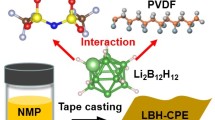
Abstract
Composite solid electrolytes (CSEs) containing polymer matrices and inorganic fillers hold promise for the next generation of solid-state batteries. However, the role of residual solvents in CSEs remains controversial. This study investigated the evolution and function of the residual solvent in a polymer-Li2B12H12 CSE. A partial reaction occurred between Li2B12H12 and solvent N, N-dimethylformamide (DMF), which produced dimethylaminomethanol (DMAM) in the CSE. Density functional theory calculations have revealed that DMAM forms stronger hydrogen bonds with polyvinylidene fluoride chains than DMF, which can have a plasticizing effect on the polymer matrix, leading to lower crystallinity and quicker segment motion. Therefore, this CSE exhibited improved Li-ion conducting properties, enabling the stable cycling of Li||LiFePO4 solid-state batteries. This study provided insights into the role of residual solvents in CSEs.
摘要
含有聚合物基质和无机填料的复合固体电解质(CSE)有望成为下一代固态电池。然而,残留溶剂在 CSE 中的作用仍存在争议。本研究调查了聚合物-Li2B12H12 CSE 中残留溶剂的演变和功能。Li2B12H12 与溶剂 N,N-二甲基甲酰胺(DMF)之间发生了部分再反应,在 CSE 中产生了二甲氨基甲醇(DMAM)。密度泛函理论计算显示,DMAM 与聚偏氟乙烯链之间形成的氢键比 DMF 更强,会对聚合物基体产生塑化作用,从而导致结晶度降低和区段运动加快。因此,这种 CSE 具有更好的锂离子传导性能,可实现锂离子固态电池的稳定循环。这项研究有助于深入了解残留溶剂在 CSE 中的作用。
Graphical abstract







Similar content being viewed by others
References
Tian YS, Zeng GB, Rutt A, Shi T, Kim H, Wang JY, Koettgen J, Sun YZ, Ouyang B, Chen TN, Lun ZY, Rong ZQ, Persson K, Ceder G. Promises and challenges of next-generation “beyond Li-ion” batteries for electric vehicles and grid decarbonization. Chem Rev. 2021;121(3):1623. https://doi.org/10.1021/acs.chemrev.0c00767.
Cano ZP, Banham D, Ye SY, Hintennach A, Lu J, Fowler M, Chen ZW. Batteries and fuel cells for emerging electric vehicle markets. Nat Energy. 2018;3(4):279. https://doi.org/10.1038/s41560-018-0108-1.
Liu J, Bao ZN, Cui Y, Dufek EJ, Goodenough JB, Khalifah P, Li QY, Liaw BY, Liu P, Manthiram A, Meng YS, Subramanian VR, Toney MF, Viswanathan VV, Whittingham MS, Xiao J, Xu W, Yang JH, Yang XQ, Zhang JG. Pathways for practical high-energy long-cycling lithium metal batteries. Nat Energy. 2019;4(3):180. https://doi.org/10.1038/s41560-019-0338-x.
Zhan HB, Liu SQ, Wang Q, Cao ML, Ma YN, Zhang CK, Li J. Development of lithium manganese iron phosphate cathode material for lithium-ion batteries. Chin J Rare Met. 2023;47(12):1669. https://doi.org/10.13373/j.cnki.cjrm.XY23060002.
Wang CW, Fu K, Kammampata SP, McOwen DW, Samson AJ, Zhang L, Hitz GT, Nolan AM, Wachsman ED, Mo YF, Thangadurai V, Hu LB. Garnet-type solid-state electrolytes: materials, interfaces, and batteries. Chem Rev. 2020;120(10):4257. https://doi.org/10.1021/acs.chemrev.9b00427.
Zhang Q, Cao DX, Ma Y, Natan A, Aurora P, Zhu HL. Sulfide-based solid-state electrolytes: synthesis, stability, and potential for all-solid-state batteries. Adv Mater. 2019;31(44):1901131. https://doi.org/10.1002/adma.201901131.
Lutz HD, Schmidt W, Haeuseler H. Chloride spinels: a new group of solid lithium electrolytes. J Phys Chem Solids. 1981;42(4):287. https://doi.org/10.1016/0022-3697(81)90142-6.
Li XN, Liang JW, Chen N, Luo J, Adair KR, Wang CH, Banis MN, Sham TK, Zhang L, Zhao SQ, Lu SG, Huang H, Li RY, Sun XL. Water-mediated synthesis of a superionic halide solid electrolyte. Angew Chem-Int Ed. 2019;58(46):16427. https://doi.org/10.1002/anie.201909805.
Matsuo M, Nakamori Y, Orimo S, Maekawa H, Takamura H. Lithium superionic conduction in lithium borohydride accompanied by structural transition. Appl Phys Lett. 2007;91(22):224103. https://doi.org/10.1063/1.2817934.
Pang YP, Wang XT, Shi XX, Xu F, Sun LX, Yang JH, Zheng SY. Solid-state prelithiation enables high-performance Li-Al-H anode for solid-state batteries. Adv Energy Mater. 2020;10(12):1902795. https://doi.org/10.1002/aenm.201902795.
Pang Y, Liu Y, Yang J, Zheng S, Wang C. Hydrides for solid-state batteries: a review. Materials Today Nano. 2022;18:100194. https://doi.org/10.1016/j.mtnano.2022.100194.
Xue ZG, He D, Xie XL. Poly(ethylene oxide)-based electrolytes for lithium-ion batteries. J Mater Chem A. 2015;3(38):19218. https://doi.org/10.1039/c5ta03471j.
