Abstract
Cyanobacteria phycobiliproteins (PBPs) are already exploited in the food industries and for biotechnological applications but not in the agricultural field. Different concentrations (0.6 – 4.8 mg/mL) of Anabaena minutissima PBPs were applied to tomato seed to study their priming effect against the soil-borne fungal pathogen Rhizoctonia solani and in promoting plant growth. PBPs increased seedling emergence and vigour, showed activity against root rot disease (67%), and enhanced plant dry weight, length, and height. Generally, no dose effect has been observed except for dry weight (55% at 4.8 mg/mL). Seed treatment primed seeds and seedlings by leading to the activation of defence responses raising phenol (26% in hypocotyls) and flavonoid (26 and 45% in hypocotyls and epicotyls, respectively) contents and chitinase (4-fold at 2.4 and 4.8 mg/mL in hypocotyls) and β-1,3-D-glucanase (up to about 2-fold at all doses in epicotyls) activities. Micro-Attenuated Total Reflection Fourier Transform Infrared revealed changes in functional groups of primed seeds, hypocotyls and exudates released into the agar because of treatment. Protein extract from PBP-primed seedlings inhibited mycelial growth (67% for epicotyl proteins) and caused morphological alterations in hyphae. This research emphasizes the potential priming role of PBPs applied by seed treatment against soil-borne pathogens.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Phycobiliproteins are brilliantly coloured, highly fluorescent proteins of the photosynthetic light-harvesting antenna complexes of cyanobacteria and other marine organisms such as red algae. They are assessor pigments capturing solar light in the range beyond chlorophyll a absorption which is then transferred to the chlorophyll photosystem (Dufossé, 2018). They are already used in cosmetics and the food industry as natural safety colourants and are also commercialized as bright fluorescent labels for immunofluorescence assay (Chen & Jiang, 2018; Sekar & Chandramohan, 2008). Phycobiliproteins are characterized by several chromophores called phycobilins bonded to their polypeptide structure. Phycobilins are open-chain tetrapyrroles from heme group-derived (Sidler, 1994) existing in different configurational and conformational isomers (Ma et al., 2020; Mroginski et al., 2004). Four main classes of phycobiliproteins are known based on their spectroscopic properties: allophycocyanin (650–655 nm), phycocyanin (615–640 nm), phycoerythrin (565–575 nm), and phycoerythrocyanin (575 nm) (Bryant, 1982; Bryant et al., 1979).
Phycobiliproteins have multiple biological activities against human and animal pathogens, such as bacteria, fungi, and viruses (Dagnino-Leone et al., 2022; Vinothkanna & Sekar, 2020) making them attractive tools for the development of new biological products in different fields. Their application in the field of plant protection has been scarcely investigated so far. Only one study has actually demonstrated on tomato fruit the antifungal activity of Anabaena minutissima phycobiliproteins against Botrytis cinerea, the agent of grey mould disease (Righini et al., 2021a). On tomato fruits again, the same authors showed the activity of phycobiliproteins extracted from the cyanobacterium Arthrospira platensis and the red alga Hydropuntia cornea against B. cinerea (Righini et al., 2020).
The use of natural alternatives to synthetic products has been recommended since 2009 by the European Regulation (EC 1107/2009), concerning the placing of plant protection products on the market and repealing Council Directives 79/117/EEC and 91/414/EEC. Moreover, the European Green Deal has the ambitious goal of reducing the application of chemical pesticides in agriculture by 50% by the year 2030. In particular, the management of soil-borne pathogens by conventional synthetic chemical fungicides and fumigants has undergone restrictions because of environmental and human health issues (Panth et al., 2020). Thus, the research and the use of natural alternative solutions for plant disease management are increasing.
Among soil-borne pathogens, the fungus Rhizoctonia solani J.G. Kühn (teleomorph: Thanatephorus cucumeris (A.B. Frank) Donk.) has a worldwide distribution (Baker, 1970) and is highly polyphagous, capable of attacking various important horticultural plant species belonging to Solanaceae, Cucurbitaceae, Fabaceae, Asteraceae and Brassicaceae family in any developmental plant stages inflicting severe yield losses (Ajayi-Oyetunde & Bradley, 2018). The pathogen can survive for a long time in the soil and on plant debris as mycelium or as resistant structures called sclerotia. Seed germination and seedling emergence are key physiological periods for plant development, and they are high-risk plant phases for pathogen attack. During these early growth stages, the management of R. solani is crucial because the fungus can cause seed rot, pre- and post-emergence damping-off and collar rot leading to seedling death.
Tomato (Solanum lycopersicum L.) is among the most widely cultivated crop in the world with 189 million tons produced and 36,600 kg/ha yield in 2021 (FAOSTAT, https://www.fao.org/faostat/en/#data, accessed on 30 January 2023). In some of the production areas, tomato collar and root rot caused by R. solani is one of the most serious diseases causing losses from the nursery phase and during the cultivation in the field and greenhouse (Nikraftar et al., 2013; Solanki et al., 2012; Taheri & Tarighi, 2012).
Given the restrictions of synthetic products for soil treatment and the highly sensible plant stages to soil-borne pathogens in the nursery phase, seed treatment with natural substances can be considered an important practice to protect seeds and seedlings against pathogen attacks. Seed treatment may act as seed priming when it promotes seed germination and improves seedling establishment, and tolerance to abiotic and biotic stresses (Mahmood et al., 2016; Song et al., 2017; Tambussi et al., 2020). Seed priming works at the seed level, from dormancy to full germination, acting on processes such as the activation of DNA repair mechanisms and antioxidant pathways, which are essential for obtaining better-quality seeds. Overall, the seed priming technique is commonly employed for commercial seed lots to boost seed quality (Paparella et al., 2015). In this scenario, the priming effect of phycobiliproteins applied to seeds for controlling soil-borne pathogens has never been documented.
Therefore, this research's main objective was to investigate the biological effect of phycobiliproteins from the cyanobacterium A. minutissima, applied as a seed treatment on tomato as a model plant. In particular, the study was focused on the priming effect i) against the soil-borne pathogen R. solani under greenhouse conditions; ii) on primary and secondary metabolites in the seedling in in vitro experiments; iii) on modifications of functional groups in treated seeds, seedlings and in root exudates by micro-ATR FT-IR. To deeper evaluate the priming effect, the crude protein extract from primed seedlings was tested against R. solani mycelium growth.
Materials and methods
Phycobiliprotein extraction, quantification, and antioxidant activity
Phycobiliproteins (PBPs) were extracted from lyophilized biomass of A. minutissima Lemmermann, 1898 (BEA 0300B) from the Banco Español de Algas (BEA) Culture Collection (Righini et al., 2021a). The biomass (7.5 g) was suspended in 100 mL of phosphate buffer (0.2 M, pH 7) and then stirred at room temperature in the dark. After 4 h, the suspension was centrifuged for 20 min at 13 °C, 5000 rpm, and the PBPs phycocyanin, allophycocyanin and phycoerythrin in the supernatant were quantified at 652, 615 and 562 nm spectrophotometrically (Bennett & Bogorad, 1973; Bryant, 1982) by using the following equations:
The antioxidant activity of the PBPs in the supernatant was determined by measuring radical scavenging activity according to the DPPH (2,2-Diphenyl-1-picrylhydrazyl) method by Blois (1958) and Pan-utai and Iamtham (2019), with minor modifications, and expressed as inhibition percentage. Phycobiliprotein extract (150 μL) was mixed with 150 μL of DPPH solution (1.27 mM in 90% methanol), thoroughly shaken, and then let stand in darkness. As a control, a mixture of 150 µL MeOH (90%) with150 µl DPPH solution was used. After 30 min of incubation at room temperature, the absorbance of the solution was measured at 517 nm using a Shimadzu 1800 UV–Vis spectrophotometer. The radical inhibition percentage was calculated by the equation:
The assay was repeated three times (n = 3).
For the following experiments, PBPs were purified by using Amicon® Ultra-4 (Millipore Corporation, Burlington, MA, USA) centrifugal filtering devices and GV Millex® Syringe Filter Unit (pore diameter 0.45 µm, Millipore Corporation, USA) and then lyophilized and stored at 4 °C until use.
Pathogen and plant material
The fungal pathogen R. solani DAFS3001 (RS) belonging to the DISTAL collection was isolated from tissues of tomato plants showing symptoms of root and crown rot (Righini et al., 2021b).
The fungus was cultured on potato dextrose agar (PDA, 4%, Difco, Laboratories, Detroit, MI, USA) at 25 °C for the greenhouse and in vitro experiments.
