Abstract
Background
Pulse pressure variation (PPV) and stroke volume variation (SVV) are dynamic preload variables that can be measured noninvasively to assess fluid responsiveness (FR) in anesthetized patients with mechanical ventilation. Few studies have examined the effectiveness of predicting FR according to the definition of FR, and assessment of inconclusive values of PPV and SVV around the cut-off value (the “grey zone”) might improve individual FR prediction. We explored the ability of noninvasive volume clamp derived measurements of PPV and SVV to predict FR using the grey zone approach, and we assessed the influence of multiple thresholds on the predictive ability of the numerical definition of FR.
Methods
Ninety patients undergoing general surgery were included in this prospective observational study and received a 500 mL fluid bolus as deemed clinically required by the attending anesthesiologist. A minimal relative increase in stroke volume index (↑SVI) was used to define FR with different thresholds from 10-25%. The PPV, SVV, and SVI were measured using the Nexfin® device that employs noninvasive volume clamp plethysmography.
Results
The area under the receiver operator characteristic curve gradually increased for PPV / SVV with higher threshold values (from 0.818 / 0.760 at 10% ↑SVI to 0.928 / 0.944 at 25% ↑SVI). The grey zone limits of both PPV and SVV changed from 9–16% (PPV) and 5–13% (SVV) at the 10% ↑SVI threshold to 18–21% (PPV) and 14–16% (SVV) at the 25% ↑SVI threshold.
Conclusion
Noninvasive PPV and SVV measurements allow an acceptable FR prediction, although the reliability of both variables is dependent on the intended increase in SVI, which improves substantially with concomitant smaller grey zones at higher ↑SVI thresholds.
Résumé
Contexte
La variation de pression différentielle (VPD) et la variation de volume d’éjection (VVE) sont des variables de précharge dynamiques qui peuvent être mesurées de façon non invasive afin d’évaluer la réponse liquidienne (RL) chez les patients anesthésiés sous ventilation mécanique. Peu d’études ont examiné l’efficacité de prédire la RL selon la valeur prédéterminée d’interruption du traitement, et l’évaluation de valeurs non concluantes de VPD et de VVE (la « zone grise ») pourrait améliorer la prédiction de la RL individuelle. Nous avons exploré l’utilisation de la méthode à volume imposé (« volume clamp method ») pour les mesures non invasives de la VPD et de la VVE afin de prédire la RL en utilisant l’approche de zone grise, et nous avons évalué l’influence de plusieurs seuils sur la capacité prédictive de la définition numérique de la RL.
Méthode
Quatre-vingt dix patients subissant une chirurgie générale ont été inclus dans cette étude observationnelle prospective et ont reçu un bolus liquidien de 500 mL lorsque l’anesthésiologiste en charge a jugé le bolus était nécessaire d’un point de vue clinique. Une augmentation relative minimale de l’indice de volume d’éjection (↑IVE) a été utilisée pour définir la RL avec différents seuils allant de 10 à 25 %. Les VPD, VVE et IVE ont été mesurés à l’aide d’un dispositif Nexfin® qui se fonde sur une pléthysmographie à volume imposé non invasive.
Résultats
La surface sous la courbe ROC a progressivement augmenté pour la VPD et la VVE avec des valeurs seuils plus élevées (de 0,818 / 0,760 à 10 % ↑IVE à 0,928 / 0,944 à 25 % ↑IVE). Les limites de zone grise de la VPD et de la VVE ont changé lorsqu’on a atteint le seuil de 10 % ↑IVE (de 9-16 % et 5-13 %, respectivement) et au seuil de 25 % ↑IVE (de 18-21 % et 14-16 %, respectivement).
