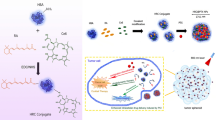
Abstract
Cisplatin resistance is the main cause for the failure of cancer therapy. To solve the problem, we proposed to develop a novel human serum albumin (HSA) nanoplatform to integrate chemotherapy, photothermal therapy (PTT) and immunotherapy. To this end, we obtained a platinum compound (C5) with significant cytotoxicity in the cisplatin-resistant SKOV-3 cells (SKOV-3/DDP), and then innovatively constructed photosensitizer (indocyanine green (ICG))-encapsulated HSA-C5 complex nanoparticles (ICG@HSA-C5 NPs). The ICG@HSA-C5 NPs exhibited excellent photothermal performances in vitro and in vivo. Importantly, the in vivo results revealed that HSA enhanced the antitumor effect of C5 and that the combination of chemotherapy and PTT could significantly inhibit cisplatin-resistant tumor growth and improved the targeting abilities of C5 and ICG, and reduced their side effects. We also confirmed that ICG@HSA-C5 NPs killed the SKOV-3/DDP cells via gasdermin E (GSDME)-mediated pyroptosis and pyroptosis-induced immune responses, thereby synergistically leading to the death of the SKOV-3/DDP cells.
Graphical abstract

摘要
顺铂耐药是癌症治疗失败的主要原因之一。为了解决这一问题, 我们提出开发一种新型的人血清白蛋白(HSA)纳米平台, 实现化疗、光热治疗(PTT)和免疫治疗一体化。为此, 我们在顺铂耐药的SKOV-3细胞(SKOV-3/DDP)中获得了具有显著细胞毒性的铂化合物(C5), 然后创新地构建了光敏剂[吲哚青绿(ICG)]包封的HSA-C5复合物纳米颗粒(ICG@HSA-C5 NPs)。ICG@HSA-C5 NPs在体外和体内均表现出良好的光热性能。重要的是, 体内实验结果显示, HSA增强了C5的抗肿瘤作用以及C5和ICG的靶向能力, 降低了C5和ICG的副作用。化疗联合PTT可显著抑制顺铂耐药肿瘤的生长。我们还证实ICG@HSA-C5 NPs通过GSDME介导的焦亡和焦亡诱导的免疫应答杀死SKOV-3/DDP细胞, 从而导致SKOV-3/DDP细胞死亡。
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Although cisplatin plays a key role in combating various cancers, it exerts severe in vivo side effects attributed to its lack of selectivity, markedly limiting its clinical applications [1]. Single chemotherapy cannot yield satisfying therapeutic efficacy due to drug resistance [2], the combination of chemotherapy with other treatment modalities has been established as a promising strategy for cancer treatment [3, 4]. To achieve combined therapy, multitarget anticancer platinum compounds with low toxicity can be designed and combined with a drug carrier.
At present, combination therapy is realized using several drugs [5]. The best strategy for avoiding the toxic and side effects due to the combination of multiple drugs [6] involves designing a single drug with simultaneous dual or multiple therapeutic functions. Metal thiosemicarbazone compounds have been extensively studied as promising next-generation metal drugs [7,8,9,10,11,12,13], which could inhibit tumor growth through multiple mechanisms and reverse Pt drug resistance [14]. Further, pyroptosis is a type of immunogenic cell death (ICD) with tremendous potential for cancer therapy [15]. Generally, the tumor-associated antigens are released by the pyroptosis-induced dying tumor cells and captured by antigen-presenting cells, such as dendritic cells (DCs), which facilitate their maturation. The mature antigen-presenting cells migrate to the lymph nodes, where they present the tumor antigens and induce T cell activation [16], which subsequently evokes the antitumor immune response of the host and improves the immunosuppressive microenvironment of solid tumors, thereby significantly facilitating cancer treatment [17]. Furthermore, photothermal therapy (PTT) has gradually become a promising antitumor modality in recent years [18]. Additionally, the combination of PTT with other therapeutic modes exhibits broad prospects for improving therapeutic efficacy and overcoming drug resistance [19]. Thus, a metal thiosemicarbazone compound with two functions, namely chemotherapy and chemotherapy-induced immunotherapy, may be designed, after which a photosensitizer may be combined with the PTT function to overcome cisplatin resistance.
Notably, the differences in the pharmacokinetic profiles, which cause inconsistent drug intake, and the undesired intensification of the toxicity of the therapeutic agent prevent successful combination therapy [20]. Therefore, two major issues must be addressed in vivo: (1) the co-delivery of the metal thiosemicarbazone compound and photosensitizer and (2) the enhancement of their targeting abilities and reduction of their side effects. The nanocarrier can deliver more therapeutic agents to tumor tissues and reduce their accumulation in undesired sites, thus improving therapeutic efficacy while minimizing side effects [21]. Further, human serum albumin (HSA) nanoparticles (NPs) have emerged as one of the most promising drug nanocarriers due to that they are endogenous proteins with advantages of nontoxicity, non-antigenicity, biodegradation and biocompatibility [22, 23].
Based on the foregoing, we developed a new Pt(II) thiosemicarbazone compound based on HSA NPs encapsulating indocyanine green (ICG), ICG@HSA NPs, as a photosensitizer for PTT to achieve the combination of chemotherapy, PTT and immunotherapy toward overcoming cisplatin resistance (Fig. 1). We conducted the following studies to verify our hypothesis: (1) we designed and synthesized five Pt(II) pyridine-2-formaldehyde thiosemicarbazone compounds (Fig. 2) and obtained a Pt(II) pyridine-2-formaldehyde thiosemicarbazone compound (C5) with remarkable cytotoxicity in cisplatin-resistant SKOV-3 cells (SKOV-3/DDP) cells (resistant to cisplatin) by investigating their structure–activity relationships in vitro; (2) we constructed a novel delivery system using the HSA−C5 complex NPs encapsulating ICG (ICG@HSA−C5 NPs); (3) we investigated and compared the antitumor performances of cisplatin, ICG@HSA NPs with laser, C5 and ICG@HSA–C5 NPs with and without laser in vivo; and (4) we confirmed the potential mechanism of C5/ICG@HSA−C5 NPs in overcoming cisplatin resistance.
2 Experimental
2.1 Materials
All commercial chemicals and reagents were provided by Innochem Company (Beijing, China) or Sigma-Aldrich and used as received. HSA was purchased from Sigma-Aldrich (Shanghai, China). Antibodies were purchased from Abcam. The purity of all of the target Pt(II) compounds used in the experiments was ≥ 95%, which was verified by high performance liquid chromatography (HPLC, Fig. S1). 1H nuclear magnetic resonance (NMR) spectra were obtained on a Bruker 400 MHz spectrometer in dimethyl sulfoxide (DMSO)-d6. Ultrapure water was used in all the experiments.
2.2 Synthesis of ligands (L1−L5)
Briefly, 10 mmol 2-pyridinecarboxaldehyde and thiosemicarbazide compounds were added to 20 ml methanol. The mixture was stirred at 65 °C for 6 h. Then, methanol was removed by slowly evaporation at room temperature to obtain acicular crystals, which were the ligands. All ligands (L1−L5) were characterized by 1H NMR and high-resolution mass spectrometry (HRMS, Figs. S1−S10).