Tomato seeds of cv. Marmande (L’Ortolano, Savini Vivai, Italy; production and purchase 2019; expiry date 31 December 2022) were used for all in vivo experiments because they showed to be very responsive to priming (Nawaz et al., 2012) and seedlings were highly sensitive to RS (Righini et al., 2022).
Seed treatment
Before treatment, seeds were first surface disinfected with ethanol (70%) for 5 min and then with a solution of NaClO (2.5%) for 3 min, then rinsed three times with sterile distilled water (Mbega et al., 2012 with modification). For treatment, the seeds were immersed in four PBP concentrations, 0.6, 1.2, 2.4 and 4.8 mg/mL, prepared by serial dilution 1:2 with sterile distilled water, and incubated in the dark overnight at room temperature. For the control (CTRL), seeds were immersed in sterile water at the same conditions. After treatment, seeds were rinsed three times with sterile distilled water, left to dry in a laminar airflow cabinet for 10 min, and then sown on a growing substrate or agar for greenhouse or in vitro experiments, respectively.
Greenhouse experiments
The biological activity of PBPs against RS root rot was determined under greenhouse conditions at 24–26 °C (day) and 20–22 °C (night), with a 12 h/12 h day/night photoperiod, 70% relative humidity. The pathogen has been inoculated in a plant-growing substrate (peat and sand, 7:3 w/w) by mixing homogenized RS colonies grown on PDA medium for 10 days at 25 °C (2% w/w, pathogen/substrate). Before the sowing, the inoculated substrate was incubated for 2 days under a black plastic film at 25 °C in a growth chamber. Fifty treated or untreated (CTRL) seeds were sown in a plastic pot (10.5 × 14 × 9.5 cm) containing the plant-growing substrate inoculated with RS (+ RS). Untreated seeds sown in not inoculated substrate (-RS) were used as the negative control. Four pots per treatment and controls were considered. The seedling emergence was assessed every two days from 4 days after sowing (DAS) until 14 DAS and the area under the emergence curve (AUEC = the integration of the fitted emergence curve between t = 4 and the endpoint 14 days) was calculated with GraphPad Prism software, version 5.01. Moreover, ten plants/pot randomly chosen, were gently removed from the substrate and their length was measured to calculate the seedling vigour index (SVI) according to Orchard (1977) as follows: SVI = seedling length (cm) × emergence percentage at 14 DAS. After 30 DAS, ten more plants/pot randomly chosen, were gently removed from the substrate, washed, and assessed for disease and plant growth parameters. The disease was evaluated visually based on root/collar necrosis as incidence (number of plants showing symptoms over the ten examined plants) and disease severity. Disease severity was rated using a 5-class scale (Righini et al., 2021a, b with modification) where: 0, absence of necrosis (0% of symptoms); 1, very slight necrosis (up to 3% of collar/root with symptoms); 2, slight necrosis (4–30% of collar/root with symptoms); 3, moderate necrosis (31–70% of collar/root with symptoms); 4, severe necrosis (> 71% of collar/root with symptoms). For plant growth parameters, plant length and height, and root length were measured on the same plants considered for the disease assessment. To determine plant dry weight, a further 10 plants for each pot, randomly chosen, were gently removed from the substrate, washed, and dried in a hoven at 70 °C for 72 h.
The experiment was repeated three times (n = 3).
In vitro experiments
These experiments were addressed to study the effect of seed treatment with PBPs at the concentrations of 0.6, 1.2, 2.4 and 4.8 mg/mL as above described (§ Seed treatment), on seed germination and seedling growth, and the content of compounds of secondary and primary metabolism such as phenols, flavonoids, chlorophylls, carotenoids and proteins. Treated seeds and the control were sown on agar medium (Oxoid Agar Bacteriological, 1.5%, Thermo Fisher Scientific, Milan, Italy) in sterilized aluminium trays covered with a thin transparent plastic film and incubated in a growth chamber at 24 ± 1 °C (day) and 21 ± 1 °C (night), with a 12 h/12 h day/night photoperiod, 70% relative humidity.
Each of the following experiments and assays was repeated three times (n = 3) under the same experimental conditions.
Seed germination and seedling growth
For seed germination and seedling growth assessments, 75 trays (23 × 10 × 5 cm), were sown (30 seeds/tray, 15 trays/treatment and control). Firstly, seed germination was recorded at 4 DAS in all trays (15 trays/treatment, replicates), considering a seed germinated when the radicle protruded more than 2 mm (Hernández-Herrera et al., 2016). For seedling growth assessment, the 15 trays/treatment and control were divided into 5 groups (3 trays/group, replicates) for the harvesting of seedlings at 5 different times, 6, 8, 10, 12 and 14 DAS. From each tray, 20 seedlings were collected, and their length was measured for the calculation of the area under the seedling length curve (AUSLC) with GraphPad Prism software, version 5.01.
Determination of phenols, flavonoids, chlorophylls, and carotenoids
Six trays/treatment and the control (30 trays, 23 × 10 × 5 cm, 30 seeds/tray) were sown with seeds treated with PBPs at the four concentrations and water as the control. The 6 trays/treatment were divided into 2 groups (3 trays/group, replicates) for the harvesting of seedlings at 2 different times, 6 and 14 DAS. From each tray, 20 seedlings were gently removed. Seedlings collected at 6 DAS from each treatment were immediately snap-frozen, lyophilized, and used for phenol and flavonoid content determination. Seedlings collected at 14 DAS were first cut to separate epicotyls from the hypocotyls, snap-frozen and then lyophilized for the determination of phenols, flavonoids, chlorophylls, and carotenoids.
Total phenol content was determined by the Folin–Ciocalteau method (López Arnaldos et al., 2001). Epicotyls or hypocotyls were ground with absolute methanol (0.1 mL/mg), incubated for 30 min on ice, and then centrifuged for 30 min at 4 °C and 12,000 rpm. Phenols were determined in the supernatants using gallic acid (Sigma Aldrich, Merk, Darmstadt, Germany) as a standard curve (Meenakshi et al., 2009). Phenolic extract (50 µL) was added with 1 mL of Na2CO3 2% and 75 µL of Folin–Ciocalteau reagent (Sigma Aldrich, Merk) and the absorbance was measured at λ = 725 nm after 15 min incubation in the dark at 25 °C. Results were expressed as mg gallic acid equivalents (GAE) per mg freeze-dried sample (FDS).
Flavonoid content was determined by following the method of Schiavon et al. (2010). One mg of epicotyls or hypocotyls was crushed with 50 µL acidified methanol solution (HCl 1%), incubated at 4 °C for 16 h and the absorbance was measured at λ = at 300 nm. Flavonoid content was expressed as mg quercetin equivalents (QE) per mg FDS.
As for the determination of chlorophylls and carotenoids, freeze-dried epicotyls (FDE) were used. Five mg of samples were crushed with MeOH (100%, 1 mL), vortex mixed 3 times, 1 min each time, and then incubated in the dark for 3 h at 23 °C. After centrifugation at 12000 rpm for 30 min, the absorbance at 665, 652 and 470 nm was measured in supernatants. The chlorophyll and carotenoid content was calculated using the following equations (Lichtenthaler & Buschmann, 2001; Wellburn, 1994):
Protein content, chitinase and β-1,3-glucanase activities and antifungal activity of proteins against R. solani
A separate experiment was carried out to study if the seed treatment with PBPs was able to elicit in seedlings a defence response in terms of the activity of pathogenesis-related proteins, chitinases and β-1,3 glucanases. Then the proteins from seedlings were tested for their antifungal activity against RS.
Three trays/treatment (replicates) and the control (15 trays, 24 × 16 × 5, 100 seeds/tray) were sown with seeds treated with PBPs at the four concentrations and water as the control. After 14 DAS of incubation at the conditions reported above, seedlings were collected from each tray, cut to separate epicotyls from the hypocotyls, weighted, and snap-frozen at – 80 °C. Three g of both epicotyls and hypocotyls for each treatment and the control were used for crude protein extraction. Samples were ground in liquid nitrogen, added with 3 mL sodium acetate buffer (20 mM pH 5.5) containing polyvinylpolypyrrolidone (1% w/v, Sigma–Aldrich Co.) and then incubated for 60 min at 4 °C under continuous gentle stirring. Samples were centrifuged twice for 20 min at 12,000 rpm and 4 °C and the supernatant was filtered by using GV Millex® Syringe Filter Unit (Millipore Corporation, USA). Protein content was determined by Bradford (1976) method. The protein content was expressed as µg protein per g of fresh weight.