Conclusion
Les mesures non invasives de la VPD et de la VVE permettent de prédire de façon acceptable la RL, bien que la fiabilité de ces deux variables dépende de l’augmentation prévue de l’IVE, qui s’améliore considérablement avec des zones grises concomitantes plus petites à des seuils plus élevés d’↑IVE.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Determination of perioperative fluid responsiveness (FR), i.e., predicting whether cardiac output (CO) increases in response to fluid administration, has been extensively studied in the past decade.1 Dynamic (preload) variables that rely on heart-lung interactions during volume-controlled mechanical ventilation2 have been shown to be good predictors of FR.1,3-5 Pulse pressure variation (PPV) and stroke volume variation (SVV) are the most well-known and validated dynamic variables1 and are usually derived by waveform analysis. Noninvasive hemodynamic monitoring devices, such as the Nexfin® (or its successor, the ClearSight® monitor; Edwards Lifesciences, Irvine, CA, USA), provide continuous measurements of arterial blood pressure and dynamic variables using volume clamp plethysmography.6-8 Multiple studies have shown that these noninvasively derived dynamic variables are as reliable as their invasively derived counterparts in predicting FR.9-11
One specific cut-off value of a dynamic variable is usually defined to distinguish fluid responders from non-responders. The use of such a “binary” analysis may often be inappropriate, especially considering different patient’s comorbidities and clinical conditions. This problem may be partially overcome using a “grey zone” approach,12-14 which identifies a range of values of dynamic variables where its predictive ability is inconclusive and where subsequent guidance of fluid administration must be directed according to traditional clinical signs (e.g., comorbidity, fluid “history”, and hemodynamic variables such as blood pressure and heart rate).
Furthermore, substantial differences exist in the literature1 regarding the chosen numerical definition of FR, which is a limitation both in comparing results from different studies investigating FR as well as in translating individual study results into clinical decision-making algorithms.
The aim of this prospective observational study was to determine the effectiveness of noninvasive PPV and SVV measurements obtained with the volume clamp device to predict FR using the grey zone approach. Secondly, because of the differences in thresholds for defining FR in the literature, we investigated the prediction of FR by PPV and SVV for a wide range of definitions of FR.
Methods
The local Medical Ethics Committee approved this prospective observational study in April 2011 (METc2011.052; University Medical Center Groningen, University of Groningen, Groningen, Netherlands), and written informed consent was obtained from the study participants. Adult patients undergoing various types of surgery (Table 1) under general anesthesia were included in the study if the following criteria were met: tracheal intubation was performed, mechanical ventilation was applied in a volume-controlled mode, and administration of a fluid bolus was deemed necessary by the attending anesthesiologist for clinical reasons (e.g., clinical signs of hypovolemia or anticipated blood loss). Patients were not included in the study if they had cardiac arrhythmia or if they underwent surgical procedures associated with altered intrathoracic or intra-abdominal pressure (e.g., laparoscopy, open-chest conditions).15
General anesthesia was induced using propofol with either sufentanil or remifentanil. Anesthesia was maintained with a target-controlled infusion of propofol or with sevoflurane in combination with a target-controlled infusion of sufentanil or remifentanil. Tracheal intubation was facilitated by the administration of rocuronium, and repeated doses of neuromuscular blocking agents were administered only if deemed necessary. Patients received 1-3 mL·kg−1·hr−1 of crystalloid solution as baseline maintenance fluids according to our standard intraoperative management.
Volume-controlled mechanical ventilation (≥ 8 mL·kg−1 lean body mass) was performed with an inspired oxygen fraction of 0.3-0.4, with the respiratory rate adjusted to maintain normocapnia (end-tidal CO2 = 34-41 mmHg).
The Nexfin finger cuff was attached to the middle phalanx of the patient’s left or right hand.
