Abstract
Background
Ultrasound has been shown to facilitate accurate identification of the intervertebral level and to predict skin-to-epidural depth in the lumbar epidural space with reliable precision. We hypothesized that we could accurately predict the skin-to-epidural depth and the intervertebral level in the thoracic spine with the use of ultrasound.
Methods
Twenty patients presenting for thoracic surgery were included in a feasibility study. The skin-to-epidural depth was measured using prepuncture ultrasound in the paramedian window, and the predicted depth was compared with the actual needle depth and the depth as measured by computed tomography. In addition, the intervertebral levels were identified by ultrasound using the “counting up” method, and the results were compared with the levels identified by anesthesiologists.
Results
The ultrasound-based depth measurements displayed a bias of 3.21 mm with 95% limits of agreement from −7.47 to 13.9 mm compared with the clinically determined needle depth. The intervertebral levels identified by the anesthesiologists and the sonographer matched in only 40% of cases.
Conclusion
Ultrasound-based measurements of skin-to-epidural depth show acceptable agreement with the actual depth observed during epidural catheterization; however, the limits of agreement are wide, which restricts the predictive value of ultrasound-based measurements. Further study is required to delineate the role of ultrasound in thoracic epidural catheterizations.
Résumé
Contexte
Il a été démontré que l’échographie permettait d’identifier de façon précise le niveau intervertébral et de prédire la profondeur entre la peau et l’espace péridural avec une bonne précision dans l’espace péridural lombaire. Nous avons émis l’hypothèse que l’échographie permettrait de prédire de façon précise la profondeur entre la peau et l’espace péridural et le niveau intervertébral dans la colonne thoracique.
Méthode
Vingt patients se présentant pour une chirurgie thoracique ont été recrutés pour cette étude de faisabilité. La profondeur entre la peau et l’espace péridural a été mesurée à l’aide d’une échographie pré-ponction dans une fenêtre paramédiane. La profondeur prédite a été comparée à la profondeur réelle de l’aiguille et à la profondeur telle que mesurée par tomodensitométrie. En outre, les niveaux intervertébraux ont été identifiés par échographie à l’aide d’une méthode de décompte vers le haut et comparés aux niveaux identifiés par les anesthésiologistes.
Résultats
Les mesures de profondeur se fondant sur l’échographie ont révélé un biais de 3,21 mm avec 95 % des limites de concordance situées entre −7,47 et 13,9 mm par rapport à la profondeur de l’aiguille déterminée de façon clinique. Les niveaux intervertébraux identifiés par l’anesthésiologiste et l’échographiste ne correspondaient que dans 40 % des cas.
Conclusion
Les mesures fondées sur l’échographie de la profondeur entre la peau et l’espace péridural concordent de façon acceptable avec la profondeur réelle observée pendant l’installation du cathéter péridural; cependant, les limites de concordance sont larges, ce qui limite la valeur prédictive des mesures fondées sur l’échographie. Des recherches plus poussées sont nécessaires afin de déterminer le rôle de l’échographie dans l’installation de péridurales thoraciques.
Similar content being viewed by others
Placement of a thoracic epidural is among the most difficult regional anesthetic techniques due to the narrow intervertebral foramen that must be identified “blindly” with needle probing. Complication rates associated with epidural insertion, such as paresthesias and blood aspiration, are reported at 0.16% and 0.67%, respectively.1 Significant nerve injury is exceedingly rare, and the rate of epidural hematoma is estimated at less than one in 150,000 epidurals2; however, complications may be catastrophic.3 Ultrasound is being used increasingly for a multitude of invasive medical procedures. Its use has been shown to increase peripheral nerve block efficacy and decrease complication rates during central venous catheterization.4,5 The ability to image anatomic structures, including nerve tissue, noninvasively in the operating room with ultrasound has revolutionized regional anesthesia over the last decade and has led to its widespread acceptance. Less is known about the utility of ultrasound for neuraxial anesthesia; however, some work has been published on the use of ultrasound for lumbar epidural placement.6-8 Early reports of real-time use of ultrasound for lumbar epidural placement are emerging,8-11 and ultrasound has been shown to improve the accurate identification of the intervertebral level using a “counting down” method from the 12th rib.12,13 Skin-to-epidural depth measured by ultrasound is highly correlated to the actual depth observed during lumbar epidural placement.6,14 Grau et al. have examined the value of ultrasound for thoracic epidural insertion. 14 They demonstrated that the ultrasound-measured distance from the skin to the epidural space showed only moderate agreement with measurements derived from magnetic resonance imaging (MRI). At present, however, there is a knowledge gap about the agreement between the depth of the thoracic epidural space determined by ultrasound and the clinically observed depth.
