperative full stomach: diagnostic study in urgent surgical adult patients. Anaesth Crit Care Pain Med. 2021;40(3):100836.
Bouvet L, Mazoit JX, Chassard D, Allaouchiche B, Boselli E, Benhamou D. Clinical assessment of the ultrasonographic measurement of antral area for estimating preoperative gastric content and volume. Anesthesiology. 2011;114(5):1086–92.
Klein SR, Hobai IA. Semaglutide, delayed gastric emptying, and intraoperative pulmonary aspiration: a case report. Can J Anesth Can Anesth [Internet]. 2023 Mar 28 [cited 2023 May 26]; Available fromhttps://doi.org/10.1007/s12630-023-02440-3
Gulak MA, Murphy P. Regurgitation under anesthesia in a fasted patient prescribed semaglutide for weight loss: a case report. Can J Anaesth J Can Anesth. 2023 Jun 6;
American Society of Anesthesiologists consensus-based guidance on preoperative management of patients (adults and children) on glucagon-like peptide-1 (GLP-1) receptor agonists [Internet]. [cited 2023 Jul 25]. Available from: https://www.asahq.org/about-asa/newsroom/news-releases/2023/06/american-society-of-anesthesiologists-consensus-based-guidance-on-preoperative
• Jones PM, Hobai IA, Murphy PM. Anesthesia and glucagon-like peptide-1 receptor agonists: proceed with caution! Can J Anaesth J Can Anesth. 2023 Jul 19; (An editorial on perioperative management of patients on GLP-1 agonists.)
Perlas A, Chan VWS, Lupu CM, Mitsakakis N, Hanbidge A. Ultrasound assessment of gastric content and volume. Anesthesiology. 2009;111(1):82–9.
Haskins SC, Kruisselbrink R, Boublik J, Wu CL, Perlas A. Gastric ultrasound for the regional anesthesiologist and pain specialist. Reg Anesth Pain Med. 2018;43(7):689–98.
Kruisselbrink R, Gharapetian A, Chaparro LE, Ami N, Richler D, Chan VWS, et al. Diagnostic accuracy of point-of-care gastric ultrasound. Anesth Analg. 2019;128(1):89.
Alakkad H, Kruisselbrink R, Chin KJ, Niazi AU, Abbas S, Chan VWS, et al. Point-of-care ultrasound defines gastric content and changes the anesthetic management of elective surgical patients who have not followed fasting instructions: a prospective case series. Can J Anaesth J Can Anesth. 2015;62(11):1188–95.
Pinto SM, Yassin M, Galang G. Cost-effectiveness analysis of routine venous doppler ultrasound for diagnosis of deep venous thrombosis at admission to inpatient rehabilitation. Am J Phys Med Rehabil. 2018;97(10):747–53.
Varrias D, Palaiodimos L, Balasubramanian P, Barrera CA, Nauka P, Melainis AA, et al. The use of point-of-care ultrasound (POCUS) in the diagnosis of deep vein thrombosis. J Clin Med. 2021;10(17):3903.
• Barrosse-Antle ME, Patel KH, Kramer JA, Baston CM. Point-of-care ultrasound for bedside diagnosis of lower extremity DVT. Chest. 2021;160(5):1853–63. (This article describes common techniques and pitfalls associated with performing a DVT POCUS.)
Kory PD, Pellecchia CM, Shiloh AL, Mayo PH, DiBello C, Koenig S. Accuracy of ultrasonography performed by critical care physicians for the diagnosis of DVT. Chest. 2011;139(3):538–42.
Pedraza García J, Valle Alonso J, Ceballos García P, Rico Rodríguez F, Aguayo López MÁ, Muñoz-Villanueva M del C. Comparison of the accuracy of emergency department-performed point-of-care-ultrasound (POCUS) in the Diagnosis of Lower-Extremity Deep Vein Thrombosis. J Emerg Med. 2018 May 1;54(5):656–64.
Lee JH, Lee SH, Yun SJ. Comparison of 2-point and 3-point point-of-care ultrasound techniques for deep vein thrombosis at the emergency department. Medicine (Baltimore). 2019May 31;98(22):e15791.
Spencer KT, Kimura BJ, Korcarz CE, Pellikka PA, Rahko PS, Siegel RJ. Focused cardiac ultrasound: recommendations from the american society of echocardiography. J Am Soc Echocardiogr. 2013;26(6):567–81.
•• Johri AM, Glass C, Hill B, Jensen T, Puentes W, Olusanya O, et al. The Evolution of cardiovascular ultrasound: a review of cardiac point-of-care ultrasound (POCUS) across specialties. Am J Med [Internet]. 2023 Mar 6 [cited 2023 Jun 5];0(0). Available from: https://www.amjmed.com/article/S0002-9343(23)00158-4/fulltext. (Describes various applications of cardiac POCUS and its evolution into a general competency across multiple medical specialties.)