A comprehensive description of the technology behind the Nexfin (and ClearSight) volume clamp technique has been reported previously.16,17 In short, the finger cuff pressure is adjusted to keep the arterial blood volume of the finger – measured by plethysmography - at a constant level (i.e., volume clamping). By using a high-speed feedback loop at 1,000 Hz, the cuff pressure is adjusted to keep the arterial wall “unloaded”, and the cuff pressure reflects the arterial blood pressure. An algorithm (Physiocal®) is designed to ascertain the “unloaded state”, which regularly compensates for any changes in vasomotor tone that might influence the pressure–volume relationship between the finger cuff and the arterial blood volume. A five-minute time period was allowed to attain maximal vascular unloading of the finger and for calibration of the Nexfin. After this period, continuous data recording was started and baseline values were determined just prior to fluid bolus administration. Also, a heart reference system, which corrects for hydrostatic pressure differences between the finger and the level of the heart, was used for continuous adjustment of the pressures to the level of the right atrium.
All measurements were performed in a hemodynamically stable phase during maintenance of anesthesia and at least 20 min after anesthesia induction. By placement of opaque tape on the monitor screen, the attending anesthesiologist was blinded to all Nexfin data other than blood pressure. All patients received a standardized single infusion of 6% hydroxyethyl starch solution 500 mL (Voluven®, Fresenius, Bad Homburg, Germany) over a five to ten-minute period. No changes in ventilator setting, table positioning, anesthetic levels, or vasoactive medications were made in a 20-min time period before data recording.
Hemodynamic data were recorded continuously and subsequently extracted using Nexfin@PC software (BMEYE, Amsterdam, Netherlands; Edwards Lifesciences, Irvine, CA, USA). All data were then imported into Microsoft Excel 2010® (Microsoft, Redmond, WA, USA) and plotted for visual inspection and removal of obvious artifacts. The data were then synchronized and pooled. Retrospectively, to provide an assessment of preoperative health status, the New York Heart Association Functional Classification and the Metabolic Equivalent Task score were also estimated.
Statistical analysis
Statistical analysis was performed using Microsoft Excel 2010, PASW Statistics 18 (SPSS® Inc, Chicago, IL, USA), and R software. The distribution of variables regarding patient characteristics and hemodynamic variables was assessed for normality using the Lilliefors test. Parametric data (Lilliefors test P > 0.05) were expressed as mean (SD) and non-parametric data (Lilliefors test P < 0.05) were expressed as median [interquartile range; IQR]. Categorical data were expressed as number of patients (%). A 30-sec median was calculated for all hemodynamic variables prior to the start and after the end of fluid administration. The paired Student’s t test was used to compare hemodynamic variables before and after fluid administration.
The correlation between both PPV and SVV and the percentage change in SVI was depicted in a scatter plot and coefficients of determination (R2) were calculated.
For each 5% step increase in SVI threshold (abbreviated as ↑SVI) throughout the range of 10-25%, patients were split into groups of fluid responders and non-responders. Receiver operator characteristic (ROC) analysis was performed for all thresholds, and areas under the ROC curve (AUROC) were calculated. To give more robust estimates of AUROC values and grey zone limits, the data were bootstrapped in order to limit the influence of outliers. Bootstrapping was performed by creating 1,000 ROC curves per ↑SVI threshold and per dynamic variable (PPV, SVV). R software was used for bootstrapping, and ROC curves were generated using the ROCR software package, and a mean ROC curve was then calculated per ↑SVI threshold.18,19 DeLong methodology was applied to test for differences between these ROC curves using the pROC package. For the interested reader, the technique of ROC curve calculation from bootstrapped data is further discussed in the online Electronic Supplementary Material.
The grey zones were calculated over the bootstrapped data as previously described12 using two methods: 1) by calculating the range of values for which sensitivity and/or specificity is < 90% (“inconclusive” results) and 2) by calculating the 95% confidence interval around the calculated optimal threshold for determining FR. These optimal thresholds were calculated using the Youden index which equals sensitivity + specificity − 1. One Youden index was calculated per bootstrap sample. In accordance with the original publication of the grey zone approach,12 the widest obtained grey zone interval obtained by one of these methods was selected as the final grey zone. At the moment of study design, no data on different SVI thresholds were available upon which to base the sample size calculation. Therefore, the sample size was calculated to ensure that at least a 10% increase in SVI - which is the lowest SVI threshold – could be detected. Pilot observations in our institution revealed a mean SVI of 37 mL·m−2 prior to fluid bolus administration. In order to allow a two-sided paired Student’s t test to detect a 10% change in SVI (with an α set at 0.05 and a β set at 0.10), we calculated that at least 82 patients should be included. We chose to include 90 patients in order to account for possible dropouts.
