Abstract
Streptomyces is a Gram-positive bacterium, belonging to the family Streptomycetaceae and order Streptomycetales. Several strains from different species of Streptomyces can be used to promote the health and growth of artificially cultured fish and shellfish by producing secondary metabolites including antibiotics, anticancer agents, antiparasitic agents, antifungal agents, and enzymes (protease and amylase). Some Streptomyces strains also exhibit antagonistic and antimicrobial activity against aquaculture-based pathogens by producing inhibitory compounds such as bacteriocins, siderophores, hydrogen peroxide, and organic acids to compete for nutrients and attachment sites in the host. The administration of Streptomyces in aquaculture could also induce an immune response, disease resistance, quorum sensing/antibiofilm activity, antiviral activity, competitive exclusion, modification in gastrointestinal microflora, growth enhancement, and water quality amelioration via nitrogen fixation and degradation of organic residues from the culture system. This review provides the current status and prospects of Streptomyces as potential probiotics in aquaculture, their selection criteria, administrative methods, and mechanisms of action. The limitations of Streptomyces as probiotics in aquaculture are highlighted and the solutions to these limitations are also discussed.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Sustainable aquaculture has recently emerged as a profitable alternative to provide proteinaceous diets to human consumers. This artificial way of rearing fish and shellfish not only helps to satisfy global demand but also contributes to the recovery of depleting natural resources. The global aquaculture production (aquatic animals only) reached a record 87.5 mt in 2020 [1] which as per the recent report of the Organization for Economic Co-operation and Development (OECD) and the Food and Agriculture Organization (FAO) of the United Nations (UN) is projected to reach 103 mt by 2030, rising by 17.7% as compared to 2020 [2]. However, the first, second, and third waves of COVID‑19 and later the arrival of Omicron and Delta variants and their sublineages (by far the most mutated and transmissible of all the variants of concern identified in the history of the COVID-19 pandemic) may affect the projected values. As far as the current situation is concerned, the world economy is on the verge of recovery from the post-pandemic crisis as it bounced back in 2021 with 5.6% growth defying the previous trends [3]. The development of COVID-19 vaccines and medications greatly reduced its impact on global production and trade [4].
The escalation of aquaculture practices has caused major disease outbreaks in the aquaculture sector due to high fish stocking densities in the ponds and a lack of hygiene, making the cultured stocks vulnerable to mortalities. The estimated annual global loss due to various epizootics is a quarter billion US dollars [5]. Especially, the outbreak of several pathogens during aquaculture resulted in fatal diseases which caused large-scale mortalities of fish and shellfish [6,7,8,9]. Recently, experiments have been conducted on the use of bacterial species as potential probiotics to treat diseases in aquaculture [10,11,12,13]. There are several non-profit and commercial probiotic products prepared from different bacterial species, for instance, Arthrobacter spp., Acinetobacter spp., Bacillus spp., Clostridium spp., Enterococcus spp., Janthinobacterium spp., Lactobacillus spp., Lactococcus spp., Pediococcus spp., Pseudomonas spp., Rhodococcus spp., Rhodopseudomonas spp., Synechocystis spp., Streptococcus spp., Streptomyces spp., and the yeast Saccharomyces cerevisiae among others [14,15,16,17]. Streptomyces, in particular, have emerged among those that demonstrated numerous beneficial effects in aquaculture, i.e., the production of industrially important enzymes and a broad range of biologically active secondary metabolites [18] such as antibiotics [19, 20], antioxidants [21], antifungal agents [22], and anticancer agents [23, 24]. In addition to producing secondary metabolites and exhibiting antimicrobial activity in aquaculture, Streptomyces strains also produce antagonistic and siderophore compounds to prevent bacterial infections and demonstrate antiviral and antibiofilm activity [25,26,27]. Other benefits of Streptomyces as potential probiotics include enhancement in the growth and survival of cultured species, disease resistance, competitive exclusion of pathogens, alteration in gastrointestinal microflora, and amelioration of water quality [28,29,30,31].
This review aims to provide detailed insight into the use of Streptomyces as a potential probiotic agent for sustainable aquaculture, including current evidence on the prospects of their use. Despite demonstrating promising results in aquaculture, Streptomyces also have a few limitations which we have discussed along with their possible solutions.
Probiotics
Background on Probiotics
The word probiotic is a combination of the Latin preposition “pro,” which means “for” and the Greek terminology “biotic” meaning “life” [32]. This term was first coined by German scientist Werner Georg Kollath in 1953 where he proposed probiotics as “active substances essential for a healthy development of life.” Later, several definitions of probiotics were proposed by researchers and research organizations. Fuller [33] defined them as “a live feed supplement that enhances the intestinal microbial balance of the host.” According to the World Health Organization (WHO), probiotics are “live microorganisms which, when administered in adequate amounts, confer a health benefit on the host” [34]. This definition is adopted as a consensus statement by the International Scientific Association for Probiotics and Prebiotics (ISAPP) [35]. Although the majority of proposed definitions of probiotics describe them as beneficial, their effect varies from species to species and host to host. As a result, it is critical to ensure that the probiotic being employed is not harmful to the host [36].
Before being considered for aquaculture practices, probiotics have shown remarkable beneficial effects on humans and terrestrial-based animal cultures. They were first tested in aquaculture in 1986 to determine their ability to escalate the growth of aquatic organisms [37]. The exact pathways of probiotic action in aquaculture are not well known; however, several possible modes of action have been proposed in recent experiments. The theoretical mechanisms of action of probiotics in aquaculture (except Streptomyces) mentioned in the literature are presented in Table 1.
Streptomyces
Taxonomic and Morphologic Background of Streptomyces
Streptomyces is a genus of kingdom Bacteria, phylum Actinomycetota, class Actinomycetes, order Streptomycetales, and family Streptomycetaceae [59]. It was first proposed in 1943 [60] and initially classified based on its morphology, chemotype, whole-cell sugar patterns, phospholipid and fatty acid profiles, and composition of the cell wall and later based on its phenotypic and genotypic constitutional traits. To date, 1147 species and 73 subspecies of Streptomyces have been validly described (www.bacterio.net).
Genus Streptomyces is a Gram-positive, multicellular, mycelial, and filiform aerophilous bacteria that mainly live as saprophytes in soil [61]. Interestingly, some exist as marine or rhizosphere symbionts, growing on thermal springs or gamma-irradiated surfaces [62]. Some Streptomyces strains are pathogens associated with humans, animals, or plants such as Streptomyces scabies that cause potato scab disease [63]. The cell wall of Streptomyces contains a simple peptidoglycan mesh surrounding the cytoplasmic membrane [64]. Morphogenesis in Streptomyces is determined by the establishment of aerial hyphae (that can differentiate into spores or arthrospores) that emerge from the substrate mycelium containing LL-diaminopimelic acid as the predominant diamino acid [65, 66]. The spores help to enhance the survival of Streptomyces in the soil during the dormant phase as Streptomyces are resistant to water and nutrient deficiencies as well as extreme temperatures [61].
The increasing interest of researchers in the use of Streptomyces as a probiotic is due to its antagonistic behavior against pathogens, effect on the host metabolism, diversity in morphology, genomic size, genetic content such as guanine + cytosine (G + C), and the size of the coding sequences. Streptomyces are also distinguished by their large linear chromosomes with 8.5–12 Mb of DNA length and high G + C content averaging between 67 and 78 mol % [66,67,68]. The large size of the Streptomyces genome can explain its ability to produce distinctive secondary metabolites at a large scale [69]. Specialized metabolite production on this scale is unique to Streptomyces, and it has been proposed that these bacteria require a diverse metabolic repertoire to support their unusual life cycle [70].
The Biological Rhythm of Streptomyces
Streptomyces are abundant in nature and remain quiescent as spores before they obtain favorable conditions for growth. Streptomyces undergo the following development cycle: (1) the initial mitotic phase (dispersal of spores during the sporulation process); (2) germination (the dispersed spores settle and germinate); (3) primary mycelium formation (development of the vegetative hyphae); (4) secondary mycelium formation (development of the aerial hyphae); and (5) sporulation (the formation of spores). The complete life cycle of Streptomyces is illustrated in Fig. 1.
Once the dispersed spore settles in a nutrient-rich environment, it exits its dormant stage and starts germinating. Germination results in sprouting spores into germ tubes, which further develop into branching filaments during vegetative growth and form a mesh of hyphae called the vegetative mycelium. The vegetative mycelium stimulates the formation of an aerial mycelium on the colony surface possibly due to limited nutrient and cell density signals [65, 71, 72]. The aerial mycelium is a reproductive structure that transforms into spore chains that mature and ultimately liberate the spores.
Understanding the mechanisms underpinning the different developmental transitions during the Streptomyces life cycle has been easier because of advancements in both genomics techniques and cell biology. Till now, the investigations have focused on the study of single-species cultures. However, it was recently unearthed that the co-culture of several Streptomyces species with yeasts leads to a novel mode of its growth and development that had not been seen previously for Streptomyces cultured alone. This novel way of Streptomyces growth is described as “exploration,” named for the ability of explorer cells to rapidly lie across solid surfaces. This process is stimulated by fungal interactions and is associated with the production of an alkaline volatile organic compound (VOC) which is capable of inducing exploration by other Streptomycetes. For detailed information regarding this novel phenomenon, please read Jones and Elliot [70].