2.2.1 2-Pyridinecarboxaldehyde-thiosemicarbazone (L1)
Yield: 83.7%. 1H NMR (400 MHz, DMSO-d6): δ (ppm, 10–6) 11.63 (s, 1H), 8.55 (d, J = 4.9, 1.8, 1.0 Hz, 1H), 8.34 (s, 1H), 8.26 (m, J = 8.0, 1.1 Hz, 1H), 8.16 (s, 1H), 8.09 (s, 1H), 7.81 (m, J = 7.8, 1.7 Hz, 1H), 7.36 (m, J = 7.5, 4.8, 1.2 Hz, 1H), where δ represents chemical shift; J represents coupling constant; s, d, t and m represent singlet, doublet, triplet, and multiplet, respectively. HRMS mass to charge ratio (m/z): 179.0170 [Molecular weight (M)-H].
2.2.2 2-Pyridinecarboxaldehyde-4-methylthiosemicarbazone (L2)
Yield: 87.9%. 1H NMR (400 MHz, DMSO-d6): δ 11.69 (s, 1H), 8.66 (q, J = 4.5 Hz, 1H), 8.56 (d, J = 4.9, 1.8, 1.0 Hz, 1H), 8.25 (m, J = 8.1, 1.1 Hz, 1H), 8.08 (s, 1H), 7.84 (m, J = 7.7, 1.6 Hz, 1H), 7.36 (d, J = 7.5, 4.9, 1.2 Hz, 1H), 3.03 (d, J = 4.6 Hz, 3H). HRMS m/z: 193.0547 (M-H).
2.2.3 2-Pyridinecarboxaldehyde-4-phenylthiosemicarbazone (L3)
Yield: 82.4%. 1H NMR (400 MHz, DMSO-d6): δ 12.03 (s, 1H), 10.25 (s, 1H), 8.59 (m, J = 4.9, 1.7, 1.0 Hz, 1H), 8.44 (m, J = 8.0, 1.1 Hz, 1H), 8.20 (s, 1H), 7.85 (m, J = 7.8, 1.7 Hz, 1H), 7.60–7.52 (m, 2H), 7.41–7.37 (m, 3H), 7.28–7.17 (m, 1H). HRMS m/z: 257.0852 [M + H].
2.2.4 2-Pyridinecarboxaldehyde-3-piperidinethiosemicarbazone (L4)
Yield: 89.0%. 1H NMR (400 MHz, DMSO-d6): δ 11.24 (s, 1H), 8.56 (m, J = 4.8, 1.4 Hz, 1H), 8.20 (s, 1H), 7.89–7.78 (m, 2H), 7.35 (m, J = 6.8, 4.9, 1.5 Hz, 1H), 3.75 (s, 4H), 1.89 (s, 4H). HRMS m/z: 257.0828 M + Na.
2.2.5 2-Pyridinecarboxaldehyde-4,4-dimethylthiosemicarbazone (L5)
Yield: 81.3%. 1H NMR (400 MHz, DMSO-d6): δ 11.18 (s, 1H), 8.57 (m, J = 4.9, 1.7, 1.0 Hz, 1H), 8.23 (s, 1H), 7.93–7.77 (m, 2H), 7.36 (m, J = 7.3, 4.9, 1.4 Hz, 1H), 3.31 (s, 6H). HRMS m/z: 209.0497 M + H.
2.3 Synthesis and characterization of Pt(II) compounds (C1−C5)
Corresponding ligands (L1−L5) 0.05 mmol and equimolar amount PtCl2(DMSO)2 were added in a glass tube, then 2 ml relevant solvent (C1: methanol; C2: ethanol; C3: methanol and dichloromethane = 1:1; C4: methanol; C5: methanol and dichloromethane = 1:1) was added to solve the solute. The glass tubes were sealed in a vacuum condition. Then, cooled slowly after the tubes were heated in an oven (C1−C3, C4: 120 °C, C5: 80 °C) for 72 h, single-crystal appeared and was collected for further study. All compounds (C1−C5) were characterized by 1H NMR and HRMS (Figs. S11−S20).
2.3.1 [Pt(L1)Cl] (C1)
Yield: 64.5%. 1H NMR (400 MHz, DMSO-d6): δ 8.72–8.68 (m, 1H), 8.30 (s, 1H), 8.14 (m, J = 7.8, 1.5 Hz, 2H), 7.73 (d, J = 7.7 Hz, 1H), 7.66 (m, J = 7.7, 5.6, 1.4 Hz, 2H). HRMS m/z: 452.0167 [M-Cl + DMSO].
2.3.2 [Pt(L2)Cl] (C2)
Yield: 67.6%. 1H NMR (400 MHz, DMSO-d6): δ 11.82 (s, 1H), 8.84–8.79 (m, 1H), 8.62–8.59 (m, 1H), 8.29 (d, J = 8.0 Hz, 1H), 8.10 (s, 1H), 7.49–7.43 (m, 1H), 3.04 (d, J = 4.6 Hz, 3H). HRMS m/z: 466.0326 [M-Cl + DMSO].
2.3.3 [Pt(L3)Cl] (C3)
Yield: 67.2%. 1H NMR (400 MHz, DMSO-d6): δ 10.44 (s, 1H), 8.78–8.73 (m, 1H), 8.67 (s, 1H), 8.19 (m, J = 7.8, 1.5 Hz, 1H), 7.80 (d, J = 7.6 Hz, 1H), 7.74 (m, J = 7.7, 6.3, 1.4 Hz, 1H), 7.61 (d, J = 7.7 Hz, 2H), 7.38–7.30 (m, 2H), 7.11–7.04 (m, 1H). HRMS m/z: 528.0480 [M-Cl + DMSO].
2.3.4 [Pt(L4)Cl] (C4)
Yield: 68.1%. 1H NMR (400 MHz, DMSO-d6): δ 8.66–8.62 (m, 1H), 8.44 (s, 1H), 8.22 (m, J = 7.8, 1.6 Hz, 1H), 7.85 (d, J = 7.9 Hz, 1H), 7.73 (m, J = 7.5, 5.3, 1.0 Hz, 1H), 3.17 (s, 4H), 1.92 (t, J = 6.6 Hz, 4H). HRMS m/z: 506.0639 [M-Cl + DMSO].
2.3.5 [Pt(L5)Cl] (C5)
Yield: 62.9%. 1H NMR (400 MHz, DMSO-d6): δ 8.63 (d, J = 5.0 Hz, 1H), 8.48 (s, 1H), 8.23 (m, J = 7.8, 1.5 Hz, 1H), 7.86 (d, J = 7.8 Hz, 1H), 7.74 (m, J = 7.5, 5.2, 1.0 Hz, 1H), 3.17 (d, J = 3.3 Hz, 6H). HRMS m/z: 480.0481 [M-Cl + DMSO].