Results
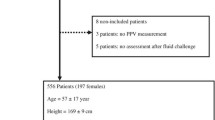
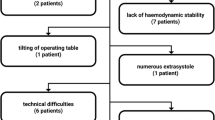
Measurements were performed in 90 patients, nine of whom were excluded for further analysis because of either new cardiac arrhythmia (n = 3, which interferes with accurate measurements of PPV and SVV) or technical difficulties with data recording (n = 4, insufficient waveform quality; n = 2, failure of the data collection system).
Patient characteristics (n = 81) are shown in Table 1.
The median [IQR] duration of fluid bolus infusion was 385 [315-529] sec. In addition, the median [IQR] tidal volume was 10.9 [10.1-11.7] mL·kg−1 of lean body mass.
In terms of the hemodynamic response to fluid administration, the mean (SD) heart rate was 68 (15) beats·min−1 before fluid administration and 67 (14) beats·min−1 in the 30 sec following the end of fluid administration (P = 0.469), while the mean arterial pressure increased from 75 (15) mmHg to 81 (17) mmHg (P < 0.0001). The SVI increased from 40 (11) mL·m−2 to 45 (10) mL·m−2 (P < 0.0001). The mean (SD) relative change of SVI after fluid administration was 16 (12)%, with a range of -11 to 46%.
Mean (SD) PPV and SVV were 14 (7)% and 12 (6)%, respectively, before volume expansion and 7 (4)% and 6 (3)%, respectively, thereafter (P < 0.0001). The relative change in SVI was correlated with PPV and SVV with an R2 value of 0.41 and 0.39, respectively; P < 0.0001 (Fig. 1).
As for the influence of the FR definition on the predictive value, the mean ROC curves and mean [99% confidence interval (CI)] AUROC values for the investigated ↑SVI thresholds are shown in Fig. 2A for PPV and in Fig. 2B for SVV. For PPV, the AUROC values of all investigated ↑SVI thresholds were significantly different from each other (P < 0.0001), except for the difference between the PPV values at the 10% and 15% ↑SVI threshold (P = 0.654). For SVV, all investigated AUROC values were significantly different from each other (P < 0.0001). The original ROC curves, from which the mean ROC curves were derived, are shown in the online Electronic Supplementary Material.
A-B ROC curves for the prediction of fluid responsiveness for PPV (2A) and SVV (2B), n = 81. Shown are the mean ROC curves and the AUROC values together with the associated 99% confidence intervals for the investigated ↑SVI thresholds. AUROC = area under the receiver operator characteristic curve; PPV = pulse pressure variation; ROC = receiver operator characteristic; SVI = stroke volume index; SVV = stroke volume variation
In addition, values of sensitivity and specificity for predicting FR at the investigated ↑SVI thresholds are shown in Fig. 3A-B for PPV (A) and SVV (B).
A-B Graph showing the sensitivity (dashed curves) and specificity (solid curves) of the PPV (3A) and SVV (3B) to predict increases in stroke volume (↑SVI) after 500 mL fluid administration, n = 81. In addition, the range of the grey zone (dashed horizontal lines) of the PPV and SVV are shown for the different ↑SVI thresholds. The small dashed horizontal line at sensitivity / specificity of 0.9 shows the intercepts of the grey zone limits for the different ↑SVI thresholds. PPV = pulse pressure variation; SVV = stroke volume variation
The grey zone limits of all investigated ↑SVI threshold values (horizontal lines) for PPV (A) and SVV (B) are displayed in Fig. 3. The grey zone limits of the PPV and SVV changed from 9-16% and from 5-13%, respectively, at the 10% ↑SVI threshold and from 18-21% and 14-16%, respectively, at the 25% ↑SVI threshold. Table 2 further summarizes the grey zone limits of both dynamic variables. In addition, the number of responders and non-responders per ↑SVI threshold are shown together with the percentage of patients having a PPV or SVV value below, within, or above the grey zone.