Selection Criteria of Streptomyces Strains as Probiotics
All strains of Streptomyces should first be analyzed through a laboratory-based screening process consisting of the following steps: (1) preliminary screening, (2) experimental screening, and (3) post-experimental screening. Considering the above methods, Hariharan and Dharmaraj [28] listed the following steps that should be followed to select Streptomyces strains as probiotics: (a) gathering preliminary details about sampling areas; (b) isolation and identification of strains; (c) conducting strain survivability tests against low pH, pepsin, bile, and pancreatin; (d) testing colonization potential (co-cultivation with pathogens to test strain dominance, hydrophobicity, hydrophilicity, and auto-aggregation); (e) conducting safety assessment of strains through antibiotic sensitivity test and nonhemolytic activity; (f) assessment of the antagonistic capacity of strains against pathogens existing in a particular environment; and (g) evaluation of the effects of probiotic strains on the host. Cost-effectiveness analysis of the probiotic strains may also be considered for their selection [73].
According to Verschuere et al. [74], selected strains should also possess the following properties: (1) nonpathogenic to the host; (2) can be administered through feed; (3) can exert targeted effect where needed; (4) effective in vivo as per in vitro findings; and (5) must not be virulent or possess antibiotic resistance genes.
Methods of Streptomyces Administration in Aquaculture
Methods for Streptomyces administration in aquaculture and their associated benefits are listed below.
-
(a)
When used via intramuscular injection technique, reduces the occurrence of white spot syndrome virus (WSSV) [75].
-
(b)
When administered/supplemented via feed, provides numerous beneficial effects [30, 76,77,78,79].
-
(c)
When added directly in the ponds as a water additive, reduces Vibrio count [80].
-
(d)
When added to inoculate or vaccinate ponds, increases the decomposition of organic matter [78].
-
(e)
When administered as bio-encapsulated Streptomyces cells, increases survival against Vibrio [77].
-
(f)
When sprayed on feed pallets as bacterial suspension, increases survival during the challenge experiment [81].
-
(g)
When administered in form of crude extract, shows average activity against fish-associated pathogens [82].
-
(h)
When added as single-cell proteins (SCPs), enhances growth [83].
Evidence shows that all species of Streptomyces can be administered as probiotics in one way or another, and there is no specificity regarding administration techniques. However, some species may not be able to withstand some administrative methods, compromising their viability. Also, the frequency of administration is vital for the proper functioning of probiotics [84].
Several in vitro experiments were also conducted to further test the capabilities of Streptomyces strains. Streptomyces when cultured in vitro on Chrome Azurol S (CAS) agar medium produced siderophore compounds and demonstrated antibacterial activity [85]. In vitro bioassays of Streptomyces strains demonstrated antibiofilm activity [86]. Similarly, seaweed-associated Streptomyces strains when co-cultured with pathogens under lab conditions competitively suppressed pathogenic strains [87]. A few of the administrative methods of Streptomyces are graphically represented in Fig. 2.
Mechanisms of Action of Streptomyces in Aquaculture
Streptomyces strains demonstrate similar mechanisms as other probiotics; however, some mechanisms are unique and only associated with Streptomyces. Listed are the detailed mechanisms exhibited by Streptomyces during different experiments and research-based studies.
Production of Bioactive, Inhibitory, and Siderophore Compounds
Streptomyces are widely recognized as important microorganisms due to their ability to produce a variety of chemical compounds [88] such as streptomycin, polyoxins, oxytetracycline, blasticidin-S, validamycin, natamycin, kusagamycin, actinovate, milbemycin, abamectin/avermectins, polynactins, emamectin benzoate, and mycostop [89]. Streptomyces can also produce antimicrobial compounds such as chalcomycin A, which was extracted from Streptomyces termitum N-15, demonstrated significant antibacterial activities when used as an antimicrobial agent against 5 different bacterial fish pathogens including Aeromonas hydrophila, Aeromonas veronii, Aeromonas sobria, Aeromonas salmonida, and Plesiomonas shigelloides [90]. Actinomycin D and Mycinamicin III glycoside isomer derived from Streptomyces strain showed antimicrobial activities against Bacillus cereus and Fusarium oxysporum [91]. Actrinomycin D, a chromophoric phenoxazine, inhibits microbial growth by being incorporated into the base pair of a double helical DNA molecule and interfering with RNA polymerase [92, 93] while Mycinamicin III, an aglycone, confers antibacterial activity against pathogens [94]. Phenazinolin D, izumiphenazine A, B, and E are bioactive compounds produced by the termite-associated strain Streptomyces showdoensis BYF17. Izumiphenazine B has strong antagonistic activity against Pseudomonas syringae pv. Actinidiae, Escherichia coli, Staphylococcus aureus, and Micrococcus tetragenus with zones of inhibition 20.6, 12.9, 12.6, and 13.3 mm, respectively. Phenazinolin D, izumiphenazine A, and E showed antagonistic activity against Staphylococcus aureus and Micrococcus tetragenus with the zone of inhibition values of 10.3, 10.6, and 11.7 mm and 15.9 and 11.2 mm, respectively [95]. Streptomyces strains in aquaculture may benefit from the ability to produce antagonistic compounds to compete for nutrients, space, and binding sites in the host (see Fig. 2). You et al. [85] found that seven Streptomyces isolates from shrimp farm sediments (Streptomyces cinerogriseus A03, A05; Streptomyces griseorubroviolaceus A26, A42; Streptomyces lavendulae A41; Streptomyces roseosporus A45; Streptomyces griseofuscus B15) can compete for iron and produce siderophore compounds to prevent pathogenic Vibrio species during in vitro challenge experiment.
Disruption of Quorum Sensing and Antibiofilm Activity
Pathogenic bacteria associated with aquaculture frequently produce many virulence factors and cause widespread mortality in fish and shellfish. Such virulence factors are induced by high cell density and abundant quorum-sensing signals. In aquaculture, some Streptomyces species have shown antiquorum sensing and anti-biofilm activities. The Streptomyces strain IM20 obtained from the gut of Indian mackerel (Rastrelliger kanagurta) isolated from Kovalam coastal area of Tamil Nadu tested for antiquorum sensing violacein production against pathogenic strain Chromobacterium violaceum MTCC 2656 and Serratia marcescens. For 6 days, strain IM20 was grown on ISP2 plates at 30 °C. After 6 days, overnight cultures of Chromobacterium violaceum MTCC 2656 and Serratia marcescens were spread on the bioassay plates and incubated for 24 h at 30 °C. As strain IM20 suppressed violet pigment production in the subjected strains without affecting bacterial growth, the antiquorum sensing screening activity resulted in the formation of turbid halo pigment-less areas [96, 97].
Streptomyces albus A66 isolated from near-shore marine sediments of the South China Sea was examined as per the screening system used by You et al. [85], disrupted the biofilm formation of Vibrio harveyi (isolated from infected white shrimp Litopenaeus vannamei) by 99.3%, and scattered the mature biofilm of Vibrio harveyi by 75.6% when used at a concentration of 2.5% (v/v). This antibiofilm activity was seen since Streptomyces metabolites reduced the number of Vibrio harveyi microcolonies by nearly tenfold and degraded the quorum sensing factor N-AHLs (N-acylated homoserine lactone) [86].
Antiviral Activity
In addition to suppressing the pathogenic bacterial growth in aquaculture, the secondary metabolites extracted from the Streptomyces have the ability to induce an antiviral effect against different aquaculture-associated viruses. Marine Streptomyces sp. VITSDK1 produced the secondary metabolite furan-2-yl acetate (C6H6O3), which demonstrated an inhibitory effect against the replication of fish nodavirus in the cell lines of Sahul Indian Grouper Eye (SIGE) with 90% cell survival when used at a minimum concentration of 20 µg mL−1 [98]. Ethyl acetate secondary metabolites extracts (unspecified) of haloalkaliphilic Streptomyces sp. AJ8 isolated from the solar salt works of Kovalam, Kanyakumari, Tamilnadu, India. This strain was incubated with white spot syndrome virus (WSSV) suspensions and injected intramuscularly into the Indian white shrimp, Fenneropenaeus indicus, according to Balasubramanian et al. [99], resulting in significant antiviral activity by reducing the occurrence of WSSV by 85% (P < 0.001) [75] (see Fig. 2).
Amelioration of Water Quality
The physicochemical status of pond water plays a crucial role in the well-being and growth of organisms in aquaculture as they are heavily dependent on their environment [52]. Deterioration of culture water mainly occurs when the metabolic waste from living organisms accumulates in the system or by the decay and decomposition of biotic material and unutilized feed. This affects the survival of the fish and shellfish against infections and diseases [100]. However, the addition of probiotic strains either in water or diet enhances water quality and improves the growth and survival of the host [52, 101]. The outcome of the bioremediation or bioaugmentation process depends greatly on the nature of the probiotics being used. Thus, probiotics should be added as per their specificity to perform bioremediation under the right environmental conditions at the correct population density to achieve the desired results.
According to Wang et al. [102], the probiotics tested on the ponds containing Penaeus vannamei during intensive farming resulted in the following beneficial effects:
-
Improved water quality.
-
Improved microbial interactions and diversity.
-
Increased beneficial microbial count, ammonifying, and protein mineralizing bacteria.
-
Increased organic matter decomposition and reduced nitrogen (N) and phosphorus (P) concentrations.
-
Higher dissolved oxygen (DO) concentration and better algal growth.