2.4 X-ray crystallography (XRC) of HSA complex
The crystallization method is described in the Supporting Information. The crystal data of the HSA complex was collected at 100 kV using the BL02U1 beamline of the Shanghai Synchrotron Radiation Facility [24]. HKL2000 was employed for data treatment [25]. The structure of the HSA complex was determined through Molecular Replacement using the CCP4 program with the initial HSA-PA (PDB: 1E7H) model. However, the initial searching model did not include the ligand. Ligands were added to the model using the Ligand Fit program in PHENIX. Manual modifications and adjustments to the PA model were performed using COOT [26, 27]. PyMOL software was utilized for depicting the crystallographic structure of the HSA complex.
2.5 In vivo evaluation of photothermal efficacy
We evaluated the photothermal efficacies of free ICG and ICG@HSA−C5 NPs in SKOV-3/DDP tumor-bearing mice. These formulations were intravenously injected into the mice at the ICG dose of 6.2 mg·kg−1 body weight, and non-injection mice was used as the control. At 8 h after administration, tumors were exposure to 808 nm laser for 300 s at a power density of 1.0 W· cm−2. During the laser irradiation period, the temperature at tumor site was recorded and imaged using an IR thermal camera.
2.6 Antitumor effect evaluation
BALB/c mice (aged 6−8 weeks, 18−20 g) were purchased from Hunan SJA Laboratory Animal Co., Ltd. (Changsha, China). All animal experiments complied with the regulations from the People’s Republic of China’s Ministry of Health (No. 55, 2001) Committee’s Care and Use of Animals. The Institutional Animal Ethical Committee (IAEC) project number is SYXK2020-0006. Unilateral SKOV-3/DDP tumor-bearing mouse model was constructed for evaluation of in vivo antitumor effect. When tumors reached 80 mm3, the mice were randomly divided into six groups (n = 5; phosphate buffered solution (PBS), cisplatin, ICG@HSA NPs with irradiation, C5, ICG@HSA−C5 NPs with and without irradiation), and were injected via tail vein once every 3 days at a ICG dose of 6.2 mg·kg·−1 or at Pt dose of 3.0 μmol·kg·−1 of body weight. Mice treated with ICG@HSA NPs and ICG@HSA−C5 NPs received laser irradiation 8 h post injection. Tumor size and body weight were measured every 3 days. The tumor volume was calculated according to the following formula: width2 × length × 0.5. All groups of mice were euthanized for 21 days after the start of treatment, the tumors and major organs were excised, then weighed and photographed. They were sliced for corresponding staining.
2.7 Statistical analysis
Data of all measurements presented as mean ± standard deviation (SD). Differences were considered to be statistically significant if the p value < 0.05.
3 Results
3.1 Design and structures of Pt(II) thiosemicarbazone compounds
On the one hand, the rational regulation of the types of metal ion and active ligand represents an effective strategy for designing anticancer metal agents [28, 29]; on the other hand, thiosemicarbazone compounds exhibit significant antitumor activity and contain sulfur and nitrogen atoms as coordination sites for metal ions. To obtain a metal agent with multiple anticancer mechanisms, we rationally designed and synthesized a series of Pt(II) pyridine-2-formaldehyde thiosemicarbazone compounds by exploiting the properties of the Pt(II) ion and thiosemicarbazone compounds. Figure 2a shows the summarized synthetic method for the Pt(II) thiosemicarbazone compounds, and their crystal structures were determined by single-crystal X-ray diffraction (XRD).
Figure 2b shows that the compounds were isomorphs and exhibited similar structures, with one Pt atom at the center, one 2-pyridinecarboxaldehyde thiosemicarbazone ligand, and one chloride ion coordinated to the Pt center. Moreover, the Pt(II) compounds contained Pt1, N1, N2, S1 and Cl1 in the same plane. The stabilities of the Pt(II) compounds were enhanced by two five-membered closed-loop rings, which were formed from the tridentate thiosemicarbazone ligand that coordinated with the Pt(II) center. Tables S1−S3 present the crystal data, selected angles and bond lengths of the compounds.
3.2 Structure–activity relationships of Pt(II) thiosemicarbazone compounds to cancer cells
The half-maximal inhibitory concentrations (IC50) of the ligands (L1−L5) and Pt(II) compounds (C1−C5) were examined in the human cervical cancer cell line (SiHa), SiHa/DDP (resistant to cisplatin), the human ovarian cancer cell line (SKOV-3), SKOV-3/DDP (resistant to cisplatin), and the human ovarian surface epithelial cells HOSE using a 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl-2H-tetrazolium bromide (MTT) assay. Table 1 reveals that the IC50 values of the Pt(II) compounds were significantly lower than those of the ligands. These results indicated that the Pt(II) thiosemicarbazone compounds exhibited higher cytotoxicity in the cancer cells than their corresponding ligands. The Pt(II) compound, which was modified by two methyl groups (C5) at the N-4 position of thiosemicarbazide, exhibited the lowest IC50 value toward the SKOV-3 cells (3.89 ± 0.84) μmol·L−1, which is 3.7-fold that of C1 (14.58 ± 0.74) μmol·L−1.
Additionally, the IC50 values of the Pt(II) compounds (C1−C5) in the drug-resistant breast cancer cell line were examined. Notably, C5 exhibited the lowest IC50 (4.23 ± 0.75) μmol·L−1 toward the cisplatin-resistant SKOV-3/DDP cells, as well as the lowest resistance factor (RF, 1.09) among the Pt(II) compounds. The platinum compounds presented a higher selectivity index (SI) compared with the corresponding ligands, indicating that the Pt compounds could selectively kill cancer cells to some extent. Concurrently, C5 exhibited the highest SI (3.28). Based on these results, C5 was selected for further study for overcoming cisplatin resistance.
3.3 Construction of a delivery system for ICG@HSA−C5 complex NPs
3.3.1 Structural basis of HSA−C5 complex
Matrix-assisted laser desorption/ionization time-of-flight mass spectrometry (MALDI-TOF–MS) and XRC were performed to confirm the formation of the HSA−C5 complex. Compared with the MALDI-TOF–MS spectrum of HSA, that of the HSA-C5 complex exhibited a ~ 400 Da increase (Fig. S22), corresponding to the molecular weight of C5 after losing a Cl ion and indicating the binding of a C5 molecule to an HSA molecule.
To further determine the binding mode and site of C5 to HSA, the crystal structure of the HSA-PA−C5 complex was resolved (Table S4). The electron-density map of the HSA-PA−C5 complex revealed that C5 bound to the IB subdomain of HSA (Fig. 3a). His146 of HSA replaced Cl of C5 and coordinated with the Pt(II) center, forming a stable HSA−C5 complex (Fig. 3a). Concurrently, the residues of the IB subdomain (Leu115, Ile142, His146, Phe149, Phe157 and Tyr161) formed hydrophobic interactions with C5 (Fig. 3b), and the HSA−C5 complex exhibited a heart-shaped structure that was similar to that of the monomer HSA (Fig. 3c).