Discussion
We found that the ability of noninvasively derived PPV and SVV measurements to discriminate between fluid responders and non-responders improved substantially for larger ↑SVI thresholds, which was also reflected by narrower grey zone limits at the higher thresholds. This finding suggests that fluid (non-)responsiveness can be predicted with more certainty with a larger increase in SVI vs a more subtle increase in SVI. These results can be used clinically to improve guidance of fluid therapy and to identify an appropriate threshold for defining FR if a clinical algorithm is designed with FR and dynamic variables as a treatment goal.
In a meta-analysis on the ability of PPV and SVV to predict FR, the mean (SD) threshold to predict FR was 12.5 (1.6)% for PPV and 11.6 (1.9)% for SVV.1 Nevertheless, the definition of FR varies widely. The influence of the height of the definition of FR on the prediction of FR was not investigated in this meta-analysis, and other studies investigating this subject are also lacking. Generally, a ↑SVI cut-off value of 10-15% is regarded as an optimal threshold for predicting FR in most clinical settings.1 Multiple studies have investigated the ability to predict FR by dynamic variables using the grey zone analysis, mostly with a threshold for defining FR set at around an equivalent 10-15% ↑SVI threshold. This yields a grey zone at about 9-14%, with 25% of patients with PPV or SVV within this range.12,13,20-22 The grey zone limits we observed in this study were comparable at these equivalent ↑SVI thresholds, although the proportion of patients with PPV and SVV values within these limits was larger (40-50%). Interestingly, the limits were narrower at the 20% and 25% ↑SVI threshold, and most importantly, the proportion of patients with either PPV or SVV within these limits decreased (Table 2).
The different ↑SVI thresholds also show marked differences with respect to sensitivity and specificity, e.g., the 25% threshold is associated with a good ability of PPV and SVV to predict FR, especially reflected by a high sensitivity (Fig. 3). In contrast, at the 10% threshold, both variables have only a moderate ability to predict FR (lower sensitivity), yet with a higher specificity (Fig. 3).
The differences between FR thresholds and their adequacy to predict FR may be partly a mathematical consequence applicable to dynamic variables derived from any monitoring device. Also, physiologic variation in SVI may account for the decreased sensitivity at lower thresholds, as more patients will be false positively identified as fluid responders.
Still, it is important to keep these different thresholds in mind when defining FR for implementation in a clinical decision-making algorithm as well as in the design of future clinical studies.
Clinically, it remains up to the anesthesiologist to decide the extent to which an increase in CO is deemed necessary in the individual patient and, more importantly, whether inadvertent hypovolemia or fluid overload should be avoided.23 For example, in a critically ill patient at risk for pulmonary edema, it may be more deleterious to administer fluids inappropriately. In contrast, in a patient with a significant aortic valve stenosis, hypovolemia would be deleterious. In the first case, the clinician should favour a high specificity (and thus a higher ↑SVI threshold), while in the second case, a high sensitivity should be favoured (with a lower ↑SVI threshold). These data strongly suggest that the observed PPV or SVV value in an individual patient will be more conclusive for higher thresholds. Concisely stated, PPV or SVV values below the limits of the grey zone indicate that fluid should not be administered, while values above the limits of the grey zone strongly suggest that the patient will benefit from fluid administration. For patients with PPV or SVV values within the grey zone, the decision whether or not to administer fluid will then be dominantly determined by patient comorbidity or other individual clinical factors, as the PPV or SVV value can be regarded as inconclusive.