Some species of Streptomyces also increase the count of heterotrophic bacteria in the culture system (see Fig. 2) when used at a proper concentration at regular intervals, which plays a significant role in accelerating the decomposition of organic waste and reduction in the level of ammonia [76, 78]. Streptomyces coelicoflavus (A6), Streptomyces diastaticus (A44), Streptomyces parvus (A56), and Streptomyces champavatii (R32) in form of biogranules effectively decompose organic matter and ameliorate shrimp culture systems [103]. In vitro, soil-isolated Streptomyces sp. MOE6 was evaluated against complex pollutants such as heavy metals and oil spills. MOE6 strain’s siderophore compound “hydroxamate” and secondary metabolites “extracellular polysaccharides” reduced hazardous pollutants in metal removal assays and emulsification activity tests [104].
Protection Against Pathogens During Challenge Experiments
Before the introduction of probiotic strains into the actual aquaculture environment, laboratory-based challenge experiments are necessary to determine the viability of probiotic strains to compete against pathogens. Multiple in vivo challenge experiments demonstrate the importance of Streptomyces as a protective agent when employed as probiotics in aquaculture. Marine sediment-derived Streptomyces sp. SH5 strain was isolated from Xinghai Bay, Dalian, China, and used for the challenge experiment in zebrafish larvae. Aeromonas hydrophila pathogenic strain was isolated from silver carp (Hypophthalmichthys molitrix) infected with Aeromonas. Prior to the challenge, zebrafish larvae were pretreated with SH5 dilutions of 1:100 or 1:1000. After 24 h of challenge, there was no mortality in the pretreated group, with 80% and 50% survival after 36 h and 72 h of challenge, respectively. There was no noticeable difference in survival rate between larvae treated with different dilution rations. Pretreatment of zebrafish larvae with SH5 effectively inhibited Aeromonas hydrophila colonies by 67.53%. Multiple factors contributed to the SH5 strain’s potential, including an improvement in zebrafish metabolism due to a reduced inflammatory response, repression of virulence factors, a reduction in pathogen colony potential, and improved immune parameters [105]. Juvenile and adult Artemia treated with Streptomyces cells at 1% concentration (v/v) through bioencapsulation ensued a higher survival rate as compared to the control group after being challenged with Vibrio pathogens at 106 CFU/mL [77]. Streptomyces CLS-28 supplemented with feed for 15 days at the same concentration, increased protection of shrimp Penaeus monodon against 12 h Vibrio challenge as median lethal dose (LD50) at 106.5 CFU/mL. Streptomyces sp. N7 and Streptomyces sp. RL8 sprayed on pelleted feed as a bacterial suspension at 1 × 108 CFU g−1 weekly increased the survival of Litopenaeus vannamei during the Vibrio challenge [81]. Ethyl acetate crude extract of Streptomyces VITNK9 evaluated for its efficacy as a protective agent against different fish-associated pathogens showed a moderate response against Aeromonas hydrophila, Edwardsiella tarda, Vibrio anguillarum, Vibrio harveyi, and Aeromonas caviae [82].
Competitive Exclusion of Pathogens from the System
In addition to the in vivo challenge, Streptomyces also competitively excluded pathogens from the culture system (see Fig. 2). The isolation of compound 1-(2-hydroperoxycyclopentyl)-4-hydroxytridecan-7-one (HCHD) with the chemical formula C18H34O4 and the molecular weight 314.46 g/mol was achieved through bioactivity-guided extraction of ethyl acetate crude extract from Streptomyces sp. VITNK9. When used at a concentration of 100 g/mL against Edwardsiellatarda and Aeromonas hydrophila, the isolated compound demonstrated significant antipathogenic activity with an inhibition zone of 19.33 ± 0.47 mm and minimal inhibitory concentration of 3.125 μg/mL and 16.66 ± 0.47 mm and 12.5 μg/mL, respectively. HCHD treatment inhibited the bacterial acetate kinase to disrupt bacterial metabolism [106]. According to these findings, bioactive extracts of Streptomyces sp. VITNK9 could competitively exclude pathogens from the system. Biogranules of Streptomyces rubrolavendulae M56 reduced the mortality rate of P. monodon (post-larvae) and the viable Vibrio count in the rearing system after 28 days of treatment. Streptomyces rubrolavendulae M56 also antagonized V. harveyi, V. alginolyticus, V. parahaemolyticus, and V. fluvialis growth during in vitro co-culture experiment [29]. Streptomyces sp. RL8 isolated from marine sediments excluded V. parahaemolyticus from the culture system when used as a water additive [80].
Modulation of Enzymatic Activities
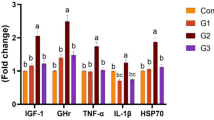
Feed utilization and digestion in cultured fish and shellfish depend on the ability of the host to produce enzymes. Probiotics can potentially produce digestive, extracellular, and antioxidant enzymes and/or modulate enzymatic activity [107,108,109]. Antioxidant enzymes protect the host against oxidative stress [110]. Soil-derived Streptomyces chartreusis KU324443 was used to prepare a basal-based diet for common carp (Cyprinus carpio) for three different experimental groups (S1, S2, and S3) at a concentration of 105, 106, and 107 CFU/g, properly blended and pelletized using a meat grinder. The prepared diets were fed to all three experimental groups for 2 months, and antioxidant enzyme activity (both in serum and skin mucus) was measured using a commercially available kit Zellbio®, Berlin, Germany. Serum antioxidant enzyme activity treatment groups showed higher superoxide dismutase (SOD) levels (P > 0.05) and moderate changes in catalase (CAT) and glutathione peroxidase (GPx). In terms of skin mucus antioxidant enzyme activity, no significant differences were observed between the treated and control groups [111]. Streptomyces’ ability to stimulate oxidative protection enzymes in the host that are hostile to oxidative stress could be attributed to the production of Exopolysaccharide (EPS). To prevent the harmful consequences of free radicals in various tissues, EPS production induces robust DPPH radical scavenging activity [112, 113]. Streptomyces also produces several hydrolytic enzymes that decompose organic matter to provide nutrients for mycelium formation. These nutrients can then be reutilized to produce spores by activating the reproduction process of aerial development [114]. Streptomyces can further secrete exoenzymes that colonize the host’s intestine to facilitate the digestion of food. For example, Streptomyces strains supplemented with feed secreted hydrolytic exoenzymes which improved the amylolytic and proteolytic activity in the digestive tract of Penaeus monodon to enhance feed utilization [77].
Stimulation in Growth and Survival
The proper utilization of feed is also essential for the development and survival of cultured fish and shellfish. Streptomyces virginiae W18 cultures were grown in AM6 medium for 6 days before being mixed with Carassius auratus feed in two different concentrations: 1:1 (group II) and 1:2 (group III). Carassius auratus was fed the prepared concentration for each group for 30 days, and the fish (n = 10/group) were randomly selected from both groups to observe their growth. In addition, fish (n = 10/group) from each group were selected for the challenge experiment and administered with 100 μL of Aeromonas veronii (1.0 × 108 CFU/mL) injection. Both groups fed W18-associated feed grew at a rate of 27.10% and 24.87%, respectively. In comparison to the control group’s 10% survival, groups challenged with Aeromonas veronii demonstrated 70% and 50% survival, respectively [115]. Streptomyces sp. supplemented with feed at a concentration of 5% fish body mass fed to Xiphophorus helleri once a day for 50 days. Absolute growth rate (AGR), specific growth rate (SGR), and relative growth rate (RGR) were all increased with overall 140.54% growth, 45% feed conversion efficiency, and 54.72% protein content [79]. Streptomyces sp. N7 supplemented feed increased the survival rate of Litopenaeus vannamei (post-larvae) compared to the control group, whereas Streptomyces sp. RL8 increased the survival rate and stimulated weight gain in Vibrio-challenged shrimp. Both strains made the host more resistant to disease when given as a feed supplement at a concentration of 108 CFU g−1 for 30 days [31].
Source of Protein to Aquaculture Species
Conventionally, animal-based proteins are used to fulfill the protein requirement of fish and shellfish in aquaculture due to a good amino acid balance and digestibility. However, probiotics based on Streptomyces are being considered an inexpensive and accessible alternative to animal-based proteins [79]. Single-cell protein (SCP) based on Streptomyces has been used as an alternative to animal-based proteins during Xiphophorus helleri culture, as it increases feed conversion and growth rate [83]. Another study demonstrated that using Streptomyces strains as SCP for 30 days of SCP-based feeding trials on Xiphophorus helleri resulted in significantly higher absolute growth rate (AGR), specific growth rate (SGR), and feed conversion ratio (FCR) than the control group [116]. SCP based on Streptomyces could thus play an important role in aquaculture nutrition and should be studied further.
Alteration in Gut Microflora
The intestinal ecology in aquaculture is important as the fish gut microbiome regulates health and determines the onset of disease [14]. A healthy gut microbiome aids in the digestion and absorption of feed, maintenance of an osmotic balance, and enhances immunity, whereas an unhealthy gut can induce various diseases and cause mortalities. Artificially altering the fish gut microflora using probiotics is the focus of researchers recently. When a dietary intervention trial of Streptomyces sp. RL8 was undertaken on white shrimp Litopenaeus vannamei, modulation in the gut microbiota and an increased Bacteriovorax population was observed, which protected shrimp against Vibrio infection [30].
A tabular representation of the specie/strain-wise mechanism of action of Streptomyces can be seen in Table 2.