ICG@HSA−C5 NPs delivery system: a experimental σA-weighted 2Fo-Fc electron density map of C5 at IB subdomain of HSA; b binding microenvironment of C5 at IB subdomain; c overall structure of HSA−C5 complex; d preparation scheme of ICG@HSA−C5 NPs; e SEM image of ICG@HSA−C5 NPs; f ICP-MS analysis to determine targeting ability of ICG@HSA−C5 NPs toward SKOV-3/DDP cells in vitro; g western blot analysis of targeting ability of ICG@HSA−C5 NPs toward SKOV-3/DDP cells in vitro; h C5 released from ICG@HSA−C5 NPs under different conditions. *p < 0.05
3.3.2 Characterization of ICG@HSA–C5 NPs
The HSA−C5 complex nanocarrier for ICG (ICG@HSA−C5 NPs) was prepared by a nanoprecipitation method as previously described with slight modification (Fig. 3d). As shown in Fig. 3e, the NPs were spherical and homogeneous, and dynamic light scattering (DLS) demonstrated the NPs with an average size about 122 nm (Fig. S23). The stability of the ICG@HSA−C5 NPs was tested by DLS after incubation in phosphate-buffered saline (PBS) at 4 and 37 °C for 7 day (Figs. S24, S25). The results exhibited relatively constant size, demonstrating good stability. In addition, the encapsulation efficiency of ICG in ICG@HSA−C5 NPs was 88.6%.
3.3.3 Feasibility of ICG@HSA−C5 NPs
An ideal drug delivery system must be capable of enhancing the cellular uptake of therapeutic agents, as well as the selective accumulation and release of these agents in tumor cells. To evaluate the uptake capability of ICG@HSA−C5 NPs in cancer cells, C5 and ICG@HSA−C5 NPs were incubated with SKOV-3/DDP and HOSE cells for 48 h. The intracellular platinum and HSA contents were detected by ICP-MS and western blotting, respectively (Fig. 3f, g). The Pt and HSA contents of the SKOV-3/DDP cells were higher than those of the HOSE cells in the ICG@HSA−C5 NP-treated group, indicating that the HSA NPs promoted the selective accumulation of C5 in the SKOV-3/DDP cells.
The fix-point release of the agents from the drug carrier represents another key parameter for evaluating the feasibility of the delivery system. Therefore, we dialyzed the ICG@HSA−C5 NPs in a buffer solution of pH = 7.4 and 4.7, which simulated a normal physiological environment and a lysosome, respectively, to investigate the release behavior of C5 from the ICG@HSA−C5 NPs. We performed ICP-MS to measure the Pt amount that was released from the ICG@HSA−C5 NPs. The data revealed that only ~ 5% C5 was released from the ICG@HSA−C5 NPs at pH = 7.4 within 72 h. However, ~ 83% C5 was released at pH = 4.7 (Fig. 3h). The studies revealed that PTT could promote the release of drugs from the NPs [30]. Expectedly, irradiation from an 808 nm laser increased the release of C5 to 91%. To confirm the release of C5 from the HSA−C5 complex, we verified the molecular weight of the HSA−C5 complex after dialysis in a buffer solution of pH = 4.7 and determined the molecular weight of C5 after its release from the HSA−C5 complex. Figure S22 shows that the molecular weight of the HSA−C5 complex decreased by ~ 400 Da, which was close to that of HSA, indicating that C5 could be released from the HSA−C5 complex at pH = 4.7. The HRMS of the products released from the HSA−C5 complex at pH = 4.7 displayed an intense signal at m/z = 402.0354 when the in-source energy was 0 eV; this weight was equal to that obtained when C5 lost a Cl ion, further indicating that C5 could be released from the HSA−C5 complex at pH = 4.7 (Fig. S26). The potential principle of the release of C5 from the HSA = C5 complex at pH = 4.7 is the protonation of His146 in an acidic environment, the decreased coordination ability of the N atom, and the reduced stability of the coordination bond between His146 and the Pt(II) center, facilitating the release of C5 from HSA−C5 complex. The results confirmed the stability and release of the ICG@HSA−C5 NPs in a normal physiological environment and an acidic lysosome, respectively.
To explore the targeting ability of the ICG@HSA−C5 NPs, we conducted tracking experiments in SKOV-3/DDP tumor-bearing mice by detecting the fluorescence signal of ICG. Figure 4a shows the significant enhancement of the fluorescence signal in the tumor site and a decreased signal in the major organs of ICG@HSA−C5 NP-treated mice compared with those treated with free ICG. A quantitative fluorescence intensity analysis revealed that the fluorescence intensity of the tumor site reached a maximum after 8 h of the intravenous injection of the ICG@HSA−C5 NPs, and this maximum was seven times that of free ICG (Fig. 4b). These results demonstrated that the ICG@HSA−C5 NPs enhanced the in vivo selective accumulations of C5 and ICG in tumor sites. After the experiment, fluorescence imaging was performed in the tumors and main organs of the mice (Fig. 4c), and the results indicated that the ICG@HSA−C5 NP-treated group exhibited strong fluorescence intensity in the tumor tissue, whereas the free ICG-treated group displayed almost zero fluorescence in the tissue. These results demonstrated that the ICG@HSA−C5 NPs could improve the selective accumulations of C5 and ICG in tumors, thereby promoting the targeting abilities of ICG and C5 after systemic administration and sequentially achieving the targeted release of drugs in tumor tissues.
In vivo targeting abilities of ICG@HSA−C5 NPs: a in vivo fluorescence images of free ICG and ICG@HSA−C5 NPs in SKOV-3/DDP-tumor–bearing mice; b fluorescence intensity analysis of image a; c ex vivo fluorescence images of major organs and tumors after injections of free ICG and ICG@HSA−C5 NPs after experiment (H: heart, Li: liver, S: spleen, Lu: lung, K: kidneys, T: tumor), ***p < 0.001
3.4 PTT performance of ICG@HSA–C5 NPs
The photothermal performance of the ICG@HSA−C5 NPs was determined using the temperature changes during 808 nm laser irradiation. First, we evaluated the effect of light exposure on cell viability. The viability of the cell decreased in a time-dependent manner when the power density was increased to 1.5 W·cm−2, however, no significant effect was observed at 1.0 W·cm−2 (Fig. S27). Therefore, 1.0 W·cm−2and 300 s were selected for the subsequent experiments. Compared with water and C5, the ICG-containing formulations, including ICG, ICG + C5 and ICG@HSA−C5 NPs, delivered better PTT performances, as confirmed by the temperature increase from 24 to 72 °C at 1.0 W·cm−2 (Fig. 5a, b), indicating that the ICG@HSA−C5 NPs exhibited good photothermal conversion ability.
In vitro and in vivo photothermal performances of ICG@HSA−C5 NPs: a infrared thermography images of water, C5, ICG, C5 + ICG and ICG@HSA−C5 NPs with a constant laser power (1.0 W·cm−2, 300 s); b quantitative analysis of temperature changes in image a; c thermal images of SKOV-3/DDP tumor-bearing mice 8 h after ICG and ICG@HSA−C5 NP injections, following exposures to 808 nm laser irradiation (1.0 W·cm−2, 300 s); d quantitative analysis of temperature changes in image c
To evaluate the effect of PTT on the viability of cancer cells, the SKOV-3/DDP cells were incubated with ICG@HSA−C5 NPs, after which the cell viability was detected by an MTT assay. The viability of the SKOV-3/DDP cells in the ICG@HSA−C5 NPs with laser group was significantly lower than those of the cells in the cisplatin, ICG@HSA NP with laser, C5, and ICG@HSA−C5 NPs without laser groups (Fig. S28). The viability of the cell decreased with the increasing concentrations of the ICG@HSA−C5 NPs (Fig. S29). With 808 nm laser irradiation, the viability of the cell decreased further compared with single chemotherapy at each concentration. These results indicated that the ICG@HSA−C5 NPs exerted remarkable cytotoxicity in the SKOV-3/DDP cells to overcome cisplatin resistance.