Levitov A, Frankel HL, Blaivas M, Kirkpatrick AW, Su E, Evans D, et al. Guidelines for the appropriate use of bedside general and cardiac ultrasonography in the evaluation of critically ill patients-part II: cardiac ultrasonography. Crit Care Med. 2016;44(6):1206–27.
Loxdale SJ, Sneyd JR, Donovan A, Werrett G, Viira DJ. The role of routine pre-operative bedside echocardiography in detecting aortic stenosis in patients with a hip fracture*. Anaesthesia. 2012;67(1):51–4.
Gerlach RM, Saha TK, Allard RV, Tanzola RC. Unrecognized tamponade diagnosed pre-induction by focused echocardiography. Can J Anesth Can Anesth. 2013;60(8):803–7.
Kobal SL, Trento L, Baharami S, Tolstrup K, Naqvi TZ, Cercek B, et al. Comparison of effectiveness of hand-carried ultrasound to bedside cardiovascular physical examination. Am J Cardiol. 2005;96(7):1002–6.
Razi R, Estrada JR, Doll J, Spencer KT. Bedside hand-carried ultrasound by internal medicine residents versus traditional clinical assessment for the identification of systolic dysfunction in patients admitted with decompensated heart failure. J Am Soc Echocardiogr. 2011;24(12):1319–24.
Li L, Yong RJ, Kaye AD, Urman RD. Perioperative point of care ultrasound (POCUS) for anesthesiologists: an overview. Curr Pain Headache Rep. 2020;24(5):20.
• Yoshida T, Yoshida T, Noma H, Nomura T, Suzuki A, Mihara T. Diagnostic accuracy of point-of-care ultrasound for shock: a systematic review and meta-analysis. Crit Care. 2023;25(27):200 (A recent meta-analysis on dependability of POCUS as a diagnostic tool in shock).
•• Bradley CA, Ma C, Hollon MM. Perioperative point of care ultrasound for hemodynamic assessment: a narrative review. Semin Cardiothorac Vasc Anesth. 2023;21:10892532231165088. (A review of perioperative POCUS for assessment, monitoring and treatment of hemodynamically unstable patients.)
Haskins SC, Tanaka CY, Boublik J, Wu CL, Sloth E. Focused cardiac ultrasound for the regional anesthesiologist and pain specialist. Reg Anesth Pain Med. 2017;42(5):632–44.
Zhang J, Critchley LAH. Inferior vena cava ultrasonography before general anesthesia can predict hypotension after induction. Anesthesiology. 2016;124(3):580–9.
Rose N, Chandra M, Nishanth CC, Srinivasan R. Preoperative ultrasonographic evaluation of subclavian vein and inferior vena cava for predicting hypotension associated with induction of general anesthesia. Anesth Essays Res. 2022;16(1):54–9.
Manzur-Sandoval D, Arteaga-Cárdenas G, Gopar-Nieto R, Lazcano-Díaz E, Rojas-Velasco G. Correlation between transhepatic and subcostal inferior vena cava ultrasonographic images for evaluating fluid responsiveness after cardiac surgery. J Card Surg. 2022;37(9):2586–91.
Turconi L, Cavalleri F, Moreno LG, Surbano M, Illescas L, Bouchacourt JP, et al. Inferior vena cava ultrasonography before general anesthesia cannot predict arterial hypotension in patients undergoing vascular surgery. Rev Esp Anestesiol Reanim Engl Ed. 2022;69(4):195–202.
Agarwal J, Panjiar P, Khanuja S, Annapureddy SKR, Saloda A, Butt KM. Correlation of preoperative inferior vena cava diameter and inferior vena cava collapsibility index with preoperative fasting status, patient demography and general anaesthesia associated hypotension: A prospective, observational study. Indian J Anaesth. 2022;66(Suppl 6):S320–7.
Orso D, Paoli I, Piani T, Cilenti FL, Cristiani L, Guglielmo N. Accuracy of ultrasonographic measurements of inferior vena cava to determine fluid responsiveness: a systematic review and meta-analysis. J Intensive Care Med. 2020;35(4):354–63.
Bughrara N, Diaz-Gomez JL, Pustavoitau A. Perioperative management of patients with sepsis and septic shock, part II: ultrasound support for resuscitation. Anesthesiol Clin. 2020;38(1):123–34.
Ávila-Reyes D, Acevedo-Cardona AO, Gómez-González JF, Echeverry-Piedrahita DR, Aguirre-Flórez M, Giraldo-Diaconeasa A. Point-of-care ultrasound in cardiorespiratory arrest (POCUS-CA): narrative review article. Ultrasound J. 2021;2(13):46.