Biotoxicity of Streptomyces Strains
García-Bernal et al. [31] evaluated the toxicity of Streptomyces sp. RL8 and N7 in Artemia salina nauplii adopting the method used by Rajabi et al. [117]. The experiment was conducted using Streptomyces spp. RL8 and N7 cell mass in five different concentrations 1, 5, 10, 50, and 100 g/L accordingly in 96-well polystyrene plates by adding 200 μL in each well. Ten (10) nauplii of Artemia salina were added per well for each concentration in triplicate and incubated at room temperature. Negative control was prepared using 10 nauplii of Artemia salina and artificially produced seawater. The toxicity of probiotic bacteria was determined by comparing the survival outcome of Artemia salina to the control group after the interval of 24, 48, and 72 h of the experiment. The addition of these concentrations in feed and oral administration caused no mortality to Artemia salina indicating the nontoxic behavior of mentioned Streptomyces strains. In the same study, he also performed the toxicity assay of the RL8 and N7 towards the post-larvae of Litopenaeus vannamei with an average weight of 0.24 ± 0.04 g. Streptomyces suspension cultures were equally sprayed on feed concentrations of 1 × 108, 1 × 109, and 1 × 1010 CFU g−1 and administered ad libitum. Ten (10) shrimps were cultured per experimental unit per treatment in triplicate according to the experimental design previously used by Purivirojkul [118] for controlling pathogenic bacteria in fairy shrimp Branchinella thailandensis culture. Survival of Litopenaeus vannamei was determined by comparing the results of this experiment with the control group after three intervals of 24, 48, and 72 h. Both strains were found innocuous to Litopenaeus vannamei as no mortality was caused during the experiment. Another experiment revealed that Streptomyces sp. MAPS15 was innocuous and nontoxic and caused no infection or mortality in Penaeus monodon [119].
Das et al. [77] have analyzed the biotoxicity of Streptomyces strains towards both nauplii and adults of Artemia salina. The toxicity test used harvested wet cell mass from three Streptomyces strains (CLS-28, CLS-39, and CLS-45). The experiment was carried out in sterile polystyrene 12-well cell culture plates. Artemia was counted and stored in five separate wells each containing 5 mL of sterile seawater with cell mass suspension concentrations of 0.1%, 0.5%, 1%, 5%, and 10%. After 72 h of incubation at 28°C, the mortality rate was determined at 24-, 48-, and 72-h intervals. The increase in cell mass concentration of Streptomyces strain CLS-39 resulted in a notably high mortality rate (F=69.71, P 0.01) for both nauplii (67.7%) and adult (64.3%) artemia.
To test, whether the Streptomyces treated fish/shellfish pose any threat to human consumers, García-Bernal et al. [120] evaluated Streptomyces strain V4 to determine its toxigenicity using the hemolytic assay. The strain was inoculated on agar plates (Cat. # 211728, BD-Bioxon, Franklin Lakes, NJ, USA) prepared with 5% of human blood and 2.5% of sodium chloride (NaCl); the plates were then incubated for 7 days at 30°C. Hemolytic activity was examined using a hemolytic Vibrio parahaemolyticus strain as a control. No hemolytic or toxic activity was observed during the experiment; however, in vivo testing in fish/shellfish is necessary for further clarity.
Drawbacks of Using Streptomyces as Probiotics in Aquaculture and Possible Solutions
The possible limitations of using Streptomyces as probiotics in aquaculture are as follows:
-
1.
Some Streptomyces strains are found in extreme environments and thus are difficult to extract.
-
2.
Culturing Streptomyces is laborious and challenging.
-
3.
Several compounds produced by Streptomyces have an unpleasant odor and taste.
-
4.
There is a risk of lateral gene transfer associated with Streptomyces.
Extreme and untapped environments are considered a hotspot of novel bacterial and fungal species with unique properties and applications, thus, attracting researchers from all around the globe. Several Streptomyces species are also extremophiles [121,122,123,124] possessing distinctive characteristics favorable to aquaculture [18, 25, 77, 125, 126]. Modern mechatronic collection devices are used to collect samples from extreme habitats [127]. For example, remote-operated submarine vehicle (ROVs) [128], robotic sampling systems (RSS), unmanned ground vehicles (UGVs), unmanned aerial vehicles (UAVs) [129], and autonomous underwater vehicles (AUVs) [130] are often used.
Culturing Streptomyces can be challenging due to a lack of standardized media and culturing methods. Streptomyces also have a slow growth rate; thus, identification requires extensive culture-dependent studies [28]. Additional experiments are needed to develop suitable and standardized laboratory procedures.
Geosmin (GSM, trans-1,10-dimethyl-trans-9-decalol) and 2-methylisoborneol (MIB (1-R-exo)-1,2,7,7-tetramethyl-bicyclo[2.2.1]heptan-2-ol) are two saturated bicyclic terpenoids produced as secondary metabolites by Streptomyces [131]. These compounds have a muddy/earthy taste and unpleasant odor [132, 133] which reduces the palatability of feed, consequently reducing the feed intake of cultured fish and shellfish [134]. Both GSM and MIB can be accumulated or absorbed in the gills, skin, and flesh up to 200–400-folds, reducing the commercial value of the fish [135]. Several techniques have been used for the remediation of these compounds from rearing water such as the use of powdered activated carbon, ozonation, and biofiltration [136]. In the case of Streptomyces, ozonation is more effective as it eradicates GSM and MIB from the rearing system via oxidation [137].
Additionally, various bacterial species are used for the biodegradation of MIB and GSM such as Pseudomonas spp., Pseudomonas aeruginosa, Pseudomonas putida, Enterobacter spp., Candida spp., Flavobacterium multivorum, Flavobacterium spp., Slaviensisbacillus spp., Bacillus subtilis, and Bacillus cereus, Bacillus subtilis, Arthrobacter atrocyaneus, Arthrobacter globiformis, Rhodococcus moris, Chlorophenolicus strain N-1053, and Rhodococcus wratislaviensis, respectively.
Inducing genetic mutation in Streptomyces and polymerase chain reaction (PCR)-targeted Streptomyces gene replacement are other techniques used to eliminate the odorous soil geosmin. Research shows that the Cyc2 protein in Streptomyces (specifically the N-terminal domain), required for geosmin biosynthesis, can be made to be inactive or even eliminated by PCR or a double crossover [28, 138].
Lastly, the possibility of lateral transfer of antibiotic resistance genes could be another limitation of using Streptomyces as probiotics in aquaculture. Various other probiotics which are often used in aquaculture may also develop antibiotic resistance such as several species of Enterococcus [139], Lactobacillus sp. [140], and Bacillus sp. [141]. Therefore, it is suggested that preference should be given to strains that do not possess any virulence or antibiotic-resistant genes. Systematic analysis should be carried out to determine the potential risks associated with antibiotic resistance genes in the Streptomyces genome. Remedial techniques could be opted to eliminate the genetic factor from the relevant probiotic strains which facilitate antibiotic resistance. For example, protoplast formation is used as a method to eliminate resistance gene-carrying plasmids from the Lactobacillus reuteri (ATCC55730) without affecting the therapeutic characteristics of the probiotic [142].
Future Prospects
Despite several bacterial species being extensively analyzed and utilized in aquaculture practices as probiotics, members of the class Actinomycetes are rarely considered [81, 143, 144]. A Few experiments in the recent past have highlighted the potential and prospects of species belonging to the class Actinomycetes, especially, Streptomyces in promoting the overall health of aquaculture species. Most of the previously conducted experiments focused on the use of single or multi-strain Streptomyces-based probiotics and overlooked the aspects of using multi-species Streptomyces-based probiotics. Several recently published original articles indicated the importance of multi-species probiotics as an eco-friendly growth stimulator in aquaculture [145, 146]. Thus, the use of Streptomyces in combination with other bacterial species could induce promising health benefits in aquaculture and requires further consideration.
Several other non-bacterial products such as prebiotics, mushrooms, microalgae, and yeast also benefited aquaculturists in maintaining healthy and sustainable aquaculture practices. Recently, postbiotics, phytobiotics, and paraprobiotics have also emerged and gained research attention by virtue of their long shelf life, safety, and potential health-promoting benefits on the host. Streptomyces incorporation with these products may synergistically confer greater health benefits which may result in better production and growth rate in both fish and shellfish aquaculture. Therefore, further experimentation on the use of Streptomyces as a probiotic candidate in a non-conventional manner is needed to better ascertain its potential in aquaculture.
Conclusion
Maintaining a sufficient food supply for an increasing global population is an expensive and strenuous task. Sustainable aquaculture has provided an alternative to meet market demands and global trade, reducing the overexploitation of natural resources by capture fisheries.
Additionally, the recent diversification and intensification of aquaculture also necessitated the development of new technological innovations to mitigate the effects of viral epizootics prevalent in aquaculture practices and to produce high-quality livestock with lower production time. An innovative approach to using live biotherapeutics for sustainable aquaculture has emerged in recent decades.
This review focuses particularly on the role of Streptomyces strains as potential probiotics in aquaculture. Studies have revealed numerous beneficial effects of Streptomyces on reared fish and shellfish. The secondary metabolites, antagonistic, and siderophore compounds produced by Streptomyces strains exerted antimicrobial, antibiofilm, antiviral, antifungal, and antioxidative effects on the cultured species. Streptomyces also enhance disease resistance, survival, growth, enzymatic activities, bioremediation of pond water, and modify the gut microflora.