Next, we evaluated the PTT property of the ICG@HSA−C5 NPs in vivo. The tumor-bearing mice employed for the experiment were administered different formulations: ICG and the ICG@HSA−C5 NPs. The tumors were irradiated for 300 s after 8 h of being injected intravenously. The thermal images of the mice were recorded every 60 s, and the temperature changes were observed (Fig. 5c). The temperature of the tumor surface in the ICG@HSA−C5 NPs group increased by 19 °C at a power density of 1.0 W·cm−2 (Fig. 5d), and this was significantly higher than the 13 °C increase observed in the free-ICG group. These results confirmed that the ICG@HSA−C5 NPs exhibited excellent photothermal performances and could be used as promising PTT agents for cancer therapy.
3.5 Anticancer behavior of ICG@HSA NPs/C5/ICG@HSA−C5 NPs in overcoming cisplatin resistance in vivo
3.5.1 Biosafety evaluation of C5/ICG@HSA−C5 NPs in vivo
We performed an acute toxicity assessment to evaluate the safety of C5 and the ICG@HSA−C5 NPs. BALB/c mice were intravenously administered C5 and the ICG@HSA−C5 NPs, and the median lethal doses of C5 and the ICG@HSA−C5 NPs were 29.15 and 41.17 μmol·kg·−1 body weight, respectively (Tables S5, S6). We attempted the biochemical detection of serum in the BALB/c mice after intravenous injections with normal saline, C5, and the ICG@HSA−C5 NPs. The results indicated that the values obtained by the C5-treated mice were higher than those obtained by the normal saline group. The values for the ICG@HSA−C5 NP-treated group were similar to those in of the normal saline-treated group (Table S7), indicating that the ICG@HSA−C5 NPs exhibited low cardiotoxicity, nephrotoxicity and hepatotoxicity.
3.5.2 Inhibition of growth of cisplatin-resistant SKOV-3/DDP tumors
To verify the therapeutic efficacy of the ICG@HSA NPs/C5/ICG@HSA−C5 NPs in overcoming cisplatin resistance in vivo, we established a model of SKOV-3/DDP tumor-bearing BALB/c mice. The tumor-bearing mice employed for the test were intravenously injected with different formulations under anesthesia: normal saline, cisplatin, ICG@HSA NPs + Laser, C5, ICG@HSA−C5 NPs, and ICG@HSA−C5 NPs + Laser (Fig. 6a). Figure 6b−d shows the changes in the tumor with time. The cisplatin-treated mice exhibited negligible tumor growth inhibition compared with the normal saline-treated mice, with a tumor-inhibition rate of 19.5%. Further, the ICG@HSA NPs + Laser and C5 groups exhibited unsatisfactory inhibition, with tumor-inhibition rates of 45.2% and 57.0%, respectively. However, in sharp contrast with the former groups, the weight and volume of the tumors in the ICG@HSA−C5 NPs group decreased significantly, particularly in the ICG@HSA−C5 NPs + Laser group; their tumor-inhibition rates in the cisplatin-resistant tumor reached 78.0% and 90.7%, respectively.
In vivo antitumor performances of ICG@HSA NPs/C5/ICG@HSA−C5 NPs in cisplatin-resistant SKOV-3/DDP tumors; a treatment schedule; b photograph of tumors after final treatment; c tumor-growth curves during treatment; d tumor weights measured after final treatment; e hematoxylin and eosin (H&E) staining of tumors after final treatment (*p < 0.05, **p < 0.01 and ***p < 0.001)
3.5.3 In vivo side effects and bioavailability
Here, we investigated the side effects of ICG@HSA NPs/C5/ICG@HSA−C5 NPs in vivo. The results revealed that the body weights of the mice treated with ICG@HSA NPs, C5, and ICG@HSA−C5 NPs increased similarly to those of the normal saline-group (Fig. 7a), whereas the body weights of the cisplatin-treated mice were lower than those of the other groups. The weights of major organs treated with ICG@HSA NPs, C5, and ICG@HSA−C5 NPs were similar to those of the control (Fig. 7b). Moreover, the H&E assays of the main organs after the final treatment were also determined (Fig. 7c), and no discernible pathologic lesions appeared in any organ compared with the control. These results confirmed that ICG@HSA NPs, C5, and ICG@HSA−C5 NPs exerted negligible side effects in vivo.
Side effects and bioavailability of ICG@HSA NPs/C5/ICG@HSA−C5 NPs in vivo: a body weight of mice measured every three days during treatment; b average organ weight after final treatment; c H&E staining of major organs after final treatment; d Pt contents of major organs and tumors after final treatment
Additionally, the ICG@HSA−C5 NP-treated groups exhibited higher and lower Pt contents in the tumors and main organs, respectively, than the cisplatin- and C5-treated groups (Fig. 7d), indicating that HSA NPs can facilitate the selective accumulation of C5 in tumors while facilitating its decreased accumulation in organs.
3.6 Mechanisms of C5/ICG@HSA–C5 NPs overcoming cisplatin resistance
3.6.1 Pyroptosis induced by C5/ICG@HSA–C5 NPs
Recent studies revealed that chemotherapy drugs can induce pyroptosis through the caspase-3 cleavage of Gasdermin E (GSDME) [31, 32]. To confirm if C5 and the ICG@HSA−C5 NPs triggered cisplatin-resistant tumor cell pyroptosis, we examined whether the SKOV-3/DDP cells exhibited proptosis-related morphological features after C5 and ICG@HSA−C5 NP treatments. Figure 8a shows that the SKOV-3/DDP cells displayed significant pyroptosis traits, with large bubbles and cell swelling after C5 and ICG@HSA−C5 NP treatments. Thereafter, we monitored the lactate dehydrogenase (LDH) and adenosine triphosphate (ATP) leakages in the cell supernatant. Figure 8b, c confirms the higher releases of LDH and ATP in the supernatants of the C5- and ICG@HSA−C5 NP-treated groups compared with the control. The ICG@HSA−C5 NPs + Laser group exhibited the highest releases of LDH and ATP.