We hypothesized that we could use prepuncture ultrasound to predict with accuracy the skin-to-epidural depth observed during actual needle insertion. The primary objective of our study was to determine the validity of ultrasound-measured depth. To this end, we performed a feasibility study to compare the ultrasound-based measurement of the depth from the skin to the epidural space with the needle insertion depth and the computed tomography (CT) derived depth. In addition, we sought to correlate the depth measurements with biometric variables (height, weight, and body mass index [BMI]). The secondary objective of our study was to compare the precision of ultrasound with that of palpation in identifying the intervertebral level.
Methods
After approval by the Clinical Research Ethics Board of the University of British Columbia and the Vancouver Coastal Health Research Institute, we obtained written informed consent from 20 patients. We studied consecutive consenting patients who were scheduled for thoracic surgery in a tertiary care university hospital. The patients were candidates for thoracic epidural anesthesia and had undergone a chest CT scan. All cases occurred during the five-month period December 2009 to April 2010 and were analyzed over the subsequent six months. The only exclusion criterion was contraindication to the placement of an epidural. The preoperative chest CT images were obtained from the radiology archive for each patient, and the CT imaging resolution for all data sets was at 0.70 mm x 0.70 mm x 2.5 mm spacing.
Ultrasound images were taken immediately prior to surgery using a Sonix RP ultrasound machine (Ultrasonix Medical Corp., Richmond, BC, Canada) with a 3.3 MHz curvilinear-array transducer. The patients were seated upright and given leg and arm support for the ultrasound examination which took less than 15 min in all cases. To facilitate recording the vertebral levels as determined by the anesthesiologist and sonographer, a paper ruler was affixed to the patient’s skin parallel to the vertebral column and 3 cm to the left of the midline. All ultrasound scans were performed by the same individual with 30 years of sonographic experience and special expertise in spine sonography.15
Intervertebral level determination
Anesthesiologists were asked to select the desired insertion level for each patient prior to the sonographic exam. The vertebral level was identified (e.g., T6-7) by palpation and marked on the ruler. Subsequently, the sonographer established the intervertebral levels based on the “counting-up” method from the last rib.12 For a target level of T6-7, the seventh rib was identified by counting up from the 12th rib while the transducer was oriented in the sagittal direction and moved superiorly in a parasagittal plane 2 cm to the right of midline. After intervertebral identification, the transducer was moved medially along the seventh rib echo to identify the ligamentum flavum (LF) at the T6-7 intervertebral space. Since the plane was 2-3 mm from the midline sagittal plane, this plane was termed the offset-sagittal plane. The same approach was used for other target levels. Vertebral levels identified by the sonographer were similarly recorded on the ruler affixed to the patients back.
Skin-to-epidural space distance determination
At the chosen target level, the depth of skin-to-epidural space was measured using the leading (posterior) edge echo from the LF as the target. The ultrasound scanning was repeated one level above and two levels below the target level to allow for comparison between measurements in the event that the site of epidural placement differed from the initially chosen target level. An acoustic window was found by searching through the echoes from the intervertebral space by moving (2-3 mm) and/or angling (5-10°) until the characteristically bright echo from the LF was clearly visible in the image (Fig. 1). As the epidural space itself is not directly visible in ultrasound images, the adjacent echogenic LF was used as the identifiable surrogate on the ultrasound images. The echo-dense LF appears as a bright echo at the base of the laminae with an appearance and thickness that varies slightly from patient to patient. In keeping with prior publications,6,14 the distance from the skin to the posterior edge of the echo was considered the skin-to-epidural depth (Fig. 2A). The ultrasound distance measurements were taken with the probe-to-skin angle as close to perpendicular as possible and after minimizing tissue compression by reducing the probe pressure on the skin to the minimal pressure necessary for adequate imaging. This distance was measured using the electronic caliper tool on the ultrasound machine, and the measurement was recorded.