There are also limitations and uncertainties associated with the use of some Streptomyces strains in aquaculture. To avoid undesired results, following a standardized, experimentally proven procedure of strain selection is mandatory. Further research is required for a comprehensive understanding of Streptomyces strains as probiotics before their use in aquaculture practices, especially those causing adverse effects and those with the possibility of gene transfer to the gastrointestinal microflora of fish, and later to human consumers.
References
FAO (2022) The state of world fisheries and aquaculture 2022. FAO, Rome
OECD-FAO (2021) OECD-FAO Agricultural Outlook 2021–2030. OECD, Paris
UNCTAD (2022) Trade and development report update: tapering in a time of conflict
UNCTAD (2022) Impact of the Covid-19 pandemic on trade and development: lessons learned
Bondad-Reantaso MG, Subasinghe RP, Arthur JR et al (2005) Disease and health management in Asian aquaculture. Vet Parasitol 132:249–272. https://doi.org/10.1016/j.vetpar.2005.07.005
Austin B, Zhang XH (2006) Vibrio harveyi: a significant pathogen of marine vertebrates and invertebrates. Lett Appl Microbiol 43:119–124. https://doi.org/10.1111/j.1472-765X.2006.01989.x
Erkinharju T, Dalmo RA, Hansen M, Seternes T (2021) Cleaner fish in aquaculture: review on diseases and vaccination. Rev Aquac 13:189–237. https://doi.org/10.1111/raq.12470
Geng Y, Liu D, Han S et al (2014) Outbreaks of vibriosis associated with Vibrio mimicus in freshwater catfish in China. Aquaculture 433:82–84. https://doi.org/10.1016/j.aquaculture.2014.05.053
Orozova P, Sirakov I, Austin DA, Austin B (2018) Recovery of Bacillus mycoides, B. pseudomycoides and Aeromonas hydrophila from common carp (Cyprinus carpio) and rainbow trout (Oncorhynchus mykiss) with gill disease. J Fish Dis 41:125–129. https://doi.org/10.1111/jfd.12686
Wu PS, Liu CH, Hu SY (2021) Probiotic Bacillus safensis NPUST1 Administration improves growth performance, gut microbiota, and innate immunity against Streptococcus iniae in Nile tilapia (Oreochromis niloticus). Microorganisms 9:2494. https://doi.org/10.3390/microorganisms9122494
Van Doan H, Lumsangkul C, Ruangwong OU et al (2021) Effects of host-associated probiotic Bacillus altitudinis B61–34b on growth performance, immune response and disease resistance of Nile tilapia (Oreochromis niloticus) raised under biofloc system. Aquac Nutr. https://doi.org/10.1111/anu.13410
Cabrera-Stevens MJ, Sánchez-Paz A, Mendoza-Cano F et al (2022) Transcriptome analysis reveals differential gene expression associated with white spot syndrome virus resistance in the shrimp Litopenaeus vannamei fed on functional diets. Aquaculture. https://doi.org/10.1016/j.aquaculture.2021.737434
Abdel-Latif HMR, Yilmaz E, Dawood MAO et al (2022) Shrimp vibriosis and possible control measures using probiotics, postbiotics, prebiotics, and synbiotics: a review. Aquaculture 551:737951. https://doi.org/10.1016/j.aquaculture.2022.737951
Butt UD, Lin N, Akhter N et al (2021) Overview of the latest developments in the role of probiotics, prebiotics and synbiotics in shrimp aquaculture. Fish Shellfish Immunol 114:263–281. https://doi.org/10.1016/j.fsi.2021.05.003
El-Kady AA, Magouz FI, Mahmoud SA, Abdel-Rahim MM (2022) The effects of some commercial probiotics as water additive on water quality, fish performance, blood biochemical parameters, expression of growth and immune-related genes, and histology of Nile tilapia (Oreochromis niloticus). Aquaculture. https://doi.org/10.1016/j.aquaculture.2021.737249
Ringø E, van Doan H, Lee SH et al (2020) Probiotics, lactic acid bacteria and bacilli: interesting supplementation for aquaculture. J Appl Microbiol. https://doi.org/10.1111/jam.14628
Yilmaz S, Yilmaz E, Dawood MAO et al (2022) Probiotics, prebiotics, and synbiotics used to control vibriosis in fish: a review. Aquaculture 547:737514. https://doi.org/10.1016/j.aquaculture.2021.737514
Dharmaraj S (2010) Marine Streptomyces as a novel source of bioactive substances. World J Microbiol Biotechnol 26:2123–2139. https://doi.org/10.1007/s11274-010-0415-6
Bubici G (2018) Streptomyces spp. as biocontrol agents against Fusarium species. CAB Rev Perspect Agric Vet Sci Nutr Nat Resour 13:1–15. https://doi.org/10.1079/PAVSNNR201813050
Kaweewan I, Hemmi H, Komaki H, Kodani S (2020) Isolation and structure determination of a new antibacterial peptide pentaminomycin C from Streptomyces cacaoi subsp. cacaoi. J Antibiot (Tokyo) 73:224–229. https://doi.org/10.1038/s41429-019-0272-y
Tan LTH, Chan KG, Pusparajah P et al (2019) Mangrove derived Streptomyces sp. MUM265 as a potential source of antioxidant and anticolon-cancer agents. BMC Microbiol 19:38. https://doi.org/10.1186/s12866-019-1409-7
Kim YJ, Kim J, Rho J-Y (2019) Antifungal activities of Streptomyces blastmyceticus strain 12–6 against plant pathogenic fungi. Mycobiology 47:329–334. https://doi.org/10.1080/12298093.2019.1635425
Chen C, Ye Y, Wang R et al (2018) Streptomyces nigra sp. nov. is a novel actinobacterium isolated from mangrove soil and exerts a potent antitumor activity In vitro. Front Microbiol 9:1587. https://doi.org/10.3389/fmicb.2018.01587
Tangerina MMP, Furtado LC, Leite VMB et al (2020) Metabolomic study of marine Streptomyces sp.: secondary metabolites and the production of potential anticancer compounds. PLoS ONE 15:e0244385. https://doi.org/10.1371/journal.pone.0244385
Tan LTH, Lee LH, Goh BH (2019) The bioprospecting of anti-Vibrio Streptomyces species: Prevalence and applications. Prog Microb Mol Biol 2. https://doi.org/10.36877/pmmb.a0000034
Tan LTH, Chan KG, Lee LH, Goh BH (2016) Streptomyces bacteria as potential probiotics in aquaculture. Front Microbiol. https://doi.org/10.3389/fmicb.2016.00079
Tang Y, Han L, Chen X et al (2019) Dietary supplementation of probiotic Bacillus subtilis affects antioxidant defenses and immune response in grass carp under Aeromonas hydrophila challenge. Probiotics Antimicrob Proteins 11:545–558. https://doi.org/10.1007/s12602-018-9409-8
Hariharan S, Dharmaraj S (2018) Selection of new probiotics: the case of Streptomyces. In: Therapeutic, Probiotic, and Unconventional Foods. Elsevier, pp 27–54
Augustine D, Jacob JC, Philip R (2016) Exclusion of Vibrio spp. by an antagonistic marine actinomycete Streptomyces rubrolavendulae M56. Aquac Res 47:2951–2960. https://doi.org/10.1111/are.12746
Mazón-Suástegui JM, Salas-Leiva JS, Medina-Marrero R et al (2020) Effect of Streptomyces probiotics on the gut microbiota of Litopenaeus vannamei challenged with Vibrio parahaemolyticus. Microbiologyopen 9:e967. https://doi.org/10.1002/mbo3.967
García-Bernal M, Medina-Marrero R, Rodríguez-Jaramillo C et al (2018) Probiotic effect of Streptomyces spp. on shrimp (Litopenaeus vannamei) postlarvae challenged with Vibrio parahaemolyticus. Aquac Nutr 24:865–871. https://doi.org/10.1111/anu.12622
Schrezenmeir J, de Vrese M (2001) Probiotics, prebiotics, and synbiotics: approaching a definition. Am J Clin Nutr 73:361s–364s. https://doi.org/10.1093/ajcn/73.2.361s
Fuller M (1989) Probiotics in man and animals. J Appl Bacteriol 66:365–378. https://doi.org/10.1111/j.1365-2672.1989.tb05105.x
WHO/FAO (2001) Evaluation of health and nutritional properties of powder milk and live lactic acid bacteria. Food Nutr Pap 71
Hill C, Guarner F, Reid G et al (2014) The international scientific association for probiotics and prebiotics consensus statement on the scope and appropriate use of the term probiotic. Nat Rev Gastroenterol Hepatol 11:506–514. https://doi.org/10.1038/nrgastro.2014.66
Irianto A, Austin B (2002) Probiotics in aquaculture. J Fish Dis 25:633–642. https://doi.org/10.1046/j.1365-2761.2002.00422.x
Kozasa M (1986) Toyocerin (Bacillus toyoi) as growth promotor for animal feeding. Microbiol Aliment Nutrition 4:121–135
Dhanaraj M, Haniffa MA, Singh SVA et al (2010) Effect of probiotics on growth performance of koi carp (Cyprinus carpio). J Appl Aquac 22:202–209. https://doi.org/10.1080/10454438.2010.497739
Yılmaz S, Ergun S, Yigit M, Çelik EŞ (2020) Effect of combination of dietary Bacillus subtilis and trans-cinnamic acid on innate immune responses and resistance of rainbow trout, Oncorhynchus mykiss to Yersinia ruckeri. Aquac Res 51:441–454. https://doi.org/10.1111/are.14379