For chitinase and glucanase activities, the cup plate method was used (Bargabus et al., 2004 with modification; Righini et al., 2022). In glass Petri plates (14 cm diam.) filled with agarose gel (1.5%), 20 µg of proteins were added in triplicate to 7 mm-diameter wells cut in the gel containing 0.01% glycol chitin, for chitinase activity assay, or 0.5 mg/mL laminarin (from Laminaria digitata, Sigma Aldrich St Louis, MO, USA) for the assay of β-1,3-glucanase activity. As standards, chitinase from Streptomyces griseus (Sigma-Aldrich St Louis, MO, USA) or β-1,3-D-glucanase from Helix pomatia (Sigma Aldrich) were used. Plates were incubated at 37 °C for 24 h. For the chitinase activity determination, plates were irrigated with 50 mL of 500 mM Tris–HCl (pH 8.9) containing 0.01% fluorescent brightener, rinsed with distilled water 2 h later, flooded with distilled water overnight in the dark, and then observed at λ = 302 nm under a UV light source. Images of non-fluorescent lytic zones were taken with a digital camera, and then the area (mm2) of the chitinase activity lytic zone was calculated with the Quantity One 1-D analysis software v. 4.6.6 (Bio-Rad Laboratories Inc., Hercules, CA, USA). The specific chitinase activity was expressed as units. One unit corresponds to the mg of N-acetyl-D-glucosamine released/h/mg of protein in comparison to the standard. For the detection of β-1,3-glucanase activity, plates were coloured with 50 mL of 2,3,5-triphenyl tetrazolium chloride (0.15% in NaOH 1 M) for 30 min at 37 °C. Plates were then coloured with 50 mL of 2,3,5-triphenyl tetrazolium chloride (0.15% in NaOH 1 M) for 30 min at 37 °C until the pink lytic zones were visible. Images were processed by the Quantity One 1-D analysis software as above described for chitinase activity. Glucanase activity was expressed as units. One unit corresponds to the release of 1 µmol of glucose from laminarin/min in comparison to the lytic zone of the standard.
To evaluate the antifungal activity of crude protein extract on RS colony growth, the submerged colony technique was used (Righini et al., 2021a). Fungal portions (Ø 7 mm) from the edge of a 12 day-old-colony were treated for 6 h at 24 °C by immersion in 800 µL of crude proteins extracted from epicotyls and hypocotyls of seedlings from seed treated with 0.6, 1.2, 2.4, and 4.8 mg PBPs /mL and in 800 µL of water as control. Fungal portions were also immersed in sodium acetate buffer (20 mM pH 5.5) that was previously used for protein extraction. After treatment, colony portions were placed in the centre of Petri plates on PDA medium and then incubated at 24 °C in the dark. Three plates (replicates) were considered for each treatment and control. Daily measurements of fungal colony diameter were taken along two perpendicular diameters from 1 to 4 days after treatment. Results were expressed as cm of colony growth per day.
At the last assessment, microscopical observations to detect morphological alterations of hyphae were made by using a Nikon ECLIPSE TE2000 E microscope (Nikon Instruments Europe BV, Amsterdam, The Netherlands) equipped with the Nikon NIS Elements AR 2.20 software for image captures with a Nikon DXM1200F digital. For each plate, 3 small fungal pieces (approximately 9 mm2) from the edge of the RS colony were taken and observed at the microscope at 200 and 400 magnifications. For each fungal piece, ten hyphal diameter measurements were recorded. In 100 µm length of the same hyphae used for the previous measures, the number and size of vacuoles were detected. The vacuole number was evaluated by using a 4-point scale where 1 = number of vacuoles ≤ 5; 2 = from 6 to 10; 3 = from 11 to 15; 4 = ≥ 16.
Micro-Attenuated Total Reflection Fourier Transform Infrared (Micro-ATR FT-IR)
Micro-ATR FT-IR analysis was performed to detect possible modifications of functional groups in treated seeds, and seedlings grown in agar medium and identification of functional groups of compounds released from roots in the medium. Three batches (10 seeds each) of treated seeds and control were considered. Five seedlings from each tray of the in vitro experiments were collected at 6 DAS and 14 DAS. For 14 DAS seedlings, epicotyls and hypocotyls were separated. A small portion of the agar medium around hypocotyls of the harvested seedlings was removed with a sterile cork-borer (Ø 7 mm). All samples were freeze-dried and then ground in fine powder with a mortar and pestle. Micro-ATR FT-IR spectra of lyophilized samples were recorded by using a Tensor FT-IR spectrometer (Bruker Optics, Ettlingen, Germany) equipped with an accessory for analysis in micro-ATR, single reflection, and angle of 45° angle of incidence (Specac Quest ATR, Specac Ltd., Orpington, Kent, UK). The spectra were acquired (64 scans per sample or background) in the range of 4000–400 cm−1 at a resolution of 4 cm−1 and processed using the Grams/386 spectroscopic software (version 6.00, Galactic Industries Corporation, Salem, NH, USA). The second derivative was applied to some spectra to provide much more detailed information on overlapping bands and their frequencies.
Statistical analysis
All experiments were arranged in a completely randomized design. Data were analyzed by ANOVA, and, if the p-value was less than 0.05, means were separated by Tukey HSD test (p < 0.05). All analyses were performed with GraphPad Prism software, San Diego, CA, USA, v. 4.03, 2005.
Results
Phycobiliprotein quantification, and antioxidant activity
The content of the different PBPs of A. minutissima is reported in Table 1. Different concentrations of phycocyanin, allophycocyanin and phycoerythrin were detected. Phycoerythrin was 2-fold more abundant than phycocyanin and allophycocyanin.
The PBP extract of the cyanobacterium showed a percentage of the radical scavenging capacity of 20.490 ± 0.264.
Biological activity of phycobiliproteins against R. solani root rot under greenhouse conditions
Each concentration of PBP applied to seeds was effective in boosting seed emergence and seedling vigour (Table 2). All treatments significantly increased the AUEC and SVI with respect to the infected control (CTRL + RS) without differences among doses. The concentrations of 0.6 and 1.2 mg/mL gave AUEC results significantly similar to the non-infected control (CTRL-RS), with an average increase of 55.2% as compared with CTRL + RS. All concentrations gave an increase of SVI by an average of 64.4% as compared with CTRL + RS.
For what concerns the effect of treatment against root rot disease, all concentrations significantly reduced both disease severity and disease incidence in comparison with CTRL (Fig. 1). For disease severity, no differences among PBP doses were obtained, while for disease incidence, the concentrations of 2.4 and 4.8 mg/mL showed the highest efficacy (40.3% of average reduction) (Fig. 1).
Effect of seed treatment with different concentrations of Anabaena minutissima phycobiliproteins on disease severity and disease incidence of plants grown in soil infected with Rhizoctonia solani, 30 days after sowing. CTRL = plants from water-treated seeds. Columns are means of 3 independent experiments (n = 3) ± SE. ANOVA analysis: disease severity, F(4,10) = 10.20, p = 0.0015; disease incidence, F(4,10) = 52.93, p < 0.001. Different letters indicate significant differences among treatments and the control according to the Tukey test (p < 0.05)
All concentrations of PBPs significantly increased plant dry weight when compared with CTRL + RS and gave similar results to the CRTL-RS (Fig. 2). This increase was slightly related to the dose by reaching a value of 55,1% at 4.8 mg/mL. The plant length of seedlings from seeds treated with 1.2, 2.4 and 4.8 mg/mL was higher than the CTRL + RS, and root length was increased by seed treatment with 1.2 and 4.8 mg/mL by 30.5 and 41.6%, respectively (Fig. 2). The plant height was similarly increased by 0.6, 1.2 and 2.4 mg/mL concentrations at an average of 82.0% compared with CTRL + RS. This increase was comparable to the CTRL-RS (Fig. 2).
Effect of seed treatment with different concentrations of Anabaena minutissima phycobiliproteins on plant dry weight, total plant and root length, and plant height (aerial part) 30 days after sowing in soil infected with Rhizoctonia solani (RS). CTRL = control (plants from water-treated seeds). Columns are means of 3 independent experiments (n = 3) ± SE. ANOVA analysis: dry weight, F(5,12) = 13.74, p = 0.0001; total plant length, F(5,12) = 9.44, p = 0.0008; root length, F(5,12) = 22.26, p < 0.0001; plant height, F(5,12) = 5.78, p = 0.0061. Different letters indicate significant differences among treatments and the control according to the Tukey test (p < 0.05)
In vitro experiments
Seed germination and seedling growth
The germination of seeds treated with all PBP concentrations was significantly similar to the CTRL (average: 98.9% ± 1.0 and 97.8% ± 2.5 for treated and control, respectively, at 7 DAS).