Offset sagittal ultrasound image of thoracic spine structures. Laminae are visualized as wave-like structures. The ligamentum flavum produces a bright echo at the base of the laminae. The vertebral body, which forms the anterior border of the spinal canal, appears as a bright reflector below the ligamentum flavum
The skin-to-epidural depth was also measured from CT images using the LF as the anatomical landmark. The midline sagittal plane was used to make this measurement. To ensure consistency, the posterior edge of the LF was used as the target in all CT measurements (Fig. 3).
Epidural placement and angle correction
Epidural catheters (19G closed-tip multi-orifice Perifix® FX epidural catheter; B. Braun, Bethlehem, PA, USA) were placed in the operating room using a standard sterile technique with the patient seated approaching an upright position. The epidural space was identified using loss-of-resistance to saline (LOR), which was confirmed with the pressure transduction technique previously described.16 We also checked on the patients in the postoperative period and documented effective epidural analgesia for all patients. In addition, we recorded the time from needle insertion to successful LOR, the number of attempts, and any complications (dural puncture, paresthesia). Successful epidural analgesia was sought and recorded. The epidural needle is usually angled in both the sagittal (β) and transverse (α) directions (Fig. 4), and so it is termed paramedian access. In contrast, the ultrasound images are taken when the probe is nearly perpendicular to the skin. To convert the CT and ultrasound measurements to the needle trajectory, the two angles were measured and used in a conversion formula. The measurements were taken based on two photos of the inserted needle, one from left-to-right and one from superior-to-inferior. The angles β and α were calculated from these photos by averaging three repeated measurements using image processing software (MATLAB®, Natick, MA, USA). To compare the ultrasound-measured depth with the actual insertion depth, the ultrasound measurements were geometrically transformed to match the needle angle using the following formula:
where d i is the depth measured by ultrasound and d t is the transformed depth. The transformed depth is hereafter referred to as the ultrasound angle-converted depth. Angle conversion, as outlined above, was also applied to all CT measurements. The transformed depth is hereafter referred to the CT angle-converted depth.
Statistical analysis
To ensure no bias in the measurements, the different measurements were taken by different individuals. In particular, the ultrasound-measured depths were not reported to the anesthesiologists; the computed tomography images were analyzed independently after the examinations were complete, and the needle insertion angles were found independently by three different individuals.
We did not perform a sample size calculation as this was considered a feasibility study with no published comparison data available. The comparison between the actual epidural depth measured by needle insertion and the ultrasound angle-converted depth was assessed by Bland-Altman analysis. We measured the correlation between the skin-to-epidural depth and the biometric information (height, weight, BMI) using linear regression and Pearson’s coefficient.
Results
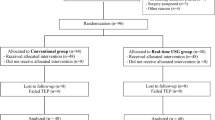
Twenty patients gave their consent to participate and completed the study (Table 1). They presented for lobectomy,17 pneumonectomy,1 or anterior mediastinal mass resection.1 Complete ultrasound studies and chest CT images were obtained for all patients, and the epidural insertions were performed by five thoracic anesthesiologists and two senior anesthesia residents. Successful thoracic epidural catheterization and analgesia were documented in all patients. Three minor complications were observed, paresthesia (n = 2) and blood aspiration (n = 1), and resolved with needle repositioning. The average number of needle insertions was 1.3 per patient, and the average duration of epidural placement was 3.3 min.
The 20 patients were characterized as follows: eight males/12 females; age [mean (standard deviation)] 62 (13) yr; weight 72 (15) kg; height 165 (9) cm; and BMI 26 (4) kg·m−2.