In vitro and in vivo pyroptosis induced by C5/ICG@HSA−C5 NPs: a representative bright-field microscopy images of SKOV-3/DDP cells treated with C5 and ICG@HSA−C5 NPs (arrows indicate pyroptotic cells); b LDH and c ATP leakages in cell supernatant after treatment; d C5- and ICG@HSA−C5 NP-induced SKOV-3/DDP cell pyroptosis detected by annexin V-FITC/PI staining; e immunofluorescence analysis of GSDME levels in tumor tissues after final treatment; f immunofluorescence analysis of GSDME-N levels in tumor tissues after final treatment; g immunofluorescence analysis of caspase-3 levels in tumor tissues after final treatment (*p < 0.05 and ***p < 0.001)
Next, we confirmed C5/ICG@HSA−C5 NP-induced pyroptosis via annexin V-fluorescein isothiocyanate (annexin V-FITC)/propidium iodide (PI) detection. As shown in Fig. 8c, the C5 and ICG@HSA−C5 NP groups exhibited significantly increased pyroptotic cells compared with the control, and the ICG@HSA−C5 NP group displayed a higher pyroptosis ratio. Moreover, when the cells were pretreated with a caspase-3 inhibitor (Z-DEVD-FMK), the pyroptotic cells decreased markedly, further indicating the caspase-3 dependence of the GSDME-mediated pyroptosis induced by C5 and the ICG@HSA−C5 NPs. Expectedly, the ICG@HSA−C5 NPs + Laser group exhibited the highest pyroptosis rate.
The in vivo proptosis process was validated by the post-treatment immunofluorescence staining of the tumor tissues. Compared with the control, the other groups exhibited significantly decreased GSDME expressions (Fig. 8e) and increased GSDME-N terminal (GSDME-N) (Fig. 8f) and caspase-3 expressions (Fig. 8g). Expectedly, the ICG@HSA−C5 NPs + Laser group displayed the most significant changes. These data confirmed that C5 and the ICG@HSA−C5 NPs could induce pyroptosis through in vitro and in vivo caspase 3-mediated GSDME cleavage.
3.6.2 Pyroptosis-induced immune response
Pyroptosis is a type of ICD that can evoke the antitumor immune response of the host [33]. To determine whether C5- and ICG@HSA−C5 NP-induced pyroptosis can trigger an immune response, we first investigated the effects of C5 and the ICG@HSA−C5 NPs on the viability of DCs, which are special antigen-presenting cells [34]. Figure S30 shows that C5 and ICG@HSA−C5 NPs exerted negligible effects on the viability of DCs at concentrations up to 30 µmol·L−1. Thereafter, DCs were cocultured with the C5- and ICG@HSA−C5 NP-pretreated SKOV-3/DDP cells in the Transwell system (Fig. 9a), and the secretion of immune response related cytokines was evaluated via enzyme-linked immunosorbent assay (ELISA). Figure 9b−d shows that the tumor necrosis factor-α (TNF-α) and interleukin 6 (IL-6) levels increased remarkably and that secretion of the immunosuppressive cytokine interleukin 10 (IL-10) decreased, indicating that C5, ICG@HSA−C5 NPs, and ICG@HSA−C5 NPs + Laser can effectively trigger DC maturation to generate in vitro immune responses.
In vitro and in vivo antitumor immune responses induced by C5/ICG@HSA−C5 NPs (G1: control, G2: C5, G3: ICG@HSA−C5 NPs, G4: ICG@HSA–C5 NPs + laser): a scheme of stimulation of DC maturation; b−d cytokine secretion in cell supernatant measured in vitro by ELISA after different treatments (n = 5); e immunofluorescence analysis of CRT and HMGB1 contents of tumor tissues after final treatment; f immunohistochemical analyses of CD8+ and CD4+ T cells in tumor tissues after final treatment; g−i cytokine secretion in serum after final treatment (n = 3, *p < 0.05, **p < 0.01, and ***p < 0.001)
Subsequently, we assessed the in vivo immune response. The extracellular release of high mobility group box 1 (HMGB1) and the cell surface exposure of calreticulin (CRT) in tumor tissues were detected by immunofluorescence. The release and exposure of HMGB1 and CRT, respectively, increased significantly in the C5 and ICG@HSA−C5 NP groups, and the ICG@HSA−C5 NP + Laser group exhibited the highest expression (Fig. 9e). Furthermore, we studied the pyroptosis-induced immune responses via the immunohistochemical analysis of the tumors after the treatment. The CD8+ and CD4+ T-cell percentages of the C5 and ICG@HSA−C5 NP groups increased significantly, and the ICG@HSA−C5 NP + Laser group exhibited the highest increase, indicating that the CD8+ and CD4+ T cells can be activated by C5 and ICG@HSA−C5 NP treatments (Fig. 9f). Additionally, cytokine secretion is crucial to antitumor immune response. Thus, we utilized an ELISA kit to evaluate the cytokine contents (TNF-α, IL-6 and IL-10) of serum (Fig. 9g−i). The results were consistent with the CD8+ and CD4+ T-cell immunohistochemical staining of the tumor, further indicating that C5 and the ICG@HSA−C5 NPs could effectively induce an immune response.
4 Discussion
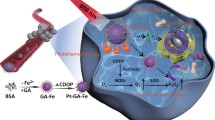
In clinical applications, cisplatin plays an important role against various cancers, although cancer cells tend to generate cisplatin resistance as it only damages nuclear DNA, which can be repaired [35]. Presently, multitarget anticancer drugs are promising in overcoming the defects of single-target anticancer drugs [36]. Thus, we designed a new Pt(II) pyridine-2-formaldehyde thiosemicarbazone compound (C5) to overcome cisplatin resistance (Fig. 2). Expectedly, C5 can directly kill the SKOV-3/DDP cells through GSDME-mediated pyroptosis (Fig. 8), as confirmed by the large bubbles and swelling of the SKOV-3/DDP cells. Conversely, the caspase-3 and GSDME-N levels increased in the C5-treated group, after which caspase-3 cleaved the GSDME-N terminal, inducing the pyroptosis of the SKOV-3/DDP cells. Pyroptosis is an ICD type that can evoke an antitumor immune response. Generally, the release and exposure of HMGB1 and CRT, respectively, increased significantly in the C5-treated group, followed by the increasing expressions of the CD8+ and CD4+ T cells in their tumor tissues (Fig. 9), indicating that pyroptosis-induced ICD activates immune responses, thereby synergistically killing the SKOV-3/DDP cells. Moreover, C5 effectively overcomes tumor cisplatin resistance and inhibits tumor growth. It is desirable to develop C5 as a promising dual-targeted lead drug that integrates chemotherapy and pyroptosis-induced immunotherapy (Fig. 10). As chemotherapy drugs only ensure limited therapeutic efficacy in most cases, combining multiple therapies represents a good strategy for achieving better therapeutic effects and overcoming cisplatin resistance.
A simple mixture of therapeutic agents may exert side effects and undesirable therapeutic effects due to discrepant pharmacokinetics, selectivity, and biodistribution in vivo. Therefore, to solve the delivery issue and improve the in vivo targeting ability of the agents, we developed a delivery system comprising an ICG-encapsulated HSA−C5 complex NP (ICG@HSA−C5 NPs) to integrate chemotherapy, immunotherapy, and PTT and overcome cisplatin resistance. The XRC results revealed that C5 was tightly bound to the hydrophobic cavity in the IB subdomain of HSA through a coordination bond and hydrophobic interactions, forming the stable HSA−C5 complex (Fig. 3), which significantly protected C5 from endogenous ions/compounds/macromolecules in the blood. Scanning electron microscopy (SEM) and DLS results demonstrated that the ICG@HSA−C5 NPs were spherical and exhibited a uniform size (Fig. 3). Additionally, the ICG@HSA−C5 NPs were stable in a normal physiological environment, and the agents could be released from the NPs in acidic tumor cells, as only ~ 5% and 83% of C5 were released from the ICG@HSA−C5 NPs at pH = 7.4 and 4.7, respectively (Fig. 3i). The in vivo tracking experiment results demonstrated that the ICG@HSA−C5 NPs could target tumor sites (Fig. 4), promoting the targeting abilities of ICG and C5 after systemic administration and sequentially achieving the targeted release of drugs in tumor tissues.