In Table 3, the results of seed treatment with PBPs on seedling length (AUSLC) throughout the 14 days of monitoring. The AUSLC showed an increase with similar values following the seed priming with 1.2, 2.4 and 4.8 mg/mL concentrations.
Seedling content of phenols, flavonoids, chlorophylls, and carotenoids
Phenol and flavonoid contents were recorded on 6 DAS seedlings at first (Fig. 3). The phenol content after seed priming with PBPs at 1.2, 2.4 and 4.8 mg/mL was higher than the CTRL, without differences among doses. The flavonoid content was significantly enhanced only by 0.6 and 1.2 mg/mL similarly.
Effect of seed treatment with different concentrations of Anabaena minutissima phycobiliproteins on phenol and flavonoid content of seedlings 6 days after sowing in agar medium. Phenols are expressed as gallic acid equivalent (GAE) and flavonoids as quercetin equivalent (QE); FDS = freeze-dried sample; CTRL = control (seedlings from water-treated seeds). Columns are means of 3 independent experiments (n = 3) ± SE. ANOVA analysis: phenols, F(4,10) = 7.54, p = 0.0045; flavonoids, F(4,10) = 20.36, p = 0.0001. Different letters indicate significant differences among treatments and the control according to the Tukey test (p < 0.05)
At 14 DAS, phenol and flavonoid contents were recorded in both epicotyls and hypocotyls (Fig. 4). The phenol content was increased only in the hypocotyl by all doses in a similar way by an average of 26.2% compared to the CTRL. Instead, flavonoids were produced by all doses in greater quantities with respect to the CTRL both in epicotyls and hypocotyls by 26.4 and 45.0% on average, respectively. No differences among doses were observed.
Effect of seed treatment with different concentrations of Anabaena minutissima phycobiliproteins on phenol and flavonoid content of seedlings 14 days after sowing in agar medium. Phenols are expressed as gallic acid equivalent (GAE) and flavonoids as quercetin equivalent (QE); FDS = freeze-dried sample; CTRL = control (seedlings from water-treated seeds). Columns are means of 3 independent experiments (n = 3) ± SE. ANOVA analysis: epicotyl phenols, F(4,10) = 2.52, p > 0.05; hypocotyl phenols, F(4,10) = 5.64, p = 0.0122; epicotyl flavonoids, F(4,10) = 5.27, p = 0.0152; hypocotyl flavonoids, F(4,10) = 5.18, p = 0.0160. Different letters indicate significant differences among treatments and the control according to the Tukey test (p < 0.05); n.s = ANOVA analysis p > 0.05
For chlorophyll content, differences among treatments and CTRL in epicotyls were insignificant, while for carotenoids a significant increase of 58.3% (µg carotenoids/mg freeze-dried epicotyls) was obtained only by seed treatment with PBPs at 0.6 mg/mL in comparison with CTRL.
Protein content, chitinase, β-1,3-glucanase and protein antifungal activities against R. solani
The protein content determined in seedlings at 14 DAS was enhanced by all treatments in both epicotyls and hypocotyls (Fig. 5). The increase in epicotyls was similar among doses by 36.0% on average. In hypocotyls, the concentration of 0.6 mg/mL gave the highest increase (78.5%) with respect to CTRL.
Effect of seed treatment with different concentrations of Anabaena minutissima phycobiliproteins on the protein content of seedlings, 14 days after sowing; CTRL = control (seedlings from water-treated seeds). Columns are means of 3 independent experiments (n = 3) ± SE. ANOVA analysis: epicotyls F(4,10) = 5.83, p = 0.0109; hypocotyl, F(4,10) = 8.67, p = 0.0027. Different letters indicate significant differences among treatments and the control according to the Tukey test (p < 0.05)
The seed treatment with PBPs also caused an increase in enzyme activities in both epicotyls and hypocotyls (Fig. 6). The chitinase activity was approximately 4-fold increased by PBPs at 2.4 and 4.8 mg/mL only in the hypocotyls in comparison to CTRL. Glucanase activity was significantly enhanced by all concentrations except for 4.8 mg/mL treatment in hypocotyls.
Chitinase and β-1,3 glucanase activities of crude protein extracts from epicotyls and hypocotyls of seedlings from seeds treated with different concentrations of Anabaena minutissima phycobiliproteins. CTRL = control (seedlings from water-treated seeds). For chitinase, one unit corresponds to the mg of N-acetyl-D-glucosamine released/h/mg of protein in comparison to the standard. For glucanase, one unit corresponds to the release of 1 µmol of glucose from laminarin/min in comparison to the standard. Columns are means of 3 independent experiments (n = 3) ± SE. ANOVA analysis: epicotyl chitinases, F(4,10) = 14.92, p = 0.0003; hypocotyl chitinases, F(4,10) = 38.12, p < 0.0001; epicotyl glucanase, F(4,10) = 12.19, p = 0.0007; hypocotyl glucanase, F(4,10) = 76.52, p = 0.0075. Different letters indicate significant differences among treatments and the control according to the Tukey test (p < 0.05)
The crude protein extract from seedlings primed with the seed treatment with the four concentrations of PBPs was able to affect RS colony growth and mycelium morphology (Fig. 7). The daily colony growth was significantly reduced by the crude protein extract from epicotyls and hypocotyls when compared to CTRL or extraction buffer. The reduction was very marked when proteins were extracted from epicotyls (67.3% compared with CTRL). The number of vacuoles (vacuole index) in hyphae of the RS colony treated with the protein extract significantly increased at the doses of 1.2, 2.4 and 4.8 mg/mL in both epicotyls and hypocotyls. Consequently, in the same hyphae segments, the vacuole size was reduced by the same concentrations. A slight increase in hyphal diameter was observed at 4.8 mg/mL in epicotyls and 2.4 and 4.8 mg/mL in hypocotyls.
Mean daily colony growth, vacuolization index, vacuole size and hyphal diameter of Rhizoctonia solani treated with crude protein extract from epicotyls and hypocotyls of seedlings from seeds treated with different concentrations of Anabaena minutissima phycobiliproteins. CTRL = control (seedlings from water-treated seeds). Columns are means of 3 independent experiments (n = 3) ± SE. ANOVA analysis: daily colony growth/epicotyl, F(5,12) = 32.75, p < 0.0001; daily colony growth/hypocotyl, F(5,12) = 9.67, p = 0.0007; vacuolization index/epicotyl, F(5,12) = 8.25, p = 0.0014; vacuolization index/hypocotyl, F(5,12) = 14.93, p = 0.0001; vacuole size/epicotyl, F(5,12) = 8.61, p = 0.0012; vacuole size/hypocotyl, F(5,12) = 7.58, p = 0.0020; hyphal diameter/epicotyl, F(5,12) = 5.50, p = 0.0074; hyphal diameter/hypocotyl, F(5,12) = 5.93, p = 0.0055. Different letters indicate significant differences among treatments and the control according to the Tukey test (p < 0.05)
Micro-ATR FT-IR of tomato seeds
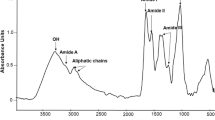
The functional groups of untreated and treated with PBP tomato seeds were analyzed in the region between 1800–1500 cm−1, where typical protein and lipid bands are located. As an example, the spectra of treated seeds at 2.4 mg/mL and 4.8 mg/mL are given in Fig. 8. In more detail, the bands at 1740 and 1710 cm− 1 are assigned to ester C-O stretching vibration in triacylglycerols and acylglycerols and C = O stretching motion in fatty acids, respectively (Lucarini et al., 2020; Ulchenko et al., 1983). A significant decrease in the relative intensity of the band at 1740 cm−1 has been observed in the spectrum of seeds treated with PBP at 4.8 mg/mL (Fig. 8 solid line) compared to untreated (dotted line) and 2.4 mg/mL (dashed line) ones. This band at lower doses (spectra not shown) is not different from that of the untreated. The presence of triacylglycerols and acylglycerols has been identified in tomato seed oil. Notably, of these compounds the unoxidized acylglycerols account for 80–90%, while the oxidized components are represented by the epoxyacylglycerols, corresponding for 0.4–0.8% (Ulchenko et al., 1983). Conversely, the band at 1710 cm−1 rose in seeds treated with PBP at 4.8 mg/mL (Fig. 8 solid line). Looking at the spectra of the primed seeds at 1.2 mg/mL (spectrum not shown) and 2.4 mg/mL (Fig. 8 dashed line), the band at 1710 cm−1 has not changed compared to that of the untreated seeds. Reverse reactions (esterification) or various transesterification reactions may be involved (Freire & Castilho, 2008). By considering the proteins, two broad bands at 1643 and 1541 cm− 1 are due to C = O stretching/C = N stretching for amide I and N–H bending/C-N stretching for amide II, respectively. The relative intensity of Amide I and Amide II decreased in seeds primed with PBP at 2.4 mg/mL (Fig. 8 dashed line). There is no variation in amide I and amide II in seed primed at doses 0.6 mg/mL and 1.2 mg/mL.