The vertebral level determined by the clinicians was always either equal to or higher than the level determined by the sonographer. The identified levels matched in only 40% of cases; they differed by one level in another 40% of cases, and they differed by two levels in the remaining 20% of cases.
The epidural insertion levels ranged from T5-6 to T8-9. The needle insertion depth was 55 mm (7 mm), and the insertion angles were β = 16° (7°) and α = 36° (8°).
The ultrasound-based depth measurements displayed a bias of 3.21 mm with 95% limits of agreement of −7.47 to 13.9 mm compared with the clinically determined needle depth, and they displayed a bias of 2.99 mm with 95% limits of agreement of −7.29 to 13.28 mm compared with the CT measured epidural depth. Fig. 5 shows the Bland-Altman analysis comparing the ultrasound and CT-based measurements of skin-to-epidural depth with the clinical needle depth for individual subjects. The correlation between the ultrasound and clinically determined needle depths was R2 = 0.65 with an average absolute difference of 4.68 mm, while the correlation between the ultrasound and the CT-based measurements was R2 = 0.69 with an average absolute difference of 4.49 mm (Table 2). Fig. 6 shows least-squares fit analysis for the ultrasound and CT-based measurements of skin-to-epidural depth compared with the clinically determined needle depth.
The correlation between the biometric variables and the skin-to-epidural depth measurements was moderate but statistically significant (Fig. 7). The correlation coefficients for weight, height, and BMI were 0.4, 0.34, and 0.19, respectively.
Discussion
This study sought to determine the utility of prepuncture ultrasound for predicting the skin-to-epidural depth for thoracic epidural placement. We showed that ultrasound can reliably image the LF in the thoracic spine using the paramedian plane. We also found an acceptable correlation between both ultrasound and CT-determined skin-to-epidural depth and the clinically determined epidural depth. The correlation coefficient, R2 = 0.65, between the needle-measured depth and the angle-converted ultrasound-measured depth is lower than that achieved in studies on the lumbar spine (R2 = 0.79-0.92).6 However, these results are in keeping with the findings by Grau et al. on the correlation ratio between ultrasound and MRI-measured depth (0.53 by paramedian, 0.54 by median, and 0.61 by transverse imaging).14 We included correlation in this analysis as it has been used regarding this topic in previous publications. However, agreement between two measurements from different modalities, e.g., CT and ultrasound, is more clinically relevant, as it identifies any inherent bias in measurements.18 In our study, the ultrasound-based measurements tended to underestimate the skin-to-epidural depth (bias 3.21 mm), perhaps due to tissue compression by the ultrasound probe or the intrinsic thickness of the LF. The range of agreement was wide (−7.47 to 13.9 mm), which limits the reliability of prepuncture ultrasound-based depth measurements for clinical decision-making during thoracic epidural catheterization.
There are limitations to our study. Patient positioning was similar but likely not identical during the ultrasound examination and the actual epidural insertion. For purposes of angle-correction, we assumed the back was a flat plane, ignoring the often marked concavity of the mid-thoracic back. The estimation of the needle angle in both the transverse and sagittal planes was also a potential source of error which we tried to minimize by taking the average of three independent measurements. The ultrasound-based skin-to-epidural depth measurements were based on the leading edge of the LF. By ignoring the thickness of the LF, a consistent bias towards smaller depths was introduced. While the sonographer attempted to keep the transducer perpendicular to the skin, an angle variation of approximately 5-10° was sometimes observed in the transverse direction. Lastly, measurement errors are possible as it is slightly more difficult to acquire an image due to the lower visibility of the LF in the thoracic spine (Fig. 2).
We demonstrated that CT images are not better at predicting skin-to-epidural depth than ultrasound as the correlation coefficients were almost identical (R2 = 0.58 vs 0.65). While there appeared to be less bias with CT-based measurements, the range of the limits of agreement was equally wide (−10.7 to 11.1). Some intrinsic error between the measurements is secondary to the fact that the depth of the LF was not considered in the ultrasound and CT measurements, although it clearly adds to the clinically observed depth.