Wang C, Liu Y, Sun G et al (2019) Growth, immune response, antioxidant capability, and disease resistance of juvenile Atlantic salmon (Salmo salar L.) fed Bacillus velezensis V4 and Rhodotorula mucilaginosa compound. Aquaculture 500:65–74. https://doi.org/10.1016/j.aquaculture.2018.09.052
Sadeghi F, Ahmadifar E, Shahriari Moghadam M et al (2021) Lemon Citrus aurantifolia peel and Bacillus licheniformis protected common carp, Cyprinus carpio, from Aeromonas hydrophila infection by improving the humoral and skin mucosal immunity, and antioxidative responses. J World Aquac Soc 52:124–137. https://doi.org/10.1111/jwas.12750
Liu H, Wang S, Cai Y et al (2017) Dietary administration of Bacillus subtilis HAINUP40 enhances growth, digestive enzyme activities, innate immune responses and disease resistance of tilapia, Oreochromis niloticus. Fish Shellfish Immunol 60:326–333. https://doi.org/10.1016/j.fsi.2016.12.003
Safari R, Adel M, Lazado CC et al (2016) Host-derived probiotics Enterococcus casseliflavus improves resistance against Streptococcus iniae infection in rainbow trout (Oncorhynchus mykiss) via immunomodulation. Fish Shellfish Immunol 52:198–205. https://doi.org/10.1016/j.fsi.2016.03.020
Newaj-Fyzul A, Adesiyun AA, Mutani A et al (2007) Bacillus subtilis AB1 controls Aeromonas infection in rainbow trout (Oncorhynchus mykiss, Walbaum). J Appl Microbiol 103:1699–1706. https://doi.org/10.1111/j.1365-2672.2007.03402.x
Li J, Tan B, Mai K (2009) Dietary probiotic Bacillus OJ and isomaltooligosaccharides influence the intestine microbial populations, immune responses and resistance to white spot syndrome virus in shrimp (Litopenaeus vannamei). Aquaculture 291:35–40. https://doi.org/10.1016/j.aquaculture.2009.03.005
Li J, Tan B, Mai K et al (2008) Immune responses and resistance against Vibrio parahaemolyticus induced by probiotic bacterium Arthrobacter XE-7 in Pacific white shrimp, Litopenaeus vannamei. J World Aquac Soc 39:477–489. https://doi.org/10.1111/j.1749-7345.2008.00188.x
Sathyapriya A (2021) Evaluation of probiotic bacteria Leucosnostoc mensenteroides against white gut disease in shrimp aquaculture. J Uni Shanghai Sci Technol 23:891–905. https://doi.org/10.51201/JUSST/21/11976
Abareethan M, Amsath A (2015) Characterization and evaluation of probiotic fish feed. Int J Pure Appl Zool 3:148–153
Balcázar JL, Vendrell D, de Blas I et al (2008) Characterization of probiotic properties of lactic acid bacteria isolated from intestinal microbiota of fish. Aquaculture 278:188–191. https://doi.org/10.1016/j.aquaculture.2008.03.014
Yilmaz S, Çoban N, Ergün S et al (2020) Combined effects of dietary Bacillus subtilis and trans-cinnamic acid on growth performance, whole body compositions, digestive enzymes and intestinal bacteria in rainbow trout (Oncorhynchus mykiss). J Zool Res 1. https://doi.org/10.30564/jzr.v1i2.1682
Sharifuzzaman SM, Rahman H, Austin DA, Austin B (2018) Properties of probiotics Kocuria SM1 and Rhodococcus SM2 isolated from fish guts. Probiotics Antimicrob Proteins 10:534–542. https://doi.org/10.1007/s12602-017-9290-x
Hura MUD, Zafar T, Borana K et al (2018) Effect of commercial probiotic Bacillus megaterium on water quality in composite culture of major carps. Int J Curr Agric Sci 8:268–273
Bhakta JN, Bhattacharya S, Lahiri S, Panigrahi AK (2022) Probiotic characterization of arsenic-resistant lactic acid bacteria for possible application as arsenic bioremediation tool in fish for safe fish food production. Probiotics Antimicrob Proteins. https://doi.org/10.1007/s12602-022-09921-9
Thurlow CM, Williams MA, Carrias A et al (2019) Bacillus velezensis AP193 exerts probiotic effects in channel catfish (Ictalurus punctatus) and reduces aquaculture pond eutrophication. Aquaculture 503:347–356. https://doi.org/10.1016/j.aquaculture.2018.11.051
Chu W, Zhou S, Zhu W, Zhuang X (2015) Quorum quenching bacteria Bacillus sp. QSI-1 protect zebrafish (Danio rerio) from Aeromonas hydrophila infection. Sci Rep 4:5446. https://doi.org/10.1038/srep05446
Zhao W, Yuan T, Piva C et al (2019) The probiotic bacterium Phaeobacter inhibens downregulates virulence factor transcription in the shellfish pathogen Vibrio coralliilyticus by N -acyl homoserine lactone production. Appl Environ Microbiol. https://doi.org/10.1128/AEM.01545-18
Zhou S, Xia Y, Zhu C, Chu W (2018) Isolation of marine Bacillus sp. with antagonistic and organic-substances-degrading activities and its potential application as a fish probiotic. Mar Drugs 16:196. https://doi.org/10.3390/md16060196
Zorriehzahra MJ, Delshad ST, Adel M et al (2016) Probiotics as beneficial microbes in aquaculture: an update on their multiple modes of action: a review. Vet Q 36:228–241. https://doi.org/10.1080/01652176.2016.1172132
Schoch CL, Ciufo S, Domrachev M et al (2020) NCBI Taxonomy: a comprehensive update on curation, resources and tools. Database. https://doi.org/10.1093/database/baaa062
Waksman SA, Henrici AT (1943) The nomenclature and classification of the actinomycetes. J Bacteriol 46:337–341. https://doi.org/10.1128/jb.46.4.337-341.1943
Samac DA, Willert AM, McBride MJ, Kinkel LL (2003) Effects of antibiotic-producing Streptomyces on nodulation and leaf spot in alfalfa. Appl Soil Ecol 22:55–66. https://doi.org/10.1016/S0929-1393(02)00109-9
Seipke RF, Kaltenpoth M, Hutchings MI (2012) Streptomyces as symbionts: an emerging and widespread theme? FEMS Microbiol Rev 36:862–876. https://doi.org/10.1111/j.1574-6976.2011.00313.x
Alam MT, Merlo ME, Takano E, Breitling R (2010) Genome-based phylogenetic analysis of Streptomyces and its relatives. Mol Phylogenet Evol 54:763–772. https://doi.org/10.1016/j.ympev.2009.11.019
Gago G, Diacovich L, Arabolaza A et al (2011) Fatty acid biosynthesis in actinomycetes. FEMS Microbiol Rev 35:475–497. https://doi.org/10.1111/j.1574-6976.2010.00259.x
Elliot MA, Buttner MJ, Nodwell JR (2007) Multicellular development in Streptomyces. Myxobacteria 419–438
Han JH, Cho MH, Kim SB (2012) Ribosomal and protein coding gene based multigene phylogeny on the family Streptomycetaceae. Syst Appl Microbiol 35:1–6. https://doi.org/10.1016/j.syapm.2011.08.007
Bérdy J (2005) Bioactive microbial metabolites: a personal view. J Antibiot 58:1–26. https://doi.org/10.1038/ja.2005.1
Law JWF, Ser HL, Duangjai A et al (2017) Streptomyces colonosanans sp. nov., a novel actinobacterium isolated from Malaysia mangrove soil exhibiting antioxidative activity and cytotoxic potential against human colon cancer cell lines. Front Microbiol. https://doi.org/10.3389/fmicb.2017.00877
Kemung HM, Tan LTH, Khan TM et al (2018) Streptomyces as a prominent resource of future anti-MRSA Drugs. Front Microbiol. https://doi.org/10.3389/fmicb.2018.02221
Jones SE, Elliot MA (2017) Streptomyces exploration: competition, volatile communication and new bacterial behaviours. Trends Microbiol 25:522–531. https://doi.org/10.1016/j.tim.2017.02.001
Flärdh K, Buttner MJ (2009) Streptomyces morphogenetics: dissecting differentiation in a filamentous bacterium. Nat Rev Microbiol 7:36–49. https://doi.org/10.1038/nrmicro1968
Dharmaraj S, Kandasamy D (2016) Antagonistic activity of marine sponges associated Actinobacteria. J Coas Life Med 4:465–474. https://doi.org/10.12980/jclm.4.2016J6-17
Sheeja MS, Selvakumar D, Dhevendaran K (2011) Antagonistic potential of Streptomyces associated with the gut of marine ornamental fishes. Middle-East J Sci Res 7:327–334
Verschuere L, Rombaut G, Sorgeloos P, Verstraete W (2000) Probiotic bacteria as biological control agents in aquaculture. Microbiol Mol Biol Rev 64:655–671. https://doi.org/10.1128/MMBR.64.4.655-671.2000
Jenifer JSCA, Donio MBS, Michaelbabu M et al (2015) Haloalkaliphilic Streptomyces spp. AJ8 isolated from solar salt works and its’ pharmacological potential. AMB Express 5:59. https://doi.org/10.1186/s13568-015-0143-2
Ajmal Khan S, Lyla PS, Das S (2006) Application of Streptomyces as a probiotic in the laboratory culture of Penaeus monodon (Fabricius). Isr J Aquacult Bamidgeh 58:198–204. https://doi.org/10.46989/001c.20439