Micro-ATR FT-IR of root exudates in agar and hypocotyl structure
The spectra of root exudates in the agar test are shown in Fig. 9. The most significant changes appeared in the agar test by seedlings germinating from seeds treated with phycobiliproteins at 1.2 mg/mL (solid line). In the second derivative spectrum, the following bands at 1745 cm−1 (C-O stretching in ester), 1710 cm−1 (C = O stretching in acids), 1667 cm−1 (Amide I) and 1546 cm−1 (Amide II) are displayed. Most of the components of the phycobiliprotein-induced radical exudate are likely to be organic acids, fatty acids, esters, and amino acids or peptides (Shi et al., 2023). Moreover, the strong band at 1639 cm−1 may be attributed to the carboxylate group in alginate as well as H2O. Conversely, in seedlings germinated from untreated seeds (Fig. 9, b) and the corresponding second derivative, no additional bands appeared.
Micro-ATR FT-IR spectra of root exudated of seedlings collected 14 days after sowing in agar test: a) seedlings germinated from seeds treated with phycobiliproteins at 1.2 mg/mL (solid line) and its second derivative above (dotted line); b) seedlings germinated from untreated seeds (solid line) and its second derivative (dotted line)
Figure 10 shows the micro-ATR FT-IR spectra of hypocotyls sampled from seedlings germinated from seeds treated with phycobiliproteins and untreated in the spectral range of 1800 to 1200 cm−1. As an example, the treatments at 1.2 (dashed line) and 2.4 (solid line) mg/mL are shown and compared with the untreated (dotted line).
In all spectra (Fig. 10) there are bands ascribable to pectins at 1737 cm−1 (C = O stretching vibration), 1633 cm−1 (COO− asymmetric stretching and Amide I), 1416 cm−1 (COO− symmetric stretching); 1370 cm−1 (CH2 bending) and 1324 cm−1 (CH ring bending); proteins at 1554 cm−1 (Amide II) and 1242 cm−1 (Amide III and C-O stretching) and aromatic ring in phenols at 1517 cm−1 (Schulz & Baranska, 2007). Treatments with phycobiliproteins at 1.2 and 2.4 mg/mL resulted in a decrease in the relative intensity of the bands at 1545 cm−1 and 1514 cm−1 compared with untreated ones. Conversely, the bands from 1416 to 1324 cm−1 increased.
Discussion
The application of natural products by seed treatment may be an eco-friendly solution to both prevent root pathogenic attacks and promote plant growth. Phycobiliproteins are already used as natural dyes in the food industries (Ma et al., 2022). For example, phycocyanins, which are the principal phycobiliproteins of the cyanobacterium A. platensis, are food additives falling under EC Regulation No 1333/2008. They are also approved as colour-safe additives for colouring ingested drugs free of certification by the United State Food and Drug Administration since 2013. Taking into consideration their safety for humans, we have already considered that phycobiliproteins can be exploited in the agricultural field for plant disease control. Previously, the antifungal activity of phycobiliproteins from A. minutissima and A. platensis and the red alga H. cornea against B. cinerea was demonstrated on tomato fruit only (Righini et al., 2020, 2021a). In both studies, different doses of the phycobiliproteins reduced grey mould disease incidence and severity. To be more specific, phycobiliproteins from A. platensis and H. cornea showed a linear positive dose–response relationship with disease incidence (Righini et al., 2020) while those from A. minutissima reduced disease incidence at all doses equally (Righini et al., 2021a). Moreover, all phycobiliproteins proved relevant antifungal activity against B. cinerea mycelial growth and spore germination (Righini et al., 2020, 2021a). Also in the present study, A. minutissima phycobiliprotein concentrations in the range of 0.6–4.8 mg/mL exhibited equal activity in reducing the disease of the soil-borne R. solani on tomato. Another approach by which the activity of compounds extracted from A. minutissima has been investigated was the application of water extract on tomato seed to induce a priming effect against R. solani disease (Righini et al., 2021b). It was indicated that the water extract was able to reduce disease severity as well as to promote plant growth in terms of seedling emergence, dry weight and collar calibre. These results are consistent with those obtained with A. minutissima phycobiliproteins in the present work. It should be also pointed out that the various compounds extracted from A. minutissima differently act on tomato seed germination. The present findings showed that cyanobacterium phycobiliproteins did not affect seed germination, while Righini et al. (2021b) demonstrated that the water extract increased it even if spectroscopic analyses revealed that the main bands of PBP and WE are both associated with proteins and conjugated compounds (Righini et al., 2021a).
In this context, the phycobiliproteins were characterized using FT-IR and Raman techniques, which revealed some important differences between H. cornea and A. platensis in secondary structure, as well as the presence of polar groups that could perhaps have influenced the overall polarity of the phycobiliproteins. Therefore, we concluded that these compounds may be considered natural compounds for the control of fungal pathogens.
Previously, we provided that seed priming with Jania adhaerens enhanced tomato plant defence against soil-borne pathogens (R. solani, Pythium ultimum and Fusarium oxysporum) under greenhouse conditions and improved seed germination and seedling emergence similar to biostimulation (Righini et al., 2022).
In this work, we deepened the activity of A. minutissima phycobiliproteins (20.5% of scavenging activity) as a seed treatment. In particular, we have shown that seed treatment with phycobiliproteins primes important metabolic and physiological changes at the pre-germinative stage, in seedlings, and plants. Specifically, the effects on seeds treated with higher concentrations of phycobiliproteins were assessed by a preliminary ATR FT-IR analysis. The most important changes were identified in proteins and lipids.
It can be hypothesized that seed treatment may lead to increased protein hydrolysis resulting in the release of free amino acids, readily available for endosperm and embryo protein synthesis in the germination process (Tully & Beevers, 1978). Conversely, the important increase in the acid group might be attributed to the lipase activity, which catalyzes the hydrolysis and breakdown of the ester bonds in triacylglycerols, thereby converting them into fatty acids. (Pereira et al., 2003).
This study has been divided into two phases carried out under greenhouse conditions and in vitro. In the first phase, we demonstrated the effectiveness of seed treatment against root rot disease caused by the soil-borne fungus R. solani. In the second phase, we have deepened the changes in the production of primary and secondary metabolites possibly involved in the plant defence responses against the pathogen in 6-day and 14-day-old seedlings. Moreover, 14-day-old seedlings were divided into epicotyls and hypocotyls to measure the metabolite dynamics in the two tissues, based on the study of Groenenboom et al. (2013).
Globally, our results provided evidence that tomato seed treatment with phycobiliproteins might be recognized as a biopriming treatment because it brings beneficial effects to plants. Actually, we obtained an increase in seedling emergence and vigour, and plant growth parameters under greenhouse conditions and an increase in seed germination and growth, primary and secondary metabolites such as proteins, phenols, flavonoids, and augmentation of the chitinase and glucanase activities. All these effects may be together involved in the R. solani disease control.
In the greenhouse experiments, the pathogen artificially inoculated into the substrate caused a drastic reduction of plant growth parameters such as the emergence that was measured as the area under the emergence curve and the seedling vigour with respect to the non-inoculated control. Despite the high pathogenic stress condition, the priming treatment was able to reduce these damages and in the case of 0.6 and 1.2 mg/mL phycobiliprotein concentrations, the seedling emergence reached values comparable to non-inoculated control. Under the same experimental conditions, we observed a positive dose-dependent response only between phycobiliprotein concentrations and plant dry weight under R. solani challenge. To be more specific, the dry weight is an indicator of energy allocation in the plant (Hickman & Pitelka, 1975).
At present, numerous research approaches on seed priming methods such as hydropriming, osmopriming, solid matrix priming and biopriming have been reviewed by Delian et al. (2017). Recently, seed priming with water extracts from A. minutissima and the two algae Ecklonia maxima and J. adhaerens protected tomato plants against R. solani root rot disease and simultaneously increased seedling dry weight Righini et al., 2021b). In addition to cyanobacteria and algae, some other organisms have proven to prime seeds against R. solani. Singh et al. (2020) demonstrated the positive effect of Pseudomonas aeruginosa on the control of the pathogen with different methods of application, on seed and leaves, alone or in combination. Plants bioprimed with P. aeruginosa were better protected from pathogen attack in all cases where the treatment also included seed priming. The priming effect was also obtained by strewing Streptomyces spp. suspensions in the soil near the root of the tomato transplanted seedlings under R. solani challenge (Ebrahimi-Zarandi et al., 2021). Streptomyces strains also showed to trigger resistance against the pathogen as well as to promote plant growth.