Recent insights have resulted in a dramatic reduction in the use of synthetic colloids, and we would not advocate systematically administering colloids to treat hypovolemia. Our conclusion on the use of PPV and SVV primarily addresses the prediction of FR, which is equally applicable to FR with crystalloids, although larger volumes may be necessary to attain the same level of intravascular volume expansion.
There were some limitations to our study. Hemodynamic variables were derived noninvasively using the volume clamp method. There are conflicting reports on the agreement of this measurement method with CO measurements derived from reference methods.8,24-28 Therefore, an important limitation of the current study is the absence of a gold standard technique for measuring CO. Most of the negative reports24,25 involved patient populations that differed from ours, that is, these other studies focused on “sicker” patients who were admitted to the intensive care unit and mostly required vasopressor support or patients who had just been weaned from cardiopulmonary bypass during cardiac surgery. In contrast, while it was the clinical decision of the attending anesthesiologist to administer colloids, we studied patients in an otherwise relatively stable hemodynamic phase. Also, we used only relative changes in SVI to define FR. We therefore expect that the possible influence of measurement bias on the observed results is substantially reduced.29 Nevertheless, the current results should be interpreted with caution because of possible inaccuracy in measuring SVI using the applied measurement method. This might by itself explain some degree of difference between the subgroups of SVI thresholds. Therefore, future studies require the application of more validated measurement methods to confirm the current results. In addition, future studies should also assess the influence of cardiac function, as we did not perform a prospective evaluation of cardiac function in this study; consequently, the degree to which our conclusions are applicable in patients with varying levels of cardiac dysfunction is unclear.
A further limitation is the fact that the FR and dynamic variables we studied were both determined from the same signal, which might lead to mathematical coupling of the investigated variables. Ideally, CO data should have been obtained using an alternative independent approach. Furthermore, we did not compare noninvasively derived PPV and SVV measurements with invasively derived dynamic variables. Yet, we have previously shown that invasive and noninvasive dynamic variables have a similar ability to predict FR.9 In addition, two reports10,28 have shown that dynamic variables derived by the volume clamp method are closely correlated with dynamic variables measured invasively before and after volume loading in post-cardiac surgical patients. Nevertheless, it was recently suggested that invasive variables may still provide the best ability to predict FR.30
In four (4.4%) out of 90 patients, we could not derive an adequate waveform quality by the volume clamp method, which is a limitation of this noninvasive technique. Finally, dynamic variables can reliably predict FR only if certain clinical conditions are met, e.g., sinus rhythm, volume-controlled mechanical ventilation with tidal volumes ≥ 8 mL·kg−1 in closed-chest conditions.31 Unfortunately, it has been previously shown that, when considering all patients presenting for any type of surgery and any form of anesthesia, a valid interpretation of FR using dynamic variables is possible in only 39% of cases.31,32 Nevertheless, all our included patients met the required clinical criteria, assuring that PPV and SVV allowed a valid interpretation of FR.
In conclusion, the ability of PPV and SVV values derived by the noninvasive volume clamp method to predict FR was adequate at all investigated ↑SVI thresholds, although with increasing thresholds, their ability similarly increases and grey zone limits narrow. Future studies should apply reference methods of measuring SVI to validate these results. Nevertheless, before a final decision can be made whether or not to administer fluids, the current data suggest that absolute values of dynamic variables should not be the only consideration. The intended increase in CO as well as patient comorbidity and patient-specific risk factors regarding fluid-related risks for hypo- or hypervolemia should also be considered for assessing FR.
References
Marik PE, Cavallazzi R, Vasu T, Hirani A. Dynamic changes in arterial waveform derived variables and fluid responsiveness in mechanically ventilated patients: a systematic review of the literature. Crit Care Med 2009; 37: 2642-7.
Pinsky MR. Heart lung interactions during mechanical ventilation. Curr Opin Crit Care 2012; 18: 256-60.