Das S, Ward LR, Burke C (2010) Screening of marine Streptomyces spp. for potential use as probiotics in aquaculture. Aquaculture 305:32–41. https://doi.org/10.1016/j.aquaculture.2010.04.001
Aftabuddin S, Abul Kashem M, Abdul Kader M et al (2013) Use of Streptomyces fradiae and Bacillus megaterium as probiotics in the experimental culture of tiger shrimp Penaeus monodon (Crustacea, Penaeidae). AACL Bioflux 6:253–267
Dharmaraj S, Dhevendaran K (2010) Evaluation of Streptomyces as a probiotic feed for the growth of ornamental fish Xiphophorus helleri. Food Technol Biotechnol 48:497–504
García-Bernal M, Ossorio-Álvarezl PA, Medina-Marrero R et al (2020) Effect of Streptomyces sp. RL8 on the survival of Artemia franciscana nauplii and resistance to Vibrio parahaemolyticus. Fish Sci 86:137–144. https://doi.org/10.1007/s12562-019-01378-0
Bernal MG, Marrero RM, Campa-Córdova ÁI, Mazón-Suástegui JM (2017) Probiotic effect of Streptomyces strains alone or in combination with Bacillus and Lactobacillus in juveniles of the white shrimp Litopenaeus vannamei. Aquacult Int 25:927–939. https://doi.org/10.1007/s10499-016-0085-y
Saha S, Ishaque NM, Burgsdorf I et al (2021) Draft genome sequence of terrestrial Streptomyces sp. Strain VITNK9, isolated from Vellore, Tamil Nadu, India, exhibiting antagonistic activity against fish pathogens. Microbiol Resour Announc. https://doi.org/10.1128/MRA.01282-20
Selvakumar D, Jyothi PM, Dhevendaran K (2013) Application of Streptomyces as a single cell protein to the juvenile fish Xiphophorus maculatus. World J Fish Mar Sci 5:582–586
Sharifuzzaman SM, Austin B (2017) Probiotics for disease control in aquaculture. In: Diagnosis and control of diseases of fish and shellfish. John Wiley & Sons, Ltd, Chichester, UK, pp 189–222
You JL, Cao LX, Liu GF et al (2005) Isolation and characterization of actinomycetes antagonistic to pathogenic Vibrio spp. from nearshore marine sediments. World J Microbiol Biotechnol 21:679–682. https://doi.org/10.1007/s11274-004-3851-3
You J, Xue X, Cao L et al (2007) Inhibition of Vibrio biofilm formation by a marine actinomycete strain A66. Appl Microbiol Biotechnol 76:1137–1144. https://doi.org/10.1007/s00253-007-1074-x
Sirajudheen TK, Dhevendaran K, Pramod Kiran RB (2014) Studies on the effect of Streptomyces associated with seaweeds as single cell protein on the growth of prawn Macrobrachium Rosenbergii and antagonism against pathogens. J Aquat Biol Fish 2:256–259
Ayswaria R, Vasu V, Krishna R (2020) Diverse endophytic Streptomyces species with dynamic metabolites and their meritorious applications: a critical review. Crit Rev Microbiol 46:750–758. https://doi.org/10.1080/1040841X.2020.1828816
Aggarwal N, Thind SK, Sharma S (2016) Role of secondary metabolites of actinomycetes in crop protection. In: Plant growth promoting actinobacteria: a new avenue for enhancing the productivity and soil fertility of grain legumes. Springer Singapore, Singapore, pp 99–121
Peng Y, Lai X, Wang P et al (2022) The isolation of a novel Streptomyces termitum and identification its active substance against fish pathogens. Reprod Breed 2:95–105. https://doi.org/10.1016/j.repbre.2022.07.002
Solanki R, Kundu A, Das P, Khanna M (2015) Characterization of antimicrobial compounds from Streptomyces sp. World J Pharm Res 4:1626–1641
Guy AL, Taylor JH (1978) Actinomycin D inhibits initiation of DNA replication in mammalian cells. Proc Natl Acad Sci 75:6088–6092. https://doi.org/10.1073/pnas.75.12.6088
Wadkins RM, Vladu B, Tung CS (1998) Actinomycin D binds to metastable hairpins in single-stranded DNA. Biochemistry 37:11915–11923. https://doi.org/10.1021/bi9809730
Kaysser L, Wemakor E, Siebenberg S et al (2010) Formation and attachment of the deoxysugar moiety and assembly of the gene cluster for caprazamycin biosynthesis. Appl Environ Microbiol 76:4008–4018. https://doi.org/10.1128/AEM.02740-09
Huang Z, Tang W, Jiang T et al (2023) Structural characterization, derivatization and antibacterial activity of secondary metabolites produced by termite-associated Streptomyces showdoensis BYF17. Pest Manag Sci. https://doi.org/10.1002/ps.7359
Vignesh A, Ayswarya S, Gopikrishnan V, Radhakrishnan M (2019) Bioactive potential of actinobacteria isolated from the gut of marine fishes. Indian J Geomarine Sci 48:1280–1285
James G, Prasannan Geetha P, Thavarool Puthiyedathu S, Vattringal Jayadradhan RK (2023) Applications of actinobacteria in aquaculture: prospects and challenges. 3 Biotech 13:42. https://doi.org/10.1007/s13205-023-03465-7
Suthindhiran K, Sarath Babu V, Kannabiran K et al (2011) Anti-fish nodaviral activity of furan-2-yl acetate extracted from marine Streptomyces spp. Nat Prod Res 25:834–843. https://doi.org/10.1080/14786419.2010.530599
Balasubramanian G, Sarathi M, Kumar SR, Hameed ASS (2007) Screening the antiviral activity of Indian medicinal plants against white spot syndrome virus in shrimp. Aquaculture 263:15–19. https://doi.org/10.1016/j.aquaculture.2006.09.037
Zokaeifar H, Babaei N, Saad CR et al (2014) Administration of Bacillus subtilis strains in the rearing water enhances the water quality, growth performance, immune response, and resistance against Vibrio harveyi infection in juvenile white shrimp, Litopenaeus vannamei. Fish Shellfish Immunol 36:68–74. https://doi.org/10.1016/j.fsi.2013.10.007
Tuan TN, Duc PM, Hatai K (2013) Overview of the use of probiotics in aquaculture. Int J Res Fish Aquac 3:89–97
Wang YB, Xu ZR, Xia MS (2005) The effectiveness of commercial probiotics in northern white shrimp Penaeus vannamei ponds. Fish Sci 71:1036–1041. https://doi.org/10.1111/j.1444-2906.2005.01061.x
Babu DT, Archana K, Kachiprath B et al (2018) Marine actinomycetes as bioremediators in Penaeus monodon rearing system. Fish Shellfish Immunol 75:231–242. https://doi.org/10.1016/j.fsi.2018.01.037
Elnahas MO, Hou L, Wall JD, Majumder ELW (2021) Bioremediation potential of Streptomyces sp. MOE6 for toxic metals and oil. Polysaccharides 2:47–68. https://doi.org/10.3390/polysaccharides2010004
Liang Q, Liu G, Guo Z et al (2022) Application of potential probiotic strain Streptomyces sp. SH5 on anti-Aeromonas infection in zebrafish larvae. Fish Shellfish Immunol 127:375–385. https://doi.org/10.1016/j.fsi.2022.06.049
Nabila M, Krishnan K (2022) Streptomyces sp. VINTK9 derived compound against fish bacterial pathogens. Biomed Biotechnol Res J (BBRJ) 6:494. https://doi.org/10.4103/bbrj.bbrj_296_22
Jose Meseguer RC (2011) Current knowledge in synbiotic use for fish aquaculture: a review. J Aquac Res Dev. https://doi.org/10.4172/2155-9546.S1-008
Dawood MAO, Koshio S, Ishikawa M et al (2016) Probiotics as an environment-friendly approach to enhance red sea bream, Pagrus major growth, immune response and oxidative status. Fish Shellfish Immunol 57:170–178. https://doi.org/10.1016/j.fsi.2016.08.038
Gupta A, Verma G, Gupta P (2016) Growth performance, feed utilization, digestive enzyme activity, innate immunity and protection against Vibrio harveyi of freshwater prawn, Macrobrachium rosenbergii fed diets supplemented with Bacillus coagulans. Aquacult Int 24:1379–1392. https://doi.org/10.1007/s10499-016-9996-x
Abarike ED, Cai J, Lu Y et al (2018) Effects of a commercial probiotic BS containing Bacillus subtilis and Bacillus licheniformis on growth, immune response and disease resistance in Nile tilapia, Oreochromis niloticus. Fish Shellfish Immunol 82:229–238. https://doi.org/10.1016/j.fsi.2018.08.037
Arghideh M, Hoseinifar SH, Ghorbani Nasrabadi R et al (2022) Evaluation of soil-derived Streptomyces chartreusis KU324443 effects as probiotic on growth performance, antioxidant enzyme activity, mucosal and serum immune parameters, and related gene expression in common carp (Cyprinus carpio) fingerlings. Aquac Nutr 2022:1–9. https://doi.org/10.1155/2022/2278130
Pfaffl MW (2001) A new mathematical model for relative quantification in real-time RT-PCR. Nucleic Acids Res 29:45e–445. https://doi.org/10.1093/nar/29.9.e45
Lin YS, Saputra F, Chen YC, Hu SY (2019) Dietary administration of Bacillus amyloliquefaciens R8 reduces hepatic oxidative stress and enhances nutrient metabolism and immunity against Aeromonas hydrophila and Streptococcus agalactiae in zebrafish (Danio rerio). Fish Shellfish Immunol 86:410–419. https://doi.org/10.1016/j.fsi.2018.11.047