Bughrara N, Herrick SL, Leimer E, Sirigaddi K, Roberts K, Pustavoitau A. Focused cardiac ultrasound and the periresuscitative period: a case series of resident-performed echocardiographic assessment using subcostal-only view in advanced life support. AA Pract. 2020;14(10):e01278.
Manson WC, Kirksey M, Boublik J, Wu CL, Haskins SC. Focused assessment with sonography in trauma (FAST) for the regional anesthesiologist and pain specialist. Reg Anesth Pain Med. 2019;44(5):540–8.
Montoya J, Stawicki SP, Evans DC, Bahner DP, Sparks S, Sharpe RP, et al. From FAST to E-FAST: an overview of the evolution of ultrasound-based traumatic injury assessment. Eur J Trauma Emerg Surg Off Publ Eur Trauma Soc. 2016;42(2):119–26.
Haskins SC, Desai NA, Fields KG, Nejim JA, Cheng S, Coleman SH, et al. Diagnosis of intraabdominal fluid extravasation after hip arthroscopy with point-of-care ultrasonography can identify patients at an increased risk for postoperative pain. Anesth Analg. 2017;124(3):791–9.
Sharma A, Bhattarai P, Sharma A. eFAST for the diagnosis of a perioperative complication during percutaneous nephrolithotomy. Crit Ultrasound J. 2018;10(1):7.
Stengel D, Leisterer J, Ferrada P, Ekkernkamp A, Mutze S, Hoenning A. Point-of-care ultrasonography for diagnosing thoracoabdominal injuries in patients with blunt trauma. Cochrane Database Syst Rev. 2018 Dec 12;12(12):CD012669.
Gaarder C, Kroepelien CF, Loekke R, Hestnes M, Dormage JB, Naess PA. Ultrasound performed by radiologists-confirming the truth about FAST in trauma. J Trauma. 2009 Aug;67(2):323–7; discussion 328–329.
Daurat A, Choquet O, Bringuier S, Charbit J, Egan M, Capdevila X. Diagnosis of postoperative urinary retention using a simplified ultrasound bladder measurement. Anesth Analg. 2015;120(5):1033–8.
Cha YH, Lee YK, Won SH, Park JW, Ha YC, Koo KH. Urinary retention after total joint arthroplasty of hip and knee: systematic review. J Orthop Surg. 2020;28(1):2309499020905134.
Palese A, Buchini S, Deroma L, Barbone F. The effectiveness of the ultrasound bladder scanner in reducing urinary tract infections: a meta-analysis. J Clin Nurs. 2010;19(21–22):2970–9.
• Wollenberg M, McConville S, Sanoja I, Schulman P, Khorashadi M, Benson M. New applications of perioperative POCUS: beyond the Big 4. Int Anesthesiol Clin. 2022 Summer;60(3):65. (A review article discussing perioperative POCUS applications beyond the common cardiac, lung and abdominal POCUS.)
• Byrne M, Singleton M, Kalagara H, Haskins SC. Perioperative point-of-care ultrasound Adv Anesth. 2021;39:189–213 (A descriptive article on common perioperative POCUS applications with clinical examples).
Goffi A, Kruisselbrink R, Volpicelli G. The sound of air: point-of-care lung ultrasound in perioperative medicine. Can J Anaesth J Can Anesth. 2018;65(4):399–416.
Smith MJ, Hayward SA, Innes SM, Miller ASC. Point-of-care lung ultrasound in patients with COVID-19 – a narrative review. Anaesthesia. 2020;75(8):1096–104.
Vetrugno L, Mojoli F, Boero E, Berchialla P, Bignami EG, Orso D, et al. Level of diffusion and training of lung ultrasound during the COVID-19 pandemic - a National Online Italian Survey (ITALUS) from the lung ultrasound working group of the Italian Society of Anesthesia, Analgesia, Resuscitation, and Intensive Care (SIAARTI). Ultraschall Med Stuttg Ger 1980. 2022 Oct;43(5):464–72.
Haskins SC, Tsui BC, Nejim JA, Wu CL, Boublik J. Lung ultrasound for the regional anesthesiologist and acute pain specialist. Reg Anesth Pain Med. 2017;42(3):289–98.
Royse AG, Lui E, Gai D, Cid X, Canty D, Wang A, et al. Three zone scanning protocol for lung ultrasound: an anatomical basis. Heart Lung Circ. 2023;32(2):247–51.
Volpicelli G, Elbarbary M, Blaivas M, Lichtenstein DA, Mathis G, Kirkpatrick AW, et al. International evidence-based recommendations for point-of-care lung ultrasound. Intensive Care Med. 2012;38(4):577–91.