Osman D, Ridel C, Ray P, et al. Cardiac filling pressures are not appropriate to predict hemodynamic response to volume challenge. Crit Care Med 2007; 35: 64-8.
Michard F, Teboul JL. Predicting fluid responsiveness in ICU patients: a critical analysis of the evidence. Chest 2002; 121: 2000-8.
Hofer CK, Cecconi M, Marx G, della Rocca G. Minimally invasive haemodynamic monitoring. Eur J Anaesthesiol 2009; 26: 996-1002.
Vos JJ, Poterman M, Mooyaart EA, et al. Comparison of continuous non-invasive finger arterial pressure monitoring with conventional intermittent automated arm arterial pressure measurement in patients under general anaesthesia. Br J Anaesth 2014; 113: 67-74.
Chen G, Meng L, Alexander B, Tran NP, Kain ZN, Cannesson M. Comparison of noninvasive cardiac output measurements using the Nexfin monitoring device and the esophageal Doppler. J Clin Anesth 2012; 24: 275-83.
Bogert LW, Wesseling KH, Schraa O, et al. Pulse contour cardiac output derived from non-invasive arterial pressure in cardiovascular disease. Anaesthesia 2010; 65: 1119-25.
Vos JJ, Kalmar AF, Struys MM, Wietasch JK, Hendriks HG, Scheeren TW. Comparison of arterial pressure and plethysmographic waveform-based dynamic preload variables in assessing fluid responsiveness and dynamic arterial tone in patients undergoing major hepatic resection. Br J Anaesth 2013; 110: 940-6.
Lansdorp B, Ouweneel D, de Keijzer A, van der Hoeven JG, Lemson J, Pickkers P. Non-invasive measurement of pulse pressure variation and systolic pressure variation using a finger cuff corresponds with intra-arterial measurement. Br J Anaesth 2011; 107: 540-5.
Zimmermann M, Feibicke T, Keyl C, et al. Accuracy of stroke volume variation compared with pleth variability index to predict fluid responsiveness in mechanically ventilated patients undergoing major surgery. Eur J Anaesthesiol 2010; 27: 555-61.
Cannesson M, Le Manach Y, Hofer CK, et al. Assessing the diagnostic accuracy of pulse pressure variations for the prediction of fluid responsiveness: a “gray zone” approach. Anesthesiology 2011; 115: 231-41.
Guinot PG, de Broca B, Bernard E, Abou Arab O, Lorne E, Dupont H. Respiratory stroke volume variation assessed by oesophageal Doppler monitoring predicts fluid responsiveness during laparoscopy. Br J Anaesth 2014; 112: 660-4.
Ray P, Le Manach Y, Riou B, Houle TT. Statistical evaluation of a biomarker. Anesthesiology 2010; 112: 1023-40.
Wyffels PA, Sergeant P, Wouters PF. The value of pulse pressure and stroke volume variation as predictors of fluid responsiveness during open chest surgery. Anaesthesia 2010; 65: 704-9.
Martina JR, Westerhof BE, van Goudoever J, et al. Noninvasive continuous arterial blood pressure monitoring with Nexfin(R). Anesthesiology 2012; 116: 1092-103.
Thiele RH, Bartels K, Gan TJ. Inter-device differences in monitoring for goal-directed fluid therapy. Can J Anesth 2015; 62: 169-81.
Sing T, Sander O, Beerenwinkel N, Lengauer T. ROCR: visualizing classifier performance in R. Bioinformatics 2005; 21: 3940-1.
©The R Foundation. The R Project for Statistical Computing. Vienna, Austria - 2012. Available from URL: http://www.R-project.org (accessed April 2015).
Monnet X, Dres M, Ferre A, et al. Prediction of fluid responsiveness by a continuous non-invasive assessment of arterial pressure in critically ill patients: comparison with four other dynamic indices. Br J Anaesth 2012; 109: 330-8.