The ICG@HSA−C5 NPs exhibited remarkable photothermal effects in vitro and in vivo (Fig. 5). The 19 °C temperature increase on the tumor surface, as observed in the ICG@HSA−C5 NP group at a power density of 1.0 W·cm−2, confirmed that the ICG@HSA−C5 NPs exhibited excellent photothermal performance and could be used as a promising PTT agent for cancer therapy. The therapeutic efficiency of the ICG@HSA−C5 NPs in cisplatin-resistant SKOV-3/DDP tumor-bearing mice was significantly superior to those of the other groups (Fig. 6), as the tumor-inhibition rate of the ICG@HSA-C5 NPs + Laser group (90.7%) was higher than those of the cisplatin (19.5%), ICG@HSA NPs + Laser (45.2%), C5 (57.0%), and ICG@HSA-C5 NPs without laser (78.0%) groups. The results confirmed that HSA enhanced the antitumor effect of C5 and that the combination of chemotherapy, immunotherapy, and PTT could significantly inhibit cisplatin-resistant tumor growth. The ICG@HSA−C5 NPs increased the selective accumulation of C5 in the tumor and decreased its accumulation in the main organs (Fig. 7), thus significantly enhancing and reducing the bioavailability and side effects, respectively, of C5 and ICG in vivo.
5 Conclusion
In summary, we developed an anticancer Pt(II) pyridine-2-formaldehyde thiosemicarbazone compound (C5) with high efficiency and low toxicity. The crystal structure of the HSA-PA-C5 complex was resolved, which revealed that C5 bound to the IB subdomain of HSA. The novel ICG@HSA−C5 NP delivery system was constructed to combinate chemotherapy, PTT and immunotherapy therapy to treat cancer, exerting significant antitumor effects on cisplatin-resistant tumors. ICG@HSA−C5 NP delivery system improving the targeting ability, and reducing the potential side effects of C5 and ICG in vivo. The mechanisms of action of ICG@HSA−C5 NPs were confirmed: kill the SKOV-3/DDP cells through GSDME-mediated pyroptosis and pyroptosis-induced antitumor immune responses, thereby synergistically killing the SKOV-3/DDP cells. Our study offered a strategy for the rational development of next-generation anticancer metal drugs with low toxicity and high efficiency and paved the way for targeted multimodality combination therapy for reversing tumor cisplatin resistance and inhibiting the all-around growth of tumors.
References
Peng K, Liang BB, Liu W, Mao ZW. What blocks more anticancer platinum complexes from experiment to clinic: major problems and potential strategies from drug design perspectives. Coord Chem Rev. 2021;449:214210. https://doi.org/10.1016/j.ccr.2021.214210.
Nam J, Son S, Ochyl LJ, Kuai R, Schwendeman A, Moon JJ. Chemo-photothermal therapy combination elicits anti-tumor immunity against advanced metastatic cancer. Nat Commun. 2018;9:1. https://doi.org/10.1038/s41467-018-03473-9.
Plana D, Palmer AC, Sorger PK. Independent drug action in combination therapy: implications for precision oncology. Cancer Discov. 2022;12(3):606. https://doi.org/10.1158/2159-8290.Cd-21-0212.
Xing S, Wang DH, Zhang HF, Liu LD, Li CC, Wei C, Liu JY, Ge NJ, Liu XY. Tumor microenvironment-responsive arsenic-loaded layered double hydroxides film with synergistic anticancer and bactericidal activity. Rare Met. 2023;43(3):1207. https://doi.org/10.1007/s12598-023-02466-y.
Salas-Benito D, Perez-Gracia JL, Ponz-Sarvise M, Rodriguez-Ruiz ME, Martinez-Forero I, Castanon E, Lopez-Picazo JM, Sanmamed MF, Melero I. Paradigms on immunotherapy combinations with chemotherapy. Cancer Discov. 2021;11(6):1353. https://doi.org/10.1158/2159-8290.Cd-20-1312.
Liu AY, Wang HS, Hou XS, Ma Y, Yang GJ, Hou YL, Ding Y. Combinatory antitumor therapy by cascade targeting of a single drug. Acta Pharm Sin B. 2020;10(4):667. https://doi.org/10.1016/j.apsb.2019.08.011.
Paterson BM, Donnelly PS. Copper complexes of bis(thiosemicarbazones): from chemotherapeutics to diagnostic and therapeutic radiopharmaceuticals. Chem Soc Rev. 2011;40(5):3005. https://doi.org/10.1039/c0cs00215a.
Jiang M, Zhang ZL, Li WJ, Man XY, Sun HB, Liang, Yang F. Developing a copper(II) agent based on His-146 and His-242 residues of human serum albumin nanoparticles: integration to overcome cisplatin resistance and inhibit the metastasis of nonsmall cell lung cancer. J Med Chem. 2022; 65(13):9447. https://doi.org/10.1021/acs.jmedchem.2c00698.
Zhang ZL, Yang TF, Zhang JZ, Li WJ, Li SH, Sun HB, Liang H, Yang F. Developing a novel indium(III) agent based on human serum albumin nanoparticles: integrating bioimaging and therapy. J Med Chem. 2022;65(7):5392. https://doi.org/10.1021/acs.jmedchem.1c01790.
Yang TF, Zhang ZL, Zhang JZ, Li YP, Li WJ. Liang H, Yang F. Developing a gallium(III) agent based on the properties of the tumor microenvironment and lactoferrin: achieving two-agent co-delivery and multi-targeted combination therapy of cancer. J Med Chem. 2023;66(1):793. https://doi.org/10.1021/acs.jmedchem.2c01684.
Zhang ZL, Zhang JZ, Yang TF, Li SH, Xu G, Liang H, Yang F. Developing an anticancer platinum(II) compound based on the uniqueness of human serum albumin. J Med Chem. 2023;66(8):5669. https://doi.org/10.1021/acs.jmedchem.3c00001.
Man XY, Yang TF, Li WJ, Li SH, Xu G, Zhang Z, Liang H, Yang F. Developing a gadolinium(III) compound based on apoferritin for targeted magnetic resonance imaging and dual-modal therapy of cancer. J Med Chem. 2023;66(11):7268. https://doi.org/10.1021/acs.jmedchem.2c01904.
Li WJ, Li SH, Zhang ZL, Xu G, Man XY, Yang F, Liang H. Developing a multitargeted anticancer palladium(II) agent based on the His-242 residue in the IIA subdomain of human serum albumin. J Med Chem. 2023;66(13):8564. https://doi.org/10.1021/acs.jmedchem.3c00248.