The short-time experiments conducted in vitro were performed for evaluating metabolic and physiological changes in phycobiliprotein primed seeds and during germination and seedling establishment which are the most critical stages for seedling survival and plant life cycle (Yao et al., 2020). In our experiment, the chlorophyll content of seedlings from seeds primed with phycobiliproteins was not significant. Likewise, Sané et al. (2021) obtained similar results in tomato seedlings grown in vitro under water stress conditions.
Concerning phenols and flavonoids, the seed treatment with phycobiliproteins led to different quantities in relation to the seedling stage and seedling portions, both epicotyls and hypocotyls. It has been shown that their accumulation is also dynamic being influenced by age and growth conditions (Dadáková et al., 2020). These secondary metabolites have antioxidant properties and accumulate in the tissues, mainly during necrotrophic pathogen infections. Moreover, many phenolics have direct antimicrobial properties and play an important role in the response to abiotic and biotic stresses. (Dadáková et al., 2020). These authors identified age-related differences in phenolic biosynthesis in the pathosystem Solanum lycopersicum and Pseudomonas syringae pv. tomato. We highlight that phenol and flavonoid pathways are widely studied; however, they are poorly investigated in the first stages of seedling development.
To assess the effect of seed priming with phycobiliproteins, we focused on the structure of hypocotyls and relative exudates released into the agar. The composition of functional groups in hypocotyls was predominantly attributable to pectins and proteins (Schulz & Baranska, 2007). No lignification process was detectable in hypocotyls derived from phycobiliprotein-primed seeds. The exudates released into the agar were influenced by the seed treatment. The main components found were most likely associated with organic acids, fatty acids, esters, and amino acids or peptides (Shi et al., 2023). No detectable exudates were found in the control in our experimental conditions. There is some evidence that tomato roots can excrete certain compounds to attract beneficial microorganisms and modulate their colonization in the rhizosphere (Haichar et al., 2014). Among tomato root exudates, organic acids influence the root colonization of certain bacteria such as BCA Bacillus amyloliquefaciens T-5 (Tan et al., 2013). In addition, the sugar and organic acid composition of tomato root exudates can influence the antifungal activity against F. oxysporum (Kravchenko et al., 2003).
In general, the protein content in the crude extract increased in hypocotyls and epicotyls without a dose–effect response as well as observed in the case of seedling vigour. However, the 0.6 mg/mL concentration mostly increased proteins in hypocotyls. Similarly, the same dose enhanced carotenoid content in epicotyls.
The chitinase activity of the crude extract was increased only in hypocotyls by 2.4 and 4.8 mg/mL. The enhancement of this activity may justify the significant reduction of disease incidence at the same concentrations in greenhouse experiments. It is known that chitinases can damage fungus cell walls leading to the failure in penetrating epidermal cell walls. Under pathogen stress conditions, Kästner et al. (1998) and Roldán Serrano et al. (2007) showed increases in chitinases in cucumber and sunflower hypocotyls. The β-1,3 glucanase activity generally increased both in epicotyls and hypocotyls without a dose-dependent manner. These enzymes are critical in triggering tomato seed germination and accumulate in seeds and seedlings (Morohashi & Matsushima, 2000). Another important physiological function of β‐1,3‐glucanase in tomato seeds is to mobilise wound‐deposited callose which is the first defence barrier against pathogenic attack (Morohashi & Matsushima, 2000). In solanaceous, the co-induction of β 1,3 glucanases and chitinases in the hypocotyl of the emerged seedling is considered an evolutionarily conserved event during seedling development (Petruzzelli et al., 2003).
The crude protein extract was very effective against the mycelial growth of R. solani and caused noticeable morphological changes in hyphae, such as vacuole number and size and hyphal diameter. We think that these alterations may be related to the co-presence of β 1,3 glucanases and chitinases in the extract. As above discussed, these enzymes interfere directly with normal pathogen development. The fungal vacuoles are dynamic cytoplasmic organelles with physiological cellular functions and an important role in the virulence of many fungal pathogens. They are also involved in cell expansion and in driving the protoplasm at the tips during hyphal elongation (Deacon, 2006). Various stresses can affect their morphology, as we observed in this study with the protein extract from seedlings grown from primed seeds with phycobiliproteins. In particular, the extract caused a vacuolar fragmentation as demonstrated by Kim et al. (2021) on Cryptococcus neoformans treated with the fungicide fluconazole. These authors also suggested that vacuoles are fragmented in response to oxidative stress caused by the fungicide.
Conclusion
This study has revealed that seed treatment with phycobiliproteins from A. minutissima can be an environmentally sustainable solution under European rules for reducing chemical use in agriculture in the forthcoming years. Our results proved that phycobiliproteins were able to prime tomato seeds and seedlings by improving emergence and vigour under R solani challenge and by protecting plants against the pathogen. In addition to biocontrol activity, phycobiliproteins stimulated the plant’s defence responses and growth with a long-term effect. The beneficial role of phycobiliproteins resulted in several physiological changes such as the accumulation of chitinase and glucanase enzymes, proteins, phenols, flavonoids and carotenoids. Altogether, these primary and secondary metabolites may be involved in the pathogen control and plant growth promotion obtained with phycobiliprotein-primed seed. To achieve a future application of phycobiliproteins as priming agents on tomato further studies are needed in field-scale experiments.
Data availability
The data presented in this study are available upon request from the corresponding author.
References
Ajayi-Oyetunde, O. O., & Bradley, C. A. (2018). Rhizoctonia solani: Taxonomy, population biology and management of Rhizoctonia seedling disease of soybean. Plant Pathology, 67, 3–17. https://doi.org/10.1111/ppa.12733
Baker, K. F. (1970). Types of Rhizoctonia diseases and their occurrence. In: Parmeter Jr., J. R. (Ed.), Rhizoctonia solani: Biology and Pathology. (pp. 125–148). University of California Press.
Bargabus, R. L., Zidack, N. K., Sherwood, J. E., & Jacobsen, B. J. (2004). Screening for the identification of potential biological control agents that induce systemic acquired resistance in sugar beet. Biological Control, 30(2), 342–350. https://doi.org/10.1016/j.biocontrol.2003.11.005
Bennett, A., & Bogorad, L. (1973). Complementary chromatic adaptation in a filamentous blue-green alga. Journal of Cell Biology, 58(2), 419–435. https://doi.org/10.1083/jcb.58.2.419
Blois, M. S. (1958). Antioxidant determinations by the use of a stable free radical. Nature, 181, 1199–1200. https://doi.org/10.1038/1811199a0
Bradford, M. M. (1976). A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Analytical Biochemistry, 72, 248–254. https://doi.org/10.1006/abio.1976.9999
Bryant, D. A. (1982). Phycoerythrocyanin and phycoerythrin: Properties and occurrence in cyanobacteria. Microbiology, 128(4), 835–844. https://doi.org/10.1099/00221287-128-4-835
Bryant, D. A., Guglielmi, G., de Marsac, N. T., Castets, A. M., & Cohen-Bazire, G. (1979). The structure of cyanobacterial phycobilisomes: A model. Archives of Microbiology, 123, 113–127. https://doi.org/10.1007/BF00446810
Chen, H., & Jiang, P. (2018). Combinational biosynthesis and characterization of fusion proteins with tandem repeats of allophycocyanin holo-α subunits, and their application as bright fluorescent labels for immunofluorescence assay. Journal of Bioscience and Bioengineering, 126(6), 778–782. https://doi.org/10.1016/j.jbiosc.2018.06.004
Dadáková, K., Heinrichová, T., Lochman, J., & Kašparovský, T. (2020). Production of defense phenolics in tomato leaves of different age. Molecules, 25(21), 4952. https://doi.org/10.3390/molecules25214952
Dagnino-Leone, J., Figueroa, C. P., Castañeda, M. L., Youlton, A. D., Vallejos-Almirall, A., Agurto-Muñoz, A., Pavón, P. J., & Agurto-Muñoz, C. (2022). Phycobiliproteins: Structural aspects, functional characteristics, and biotechnological perspectives. Computational and Structural Biotechnology Journal, 20, 1506–1527. https://doi.org/10.1016/j.csbj.2022.02.016
Deacon, J. W. (2006). Fungal Biology (4th ed.). Blackwell Publishing.