Solus-Biguenet H, Fleyfel M, Tavernier B, et al. Non-invasive prediction of fluid responsiveness during major hepatic surgery. Br J Anaesth 2006; 97: 808-16.
Nordstrom J, Hallsjo-Sander C, Shore R, Bjorne H. Stroke volume optimization in elective bowel surgery: a comparison between pulse power wave analysis (LiDCOrapid) and oesophageal Doppler (CardioQ). Br J Anaesth 2013; 110: 374-80.
De Hert SG. Assessment of fluid responsiveness: insights in a “gray zone”. Anesthesiology 2011; 115: 229-30.
Stover JF, Stocker R, Lenherr R, et al. Noninvasive cardiac output and blood pressure monitoring cannot replace an invasive monitoring system in critically ill patients. BMC Anesthesiol 2009; 9: 6.
Fischer MO, Avram R, Carjaliu I, et al. Non-invasive continuous arterial pressure and cardiac index monitoring with Nexfin after cardiac surgery. Br J Anaesth 2012; 109: 514-21.
Broch O, Renner J, Gruenewald M, et al. A comparison of the Nexfin(R) and transcardiopulmonary thermodilution to estimate cardiac output during coronary artery surgery. Anaesthesia 2012; 67: 377-83.
Fischer MO, Coucoravas J, Truong J, et al. Assessment of changes in cardiac index and fluid responsiveness: a comparison of Nexfin and transpulmonary thermodilution. Acta Anaesthesiol Scand 2013; 57: 704-12.
Bubenek-Turconi SI, Craciun M, Miclea I, Perel A. Noninvasive continuous cardiac output by the Nexfin before and after preload-modifying maneuvers: a comparison with intermittent thermodilution cardiac output. Anesth Analg 2013; 117: 366-72.
Critchley LA, Lee A, Ho AM. A critical review of the ability of continuous cardiac output monitors to measure trends in cardiac output. Anesth Analg 2010; 111: 1180-92.
Cannesson M, Le Manach Y. Noninvasive hemodynamic monitoring: no high heels on the farm; no clogs to the opera. Anesthesiology 2012; 117: 937-9.
Maguire S, Rinehart J, Vakharia S, Cannesson M. Technical communication: respiratory variation in pulse pressure and plethysmographic waveforms: intraoperative applicability in a North American academic center. Anesth Analg 2011; 112: 94-6.
Lansdorp B, Lemson J, van Putten MJ, de Keijzer A, van der Hoeven JG, Pickkers P. Dynamic indices do not predict volume responsiveness in routine clinical practice. Br J Anaesth 2012; 108: 395-401.
Funding
This study was supported solely by departmental and institutional funding.
Conflicts of interest
None declared.
Author information
Authors and Affiliations
Corresponding author
Additional information
Author contributions
Jaap Jan Vos, Michel M.R.F. Struys, Thomas W.L. Scheeren, and Alain F. Kalmar were involved in data interpretation. Marieke Poterman, Pieternel Papineau Salm, and Alain F. Kalmar were involved in data recording. Jaap Jan Vos, Kai van Amsterdam, and Alain F. Kalmar were involved in data analysis. Alain F. Kalmar was involved in patient inclusion and care. All authors were involved in writing the manuscript.
This article is accompanied by an editorial. Please see Can J Anesth 2015; 62: this issue.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Figs 1 and 2
Bootstrapping technique and receiver operator characteristic curves (PDF 557 kb)
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution-NonCommercial 4.0 International License (http://creativecommons.org/licenses/by-nc/4.0/), which permits any noncommercial use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Vos, J.J., Poterman, M., Salm, P.P. et al. Noninvasive pulse pressure variation and stroke volume variation to predict fluid responsiveness at multiple thresholds: a prospective observational study. Can J Anesth/J Can Anesth 62, 1153–1160 (2015). https://doi.org/10.1007/s12630-015-0464-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12630-015-0464-2