Chater KF, Biró S, Lee KJ et al (2010) The complex extracellular biology of Streptomyces. FEMS Microbiol Rev 34:171–198. https://doi.org/10.1111/j.1574-6976.2009.00206.x
Hu W, Yu X, Jin D et al (2021) Isolation of a new Streptomyces virginiae W18 against fish pathogens and its effect on disease resistance mechanism of Carassius auratus. Microb Pathog 161:105273. https://doi.org/10.1016/j.micpath.2021.105273
Dharmaraj S, Dhavendaran K (2015) Effect of Actinobacteria as a single cell protein on growth performance of Xiphophorus helleri. Int J Aquat Biol 3:19–24. https://doi.org/10.22034/ijab.v3i1.42
Rajabi S, Ramazani A, Hamidi M, Naji T (2015) Artemia salina as a model organism in toxicity assessment of nanoparticles. DARU J Pharm Sci. https://doi.org/10.1186/s40199-015-0105-x
Purivirojkul W (2013) Application of probiotic bacteria for controlling pathogenic bacteria in fairy shrimp Branchinella thailandensis culture. Turk J Fish Aquat Sci. https://doi.org/10.4194/1303-2712-v13_1_22
Sujith (2015) An in vivo efficacy validation and immune-modulatory potential of Streptomyces sp. J Coast Life Med 3:841–847. https://doi.org/10.12980/jclm.3.2015j5-127
García-Bernal M, Campa-Córdova ÁI, Saucedo PE et al (2015) Isolation and in vitro selection of actinomycetes strains as potential probiotics for aquaculture. Vet World 8:170–176. https://doi.org/10.14202/vetworld.2015.170-176
Subramani R, Aalbersberg W (2012) Marine actinomycetes: an ongoing source of novel bioactive metabolites. Microbiol Res 167:571–580. https://doi.org/10.1016/j.micres.2012.06.005
Rateb ME, Houssen WE, Harrison WTA et al (2011) Diverse metabolic profiles of a Streptomyces strain isolated from a hyper-arid environment. J Nat Prod 74:1965–1971. https://doi.org/10.1021/np200470u
Kamjam M, Nopnakorn P, Zhang L et al (2019) Streptomyces polaris sp. nov. and Streptomyces septentrionalis sp. nov., isolated from frozen soil. Antonie Van Leeuwenhoek 112:375–387. https://doi.org/10.1007/s10482-018-1166-x
Um S, Choi TJ, Kim H et al (2013) Ohmyungsamycins A and B: cytotoxic and antimicrobial cyclic peptides produced by Streptomyces sp. from a volcanic island. J Org Chem 78:12321–12329. https://doi.org/10.1021/jo401974g
Das S, Ward LR, Burke C (2008) Prospects of using marine actinobacteria as probiotics in aquaculture. Appl Microbiol Biotechnol 81:419–429. https://doi.org/10.1007/s00253-008-1731-8
Younis KM, Usup G, Ahmad A (2016) Secondary metabolites produced by marine Streptomyces as antibiofilm and quorum-sensing inhibitor of uropathogen Proteus mirabilis. Environ Sci Pollut Res. https://doi.org/10.1007/s11356-015-5687-9
Kamjam M, Sivalingam P, Deng Z, Hong K (2017) Deep sea actinomycetes and their secondary metabolites. Front Microbiol. https://doi.org/10.3389/fmicb.2017.00760
Pathom-aree W, Stach JEM, Ward AC et al (2006) Diversity of actinomycetes isolated from Challenger Deep sediment (10,898 m) from the Mariana Trench. Extremophiles 10:181–189. https://doi.org/10.1007/s00792-005-0482-z
Bae JH, Jo W, Park JH et al (2021) Evaluation of sampling methods for robotic sediment sampling systems. IEEE J Oceanic Eng 46:542–554. https://doi.org/10.1109/JOE.2020.3005576
Russo P, Del Bufalo A, Fini M (2015) Deep sea as a source of novel-anticancer drugs: update on discovery and preclinical/clinical evaluation in a systems medicine perspective. EXCLI J 14:228–236. https://doi.org/10.17179/excli2014-632
Dickschat JS, Bode HB, Mahmud T et al (2005) A novel type of geosmin biosynthesis in myxobacteria. J Org Chem 70:5174–5182. https://doi.org/10.1021/jo050449g
Klausen C, Nicolaisen MH, Strobel BW et al (2005) Abundance of actinobacteria and production of geosmin and 2-methylisoborneol in Danish streams and fish ponds. FEMS Microbiol Ecol 52:265–278. https://doi.org/10.1016/j.femsec.2004.11.015
Guttman L, van Rijn J (2008) Identification of conditions underlying production of geosmin and 2-methylisoborneol in a recirculating system. Aquaculture 279:85–91. https://doi.org/10.1016/j.aquaculture.2008.03.047
Auffret M, Pilote A, Proulx É et al (2011) Establishment of a real-time PCR method for quantification of geosmin-producing Streptomyces spp. in recirculating aquaculture systems. Water Res 45:6753–6762. https://doi.org/10.1016/j.watres.2011.10.020
Howgate P (2004) Tainting of farmed fish by geosmin and 2-methyl-iso-borneol: a review of sensory aspects and of uptake/depuration. Aquaculture 234:155–181. https://doi.org/10.1016/j.aquaculture.2003.09.032
Elhadi SLN, Huck PM, Slawson RM (2004) Removal of geosmin and 2-methylisoborneol by biological filtration. Water Sci Technol 49:273–280. https://doi.org/10.2166/wst.2004.0586
Gonçalves AA, Gagnon GA (2011) Ozone application in recirculating aquaculture system: an overview. Ozone Sci Eng 33:345–367. https://doi.org/10.1080/01919512.2011.604595
Gust B, Challis GL, Fowler K et al (2003) PCR-targeted Streptomyces gene replacement identifies a protein domain needed for biosynthesis of the sesquiterpene soil odor geosmin. Proc Natl Acad Sci 100:1541–1546. https://doi.org/10.1073/pnas.0337542100
Deng F, Chen Y, Zhou X et al (2021) New insights into the virulence traits and antibiotic resistance of Enterococci isolated from diverse probiotic products. Microorganisms 9:726. https://doi.org/10.3390/microorganisms9040726
Sharma P, Tomar SK, Sangwan V et al (2016) Antibiotic resistance of Lactobacillus sp. isolated from commercial probiotic preparations. J Food Saf 36:38–51. https://doi.org/10.1111/jfs.12211
Gueimonde M, Sánchez B, de los Reyes-Gavilán CG, Margolles A (2013) Antibiotic resistance in probiotic bacteria. Front Microbiol 4:202. https://doi.org/10.3389/fmicb.2013.00202
Rosander A, Connolly E, Roos S (2008) Removal of antibiotic resistance gene-carrying plasmids from Lactobacillus reuteri ATCC 55730 and characterization of the resulting daughter strain, L. reuteri DSM 17938. Appl Environ Microbiol 74:6032–6040. https://doi.org/10.1128/AEM.00991-08
Akhter N, Wu B, Memon AM, Mohsin M (2015) Probiotics and prebiotics associated with aquaculture: a review. Fish Shellfish Immunol 45:733–741. https://doi.org/10.1016/j.fsi.2015.05.038
Ninawe AS, Selvin J (2009) Probiotics in shrimp aquaculture: avenues and challenges. Crit Rev Microbiol 35:43–66. https://doi.org/10.1080/10408410802667202
Hossain MK, Hossain MM, Mim ZT et al (2022) Multi-species probiotics improve growth, intestinal microbiota and morphology of Indian major carp mrigal Cirrhinus cirrhosus. Saudi J Biol Sci 29:103399. https://doi.org/10.1016/j.sjbs.2022.103399
Hossain MK, Islam SMM, Rafiquzzaman SM et al (2022) Multi-species probiotics enhance growth of Nile tilapia (Oreochromis niloticus) through upgrading gut, liver and muscle health. Aquac Res 53:5710–5719. https://doi.org/10.1111/are.16052
Funding
This work was supported by the National Science Foundation of China (Nos. 41876148 and 81573306), the Lishui City Science and Technology Plan Project (No. 2021GYX18), and the Project of Zhejiang Chinese Medical University (No. 2021FSYYZY22).
Author information
Authors and Affiliations
Contributions
Usman Dawood Butt and Bin Wu conceived and designed the sketch of the study. Usman Dawood Butt, Sumaikah Khan, Liu Xiaowan, and Xiaoqin Zhang wrote the main manuscript text. Usman Dawood Butt, Sumaikah Khan, and Bin Wu checked the logicality and language of this manuscript. Usman Dawood Butt and Bin Wu were responsible for the overall study coordination of this manuscript. Usman Dawood Butt prepared figures and Tables 1–2. Awkash Sharma revised the whole manuscript and tables. All authors reviewed and approved the final manuscript.
Corresponding author
Ethics declarations
Competing Interests
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Butt, U.D., Khan, S., Liu, X. et al. Present Status, Limitations, and Prospects of Using Streptomyces Bacteria as a Potential Probiotic Agent in Aquaculture. Probiotics & Antimicro. Prot. 16, 426–442 (2024). https://doi.org/10.1007/s12602-023-10053-x
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12602-023-10053-x