Jiang M, Chu Y, Yang TF, Li WJ, Zhang ZL, Sun HB, Liang H, Yang F. Developing a novel indium(III) agent based on liposomes to overcome cisplatin-induced resistance in breast cancer by multitargeting the tumor microenvironment components. J Med Chem. 2021;64(19):14587. https://doi.org/10.1021/acs.jmedchem.1c01068.
Ye XY, Liang X, Chen Q, Miao QW, Chen XL, Zhang XD, Mei L. Surgical tumor-derived personalized photothermal vaccine formulation for cancer immunotherapy. ACS Nano. 2019;13(3):2956. https://doi.org/10.1021/acsnano.8b07371.
Duan XP, Chan C, Lin WB. Nanoparticle-mediated immunogenic cell death enables and potentiates cancer immunotherapy. Angew Chem Int Ed. 2019;58(3):670. https://doi.org/10.1002/anie.201804882.
Sen S, Won M, Levine MS, Noh Y, Sedgwick AC, Kim JS, Sessler JL, Arambula JF. Metal-based anticancer agents as immunogenic cell death inducers: the past, present, and future. Chem Soc Rev. 2022;51(4):1212. https://doi.org/10.1039/d1cs00417d.
Ding ZL, Gu YH, Zheng C, Gu YQ, Yang J, Li DH, Xu YN, Wang P. Organic small molecule-based photothermal agents for cancer therapy: design strategies from single-molecule optimization to synergistic enhancement. Coordin Chem Rev; 2022, 464: 214564. https://doi.org/10.1016/j.ccr.2022.214564.
Chen SY, Sun B, Miao H, Wang GN, Sun PF, Li JW, Wang WJ, Fan QL, Huang W. NIR-II dye-based multifunctional telechelic glycopolymers for NIR-IIa fluorescence imaging-guided stimuli-responsive chemo-photothermal combination therapy. Acs Mate Lett. 2020;2(2):174. https://doi.org/10.1021/acsmaterialslett.9b00480.
Samanta K, Setua S, Kumari S, Jaggi M, Yallapu MM, Chauhan SC. Gemcitabine combination nano therapies for pancreatic cancer. Pharmaceutics. 2019;11(11):1. https://doi.org/10.3390/pharmaceutics11110574.
Wang MY, Zhang L, Cai YF, Yang Y, Qu LP, Shen YT, Jin J, Zhou J, Chen JH. Bioengineered human serum albumin fusion protein as target/enzyme/pH three-stage propulsive drug vehicle for tumor therapy. ACS Nano. 2020;14(12):17405. https://doi.org/10.1021/acsnano.0c07610.
Zaragoza F. Non-covalent albumin ligands in FDA-approved therapeutic peptides and proteins. J Med Chem. 2023;66(6):3656. https://doi.org/10.1021/acs.jmedchem.2c01021.
Wang ZY, Mei J, Ni DQ, Li SM, Ye JM, Li SL, Wang YL, Liu Y. A nanoplatform self-assembled by coordination delivers siRNA for lung cancer therapy. Rare Met. 2023;42(5):1483. https://doi.org/10.1007/s12598-022-02185-w.
Wang QS, Zhang KH, Cui Y, Wang ZJ, Pan QY, Liu K, Sun B, Zhou H, Li MJ, Xu Q, Xu CY, Yu F, He JH. Upgrade of macromolecular crystallography beamline BL17U1 at SSRF. Nucl Sci Tech. 2018;29(5):1. https://doi.org/10.1007/s41365-018-0398-9.
Otwinowski Z, Minor W. Processing of X-ray diffraction data collected in oscillation mode. Method enzymol. 1997;276:307. https://doi.org/10.1016/s0076-6879(97)76066-x.
Debreczeni JE, Emsley P. Handling ligands with Coot. Acta Cryst D. 2012;68:425. https://doi.org/10.1107/s0907444912000200.
Emsley P, Lohkamp B, Scott WG, Cowtan K. Features and development of Coot. Acta Cryst D. 2010;66:486. https://doi.org/10.1107/s0907444910007493.
Jin GQ, Chau CV, Arambula JF, Gao S, Sessler JL, Zhang JL. Lanthanide porphyrinoids as molecular theranostics. Chem Soc Rev. 2022;51(14):6177. https://doi.org/10.1039/d2cs00275b.
Paprocka R, Wiese-Szadkowska M, Janciauskiene S, Kosmalski T, Kulik M, Helmin-Basa A. Latest developments in metal complexes as anticancer agents. Chem Soc Rev. 2022;452: 214307. https://doi.org/10.1016/j.ccr.2021.214307.
Xu JT, Han W, Jia T, Dong SM, Bi HT, Yang D, He F, Dai YL, Gai SL, Yang PP. Bioresponsive upconversion nanostructure for combinatorial bioimaging and chemo-photothermal synergistic therapy, Chem Eng J. 2018;342:446. https://doi.org/10.1016/j.cej.2018.02.109.
Wang YP, Gao WQ, Shi XY, Ding JJ, Liu W, He HB, Wang K, Shao F. Chemotherapy drugs induce pyroptosis through caspase-3 cleavage of a gasdermin. Nature. 2017;547(7661):99. https://doi.org/10.1038/nature22393.
De Schutter E, Croes L, Ibrahim J, Pauwels P. Op de Beeck K, Vandenabeele P, Van Camp G. GSDME and its role in cancer: from behind the scenes to the front of the stage. Int J Cancer. 2021;148(12):2872. https://doi.org/10.1002/ijc.33390.
Loveless R, Bloomquist R, Teng Y. Pyroptosis at the forefront of anticancer immunity. J Exp Clin Cancer Res. 2021;40(1):264. https://doi.org/10.1186/s13046-021-02065-8.
Chen HR, Wu FX, Xie XY, Wang W, Li QQ, Tu LP, Li B, Kong XG, Chang Y. Hybrid nanoplatform: enabling a precise antitumor strategy via dual-modal imaging-guided photodynamic/chemo-/immunosynergistic therapy. ACS Nano. 2021;15(12):20643. https://doi.org/10.1021/acsnano.1c09635.
Cao JJ, Zheng Y, Wu XW, Tan CP, Chen MH, Wu N, Ji LN, Mao ZW. Anticancer cyclometalated iridium(III) complexes with planar ligands: mitochondrial DNA damage and metabolism disturbance. J Med Chem. 2019;62(7):3311. https://doi.org/10.1021/acs.jmedchem.8b01704.
Raghavendra NM, Pingili D, Kadasi S, Mettu A, Prasad SVUM. Dual or multi-targeting inhibitors: the next generation anticancer agents. Eur J Med Chem. 2018;143:1277. https://doi.org/10.1016/j.ejmech.2017.10.021.
Acknowledgements
This study was financially supported by the Natural Science Foundation of China (No. 22077021) and the Natural Science Foundation of Guangxi (No. 2022GXNSFGA035003).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interests
The authors declare that they have no conflict of interest.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Man, XY., Sun, ZW., Li, SH. et al. Development of a Pt(II) compound based on indocyanine green@human serum albumin nanoparticles: integrating phototherapy, chemotherapy and immunotherapy to overcome tumor cisplatin resistance. Rare Met. (2024). https://doi.org/10.1007/s12598-024-02759-w
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s12598-024-02759-w