The sonographic appearances of the thoracic and lumbar vertebrae are comparable. The thoracic vertebrae are closer to the skin’s surface (3.9 cm vs 5.1 cm),6 and their laminar surfaces lie more perpendicular to the sound beam than those of the lumbar which rise slightly from superior to inferior margins. The LF has the same appearance and is seen in the same location (at the superior margin of the lamina) in both the lumbar and thoracic regions.
Target intervertebral spaces are recommended for specific surgical procedures, which explains why correct identification of the intervertebral space is important for the quality of analgesia.17 Since ultrasound has previously been shown to allow a more accurate determination of the lumbar intervertebral level than a clinical exam, it was considered the “gold standard” in our study.12 However, since we did not use x-ray guidance, we were not guaranteed the actual spinal level, only the difference between the levels determined by the sonographer and the clinician. The vertebral level determined by the clinicians was always equal to or higher than the level determined by the sonographer, meaning that the clinicians tended to err on the side of being too caudad for the intended vertebral level. A difference of one intervertebral level is unlikely clinically relevant; however, a variance of two levels, which occurred in 20% of our patients, suggests that routine ultrasound may improve the quality of postoperative epidural analgesia.
The correlation between the biometric variables and the skin-to-epidural depth was moderate but statistically significant. The correlation between weight and BMI with skin-to-epidural depth is intuitive, whereas the correlation of height with skin-to-epidural depth appears somewhat surprising. The difference between ultrasound-measured and needle-measured depth, however, is not well correlated with the BMI (R2 = 0.22), meaning that the error is not larger for obese patients. Similar results were obtained by Balki et al. 19 A larger sample size, including specific recruitment criteria for obesity, would be required to make more definitive statements about ultrasound-measured depth accuracy in obese patients.
Ultrasound imaging was performed exclusively in an offset sagittal plane. The transverse plane has been shown to produce slightly better correlation coefficients for both the thoracic and lumbar spine.14,19,20 However, we purposely chose the offset sagittal plane for the thoracic spine, as it provides superior visibility of the LF along with more easily identifiable landmarks, which should aid the inexperienced sonographer.21,22
We chose an expert sonographer to eliminate a possible source of error in this feasibility study, and it is our view that this should not limit the clinical utility of these findings, as proof of principle is of foremost importance for acceptance by clinicians. Our group has work underway on new ultrasound technology to address the issue of ultrasound operation and interpretation by non-expert users.
Conclusion
Ultrasound measurement at the thoracic level differs frequently with the level identified by manual palpation. Moreover, the use of ultrasound facilitates consistent visualization of the LF at the target level. Ultrasound-based measurements of skin-to-epidural depth show acceptable agreement with the actual depth observed during epidural catheterization; however, the limits of agreement are wide, which limits the predictive value of ultrasound-based measurements. Further study is required to delineate the role of ultrasound in thoracic epidural catheterizations.
References
Tanaka K, Watanabe R, Harada T, Dan K. Extensive application of epidural anesthesia and analgesia in a university hospital: incidence of complications related to technique. Reg Anesth 1993; 18: 34-8.
Horlocker TT, Wedel DJ, Benzon H, et al. Regional anesthesia in the anticoagulated patient: defining the risks (the Second ASRA Consensus Conference on Neuraxial Anesthesia and Anticoagulation). Reg Anesth Pain Med 2003; 28: 172-97.
Visser WA, Kolling JB, Groen GJ, et al. Persistent cortical blindness after a thoracic epidural test dose of bupivacaine. Anesthesiology 2010; 112: 493-5.
Wigmore TJ, Smythe JF, Hacking JF, Raobaikady R, MacCallum NS. Effect of the implementation of NICE guidelines for ultrasound guidance on the complication rates associated with central venous catheter placement in patients presenting for routine surgery in a tertiary referral centre. Br J Anaesth 2007; 99: 662-5.
Marhofer P, Greher M, Kapral S. Ultrasound guidance in regional anaesthesia. Br J Anaesth 2005; 94: 7-17.
Tran D, Kamani AA, Lessoway VA, Peterson C, Hor KW, Rohling RN. Preinsertion paramedian ultrasound guidance for epidural anesthesia. Anesth Analg 2009; 109: 661-7.