Delian, E., Bădulescu, L., Dobrescu, A., Chira, L., & Lagunovschi-Luchian, V. (2017). A brief overview of seed priming benefits in tomato. Romanian Biotechnological Letters, 22, 12505–12513.
Dufossé, L. (2018). Microbial pigments from bacteria, yeasts, fungi, and microalgae for the food and feed industries. In A. M. Grumezescu & A. M. Holban (Eds.), Natural and artificial flavoring agents and food dyes (pp. 113–132). Academic Press. https://doi.org/10.1016/B978-0-12-811518-3.00004-1
Ebrahimi-Zarandi, M., Bonjar, G. H. S., Riseh, R. S., El-Shetehy, M., Saadoun, I., & Barka, E. A. (2021). Exploring two Streptomyces species to control Rhizoctonia solani in tomato. Agronomy, 11, 1384. https://doi.org/10.3390/agronomy11071384
Freire, G. D. M., & Castilho, F. L. (2008). Lipases em Biocatálise. In Bon et al. (Eds.), Enzimas em biotecnologia: Produção, Aplicação e Mercado. (1st ed., pp. 348-369). Interciência.
Groenenboom, M., Gomez-Roldan, V., Stigter, H., Astola, L., van Daelen, R., Beekwilder, J., Bovy, A., Hall, R., & Molenaar, J. (2013). The flavonoid pathway in tomato seedlings: transcript abundance and the modeling of metabolite dynamics. PLoS ONE, 8(7), e68960. https://doi.org/10.1371/journal.pone.0068960
Haichar, F. Z., Santaella, C., Heulin, T., & Achouak, W. (2014). Root exudates mediated interactions belowground. Soil Biology and Biochemistry, 77, 69–80. https://doi.org/10.1016/j.soilbio.2014.06.017
Hernández-Herrera, R. M., Santacruz-Ruvalcaba, F., Zañudo-Hernández, J., & Hernández-Carmona, G. (2016). Activity of seaweed extracts and polysaccharide-enriched extracts from Ulva lactuca and Padina gymnospora as growth promoters of tomato and mung bean plants. Journal of Applied Phycology, 28, 2549–2560. https://doi.org/10.1007/s10811-015-0781-4
Hickman, J. C., & Pitelka, L. F. (1975). dry weight indicates energy allocation in ecological strategy analysis of plants. Oecologia, 21, 117–121.
Kästner, B., Tenhaken, R., & Kauss, H. (1998). Chitinase in cucumber hypocotyls is induced by germinating fungal spores and by fungal elicitor in synergism with inducers of acquired resistance. The Plant Journal, 13(4), 447–454. https://doi.org/10.1046/j.1365-313X.1998.00045.x
Kim, D., Song, M., Do, E., Choi, Y., Kronstad, J. W., & Jung, W. H. (2021). Oxidative stress causes vacuolar fragmentation in the human fungal pathogen Cryptococcus neoformans. Journal of Fungi, 7(7), 523. https://doi.org/10.3390/jof7070523
Kravchenko, L. V., Azarova, T. S., Shaposhnikov, A. I., Makarova, N. M., & Tikhonovich, I. A. (2003). Root exudates of tomato plants and their effect on the growth and antifungal activity of Pseudomonas strains. Microbiology, 72, 37–41. https://doi.org/10.1023/A:1022269821379
Lichtenthaler, H. K., & Buschmann, C. (2001). Extraction of photosynthetic tissues: chlorophylls and carotenoids. Current Protocols in Food Analytical Chemistry, 1, F4.2.1-F4.2.6. https://doi.org/10.1002/0471142913.faf0402s01
López Arnaldos, T., Muñoz, R., Ferrer, M. A., & Calderón, A. A. (2001). Changes in phenol content during strawberry (Fragaria× ananassa, cv. Chandler) callus culture. Physiologia Plantarum, 113(3), 315–322. https://doi.org/10.1034/j.1399-3054.2001.1130303.x
Lucarini, M., Durazzo, A., Kiefer, J., Santini, A., Lombardi-Boccia, G., Souto, E. B., Romani, A., Lampe, A., Ferrari Nicoli, S., Gabrielli, P., Bevilacqua, N., Campo, M., Morassut, M., & Cecchini, F. (2020). Grape seeds: Chromatographic profile of fatty acids and phenolic compounds and qualitative analysis by FTIR-ATR Spectroscopy. Foods, 9, 10. https://doi.org/10.3390/foods9010010
Ma, J., You, X., Sun, S., Wang, X., Qin, S., & Sui, S.-F. (2020). Structural basis of energy transfer in Porphyridium purpureum phycobilisome. Nature, 579, 146–151. https://doi.org/10.1038/s41586-020-2020-7
Ma, J., Hu, J., Sha, X., Meng, D., & Yang, R. (2022). Phycobiliproteins, the pigment-protein complex form of natural food colorants and bioactive ingredients. Critical Reviews in Food Science and Nutrition. https://doi.org/10.1080/10408398.2022.2128714
Mahmood, A., Turgay, O. C., Farooq, M., & Hayat, R. (2016). Seed biopriming with plant growth promoting rhizobacteria: A review. FEMS Microbiology Ecology, 92(8), 1–14. https://doi.org/10.1093/femsec/fiw112
Mbega, E. R., Mortensen, C. N., Mabagala, R. B., & Wulff, E. G. (2012). The effect of plant extracts as seed treatments to control bacterial leaf spot of tomato in Tanzania. Journal of General Plant Pathology, 78, 277–286. https://doi.org/10.1007/s10327-012-0380-z
Meenakshi, S., Gnanambigai, D. M., Mozhi, S. T., Arumugam, M., & Balasubramanian, T. (2009). Total flavonoid and in vitro antioxidant activity of two seaweeds of Rameshwaram coast. Global Journal of Pharmacology, 3(2), 59–62. ISSN 1992-0075.
Morohashi, Y., & Matsushima, H. (2000). Development of β-1, 3-glucanase activity in germinated tomato seeds. Journal of Experimental Botany, 51(349), 1381–1387. https://doi.org/10.1093/jexbot/51.349.1381
Mroginski, M. A., Murgida, D. H., von Stetten, D., Kneip, C., Mark, F., & Hildebrandt, P. (2004). Determination of the chromophore structures in the photoinduced reaction cycle of phytochrome. Journal of the American Chemical Society, 126, 16734–16735. https://doi.org/10.1021/ja043959l
Nawaz, A., Amjad, M., Khan, S. M., Afzal, I., Ahmed, T., Iqbal, Q., & Iqbal, J. (2012). Tomato seed invigoration with cytokinins. Journal of Animal and Plant Sciences, 23, 121–128. ISSN: 1018-7081.
Nikraftar, F., Taheri, P., Rastegar, M. F., & Tarighi, S. (2013). Tomato partial resistance to Rhizoctonia solani involves antioxidative defense mechanisms. Physiological Molecular Plant Pathology, 81, 74–83. https://doi.org/10.1016/j.pmpp.2012.11.004
Orchard, T. (1977). Estimating the parameters of plant seedling emergence. Seed Science and Technology, 5, 61–69. ISSN/ISBN: 0251-0952.