Lichtenstein DA. BLUE-protocol and FALLS-protocol: two applications of lung ultrasound in the critically ill. Chest. 2015;147(6):1659–70.
•• Kalagara H, Coker B, Gerstein NS, Kukreja P, Deriy L, Pierce A, et al. Point-of-care ultrasound (POCUS) for the cardiothoracic anesthesiologist. J Cardiothorac Vasc Anesth. 2022;36(4):1132–47. (This article discusses multiple applications and the utility of POCUS in perioperative care and intensive care.)
Gottesman E, McCool FD. Ultrasound evaluation of the paralyzed diaphragm. Am J Respir Crit Care Med. 1997;155(5):1570–4.
Tsui JJ, Tsui BCH. A novel systematic ABC approach to diaphragmatic evaluation (ABCDE). Can J Anaesth J Can Anesth. 2016;63(5):636–7.
Lichtenstein D, Goldstein I, Mourgeon E, Cluzel P, Grenier P, Rouby JJ. Comparative diagnostic performances of auscultation, chest radiography, and lung ultrasonography in acute respiratory distress syndrome. Anesthesiology. 2004;100(1):9–15.
Ebrahimi A, Yousefifard M, Mohammad Kazemi H, Rasouli HR, Asady H, Moghadas Jafari A, et al. Diagnostic accuracy of chest ultrasonography versus chest radiography for identification of pneumothorax: a systematic review and meta-analysis. Tanaffos. 2014;13(4):29–40.
Chan KK, Joo DA, McRae AD, Takwoingi Y, Premji ZA, Lang E, et al. Chest ultrasonography versus supine chest radiography for diagnosis of pneumothorax in trauma patients in the emergency department. Cochrane Database Syst Rev. 2020 Jul 23;7(7):CD013031.
Maw AM, Hassanin A, Ho PM, McInnes MDF, Moss A, Juarez-Colunga E, et al. Diagnostic accuracy of point-of-care lung ultrasonography and chest radiography in adults with symptoms suggestive of acute decompensated heart failure: a systematic review and meta-analysis. JAMA Netw Open. 2019;2(3):e190703.
Ye X, Xiao H, Chen B, Zhang S. Accuracy of lung ultrasonography versus chest radiography for the diagnosis of adult community-acquired pneumonia: review of the literature and meta-analysis. PLoS ONE. 2015;10(6):e0130066.
Bignami E, Maffezzoni M, Bellini V. Lung ultrasound in thoracic anesthesia: which uses? J Cardiothorac Vasc Anesth. 2021;35(2):374–5.
Koziarz A, Sne N, Kegel F, Nath S, Badhiwala JH, Nassiri F, et al. Bedside optic nerve ultrasonography for diagnosing increased intracranial pressure: a systematic review and meta-analysis. Ann Intern Med. 2019;171(12):896–905.
• Propst SL, Kirschner JM, Strachan CC, Roumpf SK, Menard LM, Sarmiento EJ, et al. Ocular point-of-care ultrasonography to diagnose posterior chamber abnormalities: a systematic review and meta-analysis. JAMA Netw Open. 2020;3(2):e1921460. (A good review and meta-analysis of Ocular POCUS.)
Yamaguchi E, Ferraz A, Lange MC. Optic nerve sheath diameter and ophthalmic artery doppler in pregnant patients. Can ocular sonography play a role as a point-of-care ultrasound for anaesthesiologists?: a pilot study. Eur J Anaesthesiol. 2023 Jul 1;40(7):531–4.
•• Haskings EM, Eissa M, Allard RV, MirGhassemi A, McFaul CM, Miller EC. Point-of-care ultrasound use in emergencies: what every anaesthetist should know. Anaesthesia. 2023;78(1):105–18. (Discusses POCUS applications during perioperative emergencies like shock, cardiac arrest, and hypoxemia.)
Acknowledgements
We would like to thank Cleveland Clinic and Ms. Nandita Senthil for their assistance with illustrations.
Author information
Authors and Affiliations
Contributions
S.A, K.B, P.A.S, and H.K wrote the main manuscript. S.K prepared figures 1-4 and 6-21, S.A prepared figure 5, H.K prepared figures 22-23. All authors reviewed the manuscript.
Corresponding author
Ethics declarations
Competing interest
The corresponding author (PK) is a section editor for this journal. All other authors declare no competing or conflict of interests.
Human and animal rights
This article does not contain any studies with human or animal subjects performed by any of the authors.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Arumugam, S., Kolli, S., Brakoniecki, K. et al. Point-of-care Ultrasound (POCUS) for the Regional Anesthesiologist. Curr Anesthesiol Rep 14, 231–248 (2024). https://doi.org/10.1007/s40140-024-00622-3
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40140-024-00622-3