Kil HK, Cho JE, Kim WO, Koo BN, Han SW, Kim JY. Prepuncture ultrasound-measured distance: an accurate reflection of epidural depth in infants and small children. Reg Anesth Pain Med 2007; 32: 102-6.
Grau T, Leipold RW, Fatehi S, Martin E, Motsch J. Real-time ultrasonic observation of combined spinal-epidural anaesthesia. Eur J Anaesthesiol 2004; 21: 25-31.
Galiano K, Obwegeser AA, Bodner G, et al. Real-time sonographic imaging for periradicular injections in the lumbar spine: a sonographic anatomic study of a new technique. J Ultrasound Med 2005; 24: 33-8.
Tran D, Kamani AA, Al-Attas E, Lessoway VA, Massey S, Rohling RN. Single-operator real-time ultrasound-guidance to aim and insert a lumbar epidural needle. Can J Anesth 2010; 57: 313-21.
Willschke H, Marhofer P, Bosenberg A, et al. Epidural catheter placement in children: comparing a novel approach using ultrasound guidance and a standard loss-of-resistance technique. Br J Anaesth 2006; 97: 200-7.
Furness G, Reilly MP, Kuchi S. An evaluation of ultrasound imaging for identification of lumbar intervertebral level. Anaesthesia 2002; 57: 277-80.
Cork RC, Kryc JJ, Vaughan RW. Ultrasonic localization of the lumbar epidural space. Anesthesiology 1980; 52: 513-6.
Grau T, Leipold RW, Delorme S, Martin E, Motsch J. Ultrasound imaging of the thoracic epidural space. Reg Anesth Pain Med 2002; 27: 200-6.
Ledsome JR, Lessoway V, Susak LE, Gagnon FA, Gagnon R, Wing PC. Diurnal changes in lumbar intervertebral distance. measured using ultrasound. Spine (Phila Pa 1976) 1996; 21: 1671-5.
Lennox PH, Umedaly HS, Grant RP, White SA, Fitzmaurice BG, Evans KG. A pulsatile pressure waveform is a sensitive marker for confirming the location of the thoracic epidural space. J Cardiothorac Vasc Anesth 2006; 20: 659-63.
Visser WA, Lee RA, Gielen MJ. Factors affecting the distribution of neural blockade by local anesthetics in epidural anesthesia and a comparison of lumbar versus thoracic epidural anesthesia. Anesth Analg 2008; 107: 708-21.
Bland JM, Altman DG. Measuring agreement in method comparison studies. Stat Methods Med Res 1999; 8: 135-60.
Balki M, Lee Y, Halpern S, Carvalho JC. Ultrasound imaging of the lumbar spine in the transverse plane: the correlation between estimated and actual depth to the epidural space in obese parturients. Anesth Analg 2009; 108: 1876-81.
Arzola C, Davies S, Rofaeel A, Carvalho JC. Ultrasound using the transverse approach to the lumbar spine provides reliable landmarks for labor epidurals. Anesth Analg 2007; 104: 1188-92.
Margarido CB, Arzola C, Balki M, Carvalho JC. Anesthesiologists’ learning curves for ultrasound assessment of the lumbar spine. Can J Anesth 2010; 57: 120-6.
Grau T, Leipold RW, Horter JF, Conradi R, Martin EO, Motsch J. Paramedian access to the epidural space: the optimum window for ultrasound imaging. J Clin Anesth 2001; 13: 213-7.
Acknowledgements
The authors sincerely thank Dr. D. E. Griesdale for his helpful comments on the manuscript.
Competing interests
None declared.
Financial support
Supported by a Collaborative Health Research Project jointly funded by the Canadian Institutes for Health Research and the Natural Sciences and Engineering Research Council of Canada.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Rasoulian, A., Lohser, J., Najafi, M. et al. Utility of prepuncture ultrasound for localization of the thoracic epidural space. Can J Anesth/J Can Anesth 58, 815–823 (2011). https://doi.org/10.1007/s12630-011-9548-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12630-011-9548-9