Panth, M., Hassler, S. C., & Baysal-Gurel, F. (2020). Methods for management of soilborne diseases in crop production. Agriculture, 10(1), 16. https://doi.org/10.3390/agriculture10010016
Pan-utai, W., & Iamtham, S. (2019). Physical extraction and extrusion entrapment of C-phycocyanin from Arthrospira platensis. Journal of King Saud University-Science, 31(4), 1535–1542. https://doi.org/10.1016/j.jksus.2018.05.026
Paparella, S., Araújo, S. S., Rossi, G., Wijayasinghe, M., Carbonera, D., & Balestrazzi, A. (2015). Seed priming: State of the art and new perspectives. Plant Cell Reports, 34, 1281–1293. https://doi.org/10.1007/s00299-015-1784-y
Pereira, E. P., Zanin, G. M., & Castro, H. F. (2003). Immobilization and catalytic properties of lipase on chitosan for hydrolysis and etherification reactions. Brazilian Journal of Chemical Engineering, 20(4), 343–355. https://doi.org/10.1590/S0104-66322003000400002
Petruzzelli, L., Müller, K., Hermann, K., & Leubner-Metzger, G. (2003). Distinct expression patterns of β-1,3-glucanases and chitinases during the germination of Solanaceous seeds. Seed Science Research, 13(2), 139–153. https://doi.org/10.1079/SSR2003132
Righini, H., Francioso, O., Di Foggia, M., Quintana, A. M., & Roberti, R. (2020). Preliminary study on the activity of phycobiliproteins against Botrytis cinerea. Marine Drugs, 18(12), 600. https://doi.org/10.3390/md18120600
Righini, H., Francioso, O., Di Foggia, M., Martel Quintana, A., & Roberti, R. (2021a). Assessing the potential of the terrestrial cyanobacterium Anabaena minutissima for controlling Botrytis cinerea on tomato fruits. Horticulturae, 7, 210. https://doi.org/10.3390/horticulturae7080210
Righini, H., Francioso, O., Di Foggia, M., Prodi, A., Martel Quintana, A., & Roberti, R. (2021b). Tomato seed biopriming with water extracts from Anabaena minutissima, Ecklonia maxima and Jania adhaerens as a new agro-ecological option against Rhizoctonia solani. Scientia Horticulturae, 281, 109921. https://doi.org/10.1016/j.scienta.2021.109921
Righini, H., Roberti, R., Cetrullo, S., Flamigni, F., Quintana, A. M., Francioso, O., Panichi, V., Cianchetta, S., & Galletti, S. (2022). Jania adhaerens primes tomato seed against soil-borne pathogens. Horticulturae, 8(8), 746. https://doi.org/10.3390/horticulturae8080746
Roldán Serrano, A., Luna del Castillo, J., Jorrín Novo, J., Fernández Ocaña, A., & Rodriguez, G. (2007). Chitinase and peroxidase activities in sunflower hypocotyls: Effects of BTH and inoculation with Plasmopara halstedii. Biologia Plantarum, 51(1), 149–152. https://doi.org/10.1007/s10535-007-0028-6
Sané, A. K., Diallo, B., Kane, A., Sagna, M., Sané, D., & Sy, M. O. (2021). In vitro germination and early vegetative growth of five tomato (Solanum lycopersicum L.) varieties under water stress conditions. American Journal of Plant Sciences, 12, 1478–1502. https://doi.org/10.4236/ajps.2021.1210105
Schiavon, M., Pizzeghello, D., Muscolo, A., Vaccaro, S., Francioso, O., & Nardi, S. (2010). High molecular size humic substances enhance phenylpropanoid metabolism in maize (Zea mays L.). Journal of Chemical Ecology, 36, 662–669. https://doi.org/10.1007/s10886-010-9790-6
Schulz, H., & Baranska, M. (2007). Identification and quantification of valuable plant substances by IR and Raman spectroscopy. Vibrational Spectroscopy, 43(1), 13–25. https://doi.org/10.1016/j.vibspec.2006.06.001
Sekar, S., & Chandramohan, M. (2008). Phycobiliproteins as a commodity: Trends in applied research, patents and commercialization. Journal of Applied Phycology, 20, 113–136. https://doi.org/10.1007/s10811-007-9188-1
Shi, R., Liu, W., Lian, Y., Zeb, A., & Wang, Q. (2023). Type-dependent effects of microplastics on tomato (Lycopersicon esculentum L.): focus on root exudates and metabolic reprogramming. Science of The Total Environment, 859(Part 1), 160025. https://doi.org/10.1016/j.scitotenv.2022.160025
Sidler, W. A. (1994). Phycobilisome and Phycobiliprotein Structures. In D. A. Bryant (Ed.), The molecular biology of cyanobacteria (pp. 139–216). Springer. https://doi.org/10.1007/978-94-011-0227-8_7
Singh, S., Singh, U. B., Malviya, D., Paul, S., Sahu, P. K., Trivedi, M., Diby, P., & Saxena, A. K. (2020). Seed biopriming with microbial inoculant triggers local and systemic defense responses against Rhizoctonia solani causing banded leaf and sheath blight in maize (Zea mays L.). International Journal of Environmental Research and Public Health, 17(4), 1396. https://doi.org/10.3390/ijerph17041396
Solanki, M. K., Robert, A. S., Singh, R. K., Kumar, S., Pandey, A. K., Srivastava, A. K., & Arora, D. K. (2012). Characterization of mycolytic enzymes of Bacillus strains and their bio-protection role against Rhizoctonia solani in tomato. Current Microbiology, 65, 330–336. https://doi.org/10.1007/s00284-012-0160-1
Song, G. C., Choi, H. K., Kim, Y. S., Choi, J. S., & Ryu, C. M. (2017). Seed defense biopriming with bacterial cyclodipeptides triggers immunity in cucumber and pepper. Scientific Reports, 7, 14209. https://doi.org/10.1038/s41598-017-14155-9
Taheri, P., & Tarighi, S. (2012). The role of pathogenesis-related proteins in the tomato-Rhizoctonia solani interaction. Journal of Botany, 1–6. https://doi.org/10.1155/2012/137037
Tambussi, E. A., Guiamet, J. J., & Bartoli, C. G. (2020). Cross-tolerance to abiotic stress at different levels of organizations: Prospects for scaling-up from laboratory to field. In M. A. Hossain, F. Liu, D. J. Burritt, M. Fujita, & B. Huang (Eds.), Priming-mediated stress and cross-stress tolerance in crop plants (pp. 317–327). Academic Press.
Tan, S., Yang, C., Mei, X., Shen, S., Raza, W., Shen, Q., & Xu, Y. (2013). The effect of organic acids from tomato root exudates on rhizosphere colonization of Bacillus amyloliquefaciens T-5. Applied Soil Ecology, 64, 15–22. https://doi.org/10.1016/j.apsoil.2012.10.011
Tully, R. E., & Beevers, H. (1978). Protease and peptidases of castor bean endosperm. Enzyme characterization and changes during germination. Plant Physiology, 62(5), 726–750. https://doi.org/10.1104/pp.62.5.746
Ulchenko, N. T., Gigienova, É. I., & Umarov, A. U. (1983). Neutral lipids of the oil of tomato seeds. Chemistry of Natural Compounds, 19, 262–265. https://doi.org/10.1007/BF00579754)
Vinothkanna, A., & Sekar, S. (2020). Diagnostic applications of phycobiliproteins. In E. Jacob-Lopes, M. Queiroz, & L. Zepka (Eds.), Pigments from microalgae handbook (pp. 585–610). Springer. https://doi.org/10.1007/978-3-030-50971-2_24
Wellburn, A. R. (1994). The spectral determination of chlorophylls a and b, as well as total carotenoids, using various solvents with spectrophotometers of different resolution. Journal of Plant Physiology, 144(3), 307–313. https://doi.org/10.1016/S0176-1617(11)81192-2
Yao, Y., Xin, M., Ni, Z., & Sun, Q. (2020). Importance of small RNA in plant seed germination. In P. Guleria & V. Kumar (Eds.), Plant Small RNA. Biogenesis, Regulation and Application (pp. 117–123). Academic Press.
Acknowledgements
We thank dr. David Baldo (DISTAL, University on Bologna, Italy) for technical assistance in the microscopical observations.
Funding
Open access funding provided by Alma Mater Studiorum - Università di Bologna within the CRUI-CARE Agreement. This research was funded by the University of Bologna (RFO, 2021–2022) and by Interreg MAC Program 2014–2020, grant number MAC2/1.1b/269: REBECA-CCT.
Author information
Authors and Affiliations
Contributions
Conceptualization: Righini H., Roberti R. and Francioso O.; methodology: Righini H., Francioso O.; Roberti R., Martel Quintana A.; Zuffi V. and Cappelletti E.; formal analysis: Righini H., Roberti R. and Francioso O.; data curation, Righini H., Roberti R. and Francioso O.; original draft preparation, Righini H., Roberti R.; Martel Quintana A. and Francioso O.; review and editing: Righini H., Roberti R., Francioso O., Martel Quintana A. and Gómez Pinchetti J. L.; funding acquisition: Martel Quintana A., Roberti R. and Francioso O.
All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Competing interests
Non-financial interests Roberta Roberti is a member of the editorial board and guest editor together with Hillary Righini of the collection “Algae and cyanobacteria: prospects and challenges for plant disease management” and receives no compensation.
Consent for publication
Not applicable.
Conflicts of interest
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
Non-financial interests
Roberta Roberti is a member of the editorial board and guest editor together with Hillary Righini of the collection “Algae and cyanobacteria: prospects and challenges for plant disease management” and receives no compensation.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Righini, H., Francioso, O., Quintana, A.M. et al. New insight on tomato seed priming with Anabaena minutissima phycobiliproteins in relation to Rhizoctonia solani root rot resistance and seedling growth promotion. Phytoparasitica 51, 763–781 (2023). https://doi.org/10.1007/s12600-023-01056-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12600-023-01056-z