Fu CY, Iacob M, Sheima Y, Battaglia C, Duchene L, Seidl L, Opris DM, Remhof A. A highly elastic polysiloxane-based polymer electrolyte for all-solid-state lithium metal batteries. J Mater Chem A. 2021;9(19):11794. https://doi.org/10.1039/d1ta02689e.
Wu N, Chien PH, Qian YM, Li YT, Xu HH, Grundish NS, Xu BY, Jin HB, Hu YY, Yu GH, Goodenough JB. Enhanced surface interactions enable fast Li+ conduction in oxide/polymer composite electrolyte. Angew Chem-Int Ed. 2020;59(10):4131. https://doi.org/10.1002/anie.201914478.
Croce F, Appetecchi GB, Persi L, Scrosati B. Nanocomposite polymer electrolytes for lithium batteries. Nature. 1998;394(6692):456. https://doi.org/10.1038/28818.
Tan SJ, Zeng XX, Ma Q, Wu XW, Guo YG. Recent advancements in polymer-based composite electrolytes for rechargeable lithium batteries. Electrochem Energ Rev. 2018;1(2):113. https://doi.org/10.1007/s41918-018-0011-2.
Fan MZ, Sun BZ, Jiang JY, Pan JY, Hu PH. Enhanced energy density in polyetherimide nanocomposite film at high temperature induced by electrospun BaZrTiO3 nanofibers. Rare Met. 2023;42(6):1912. https://doi.org/10.1007/s12598-022-02241-5.
Wang X, Shen X, Zhang P, Zhou AJ, Zhao JB. Promoted Li+ conduction in PEO-based all-solid-state electrolyte by hydroxyl-modified glass fiber fillers. Rare Met. 2023;42(3):875. https://doi.org/10.1007/s12598-022-02218-4.
Wei WQ, Liu BQ, Wang YQ, Yan K, Zhang H, Qi YS. Silicon-carbide fiber-reinforced polymer electrolyte for all-solid-state lithium-metal batteries. Rare Met. 2022;41(11):3774. https://doi.org/10.1007/s12598-022-02081-3.
Liu YN, Xiao Z, Zhang WK, Zhang J, Huang H, Gan YP, He XP, Kumar GG, Xia Y. Poly(m-phenylene isophthalamide)-reinforced polyethylene oxide composite electrolyte with high mechanical strength and thermostability for all-solid-state lithium metal batteries. Rare Met. 2022;41(11):3762. https://doi.org/10.1007/s12598-022-02065-3.
Yang TQ, Wang C, Zhang WK, Xia Y, Gan YP, Huang H, He XP, Zhang J. Composite polymer electrolytes reinforced by a three-dimensional polyacrylonitrile/ Li0.33La0.557TiO3 nanofiber framework for room-temperature dendrite-free all-solid-state lithium metal battery. Rare Met. 2022;41(6):1870. https://doi.org/10.1007/s12598-021-01891-1.
Stephan AM, Nahm KS, Kulandainathan MA, Ravi G, Wilson J. Poly(vinylidene fluoride-hexafluoropropylene) (PVDF-HFP) based composite electrolytes for lithium batteries. Eur Polymer J. 2006;42(8):1728. https://doi.org/10.1016/j.eurpolymj.2006.02.006.
Cong LN, Li YA, Lu W, Jie J, Liu YL, Sun LQ, Xie HM. Unlocking the poly(vinylidene fluoride-co-hexafluoropropylene)/Li10GeP2S12 composite solid-state electrolytes for dendrite-free Li metal batteries assisting with perfluoropolyethers as bifunctional adjuvant. J Power Sources. 2020;446:227365. https://doi.org/10.1016/j.jpowsour.2019.227365.
Zhang H, Huang L, Xu H, Zhang X, Chen Z, Gao C, Lu C, Liu Z, Jiang M, Cui GJe. A polymer electrolyte with a thermally induced interfacial ion-blocking function enables safety-enhanced lithium metal batteries. eScience. 2022;2(2):201. https://doi.org/10.1016/j.esci.2022.03.001.
Liang HM, Wang L, Wang AP, Song YZ, Wu YZ, Yang Y, He XM. Tailoring practically accessible polymer/inorganic composite electrolytes for all-solid-state lithium metal batteries: a review. Nano-micro Letters. 2023;15(1):42. https://doi.org/10.1007/s40820-022-00996-1.
Wieczorek W, Lipka P, Zukowska G, Wycislik H. Ionic interactions in polymeric electrolytes based on low molecular weight poly(ethylene glycol)s. J Phys Chem B. 1998;102(36):6968. https://doi.org/10.1021/jp981397k.
Falco M, Castro L, Nair JR, Bella F, Barde F, Meligrana G, Gerbaldi C. UV-cross-linked composite polymer electrolyte for high-rate, ambient temperature lithium batteries. Acs Appl Energ Mater. 2019;2(3):1600. https://doi.org/10.1021/acsaem.8b02185.
Zheng J, Hu YY. New insights into the compositional dependence of Li-ion transport in polymer-ceramic composite electrolytes. ACS Appl Mater Interfaces. 2018;10(4):4113. https://doi.org/10.1021/acsami.7b17301.
Sang JW, Tang B, Pan KC, He YB, Zhou Z. Current status and enhancement strategies for all-solid-state lithium batteries. Acc Mater Res. 2023. https://doi.org/10.1021/accountsmr.2c00229.
Gao LX, Tang B, Jiang HY, Xie ZJ, Wei JP, Zhou Z. Fiber-reinforced composite polymer electrolytes for solid-state lithium batteries. Adv Sustain Syst. 2022;6(3):2100389. https://doi.org/10.1002/adsu.202100389.
Zhu JX, He S, Tian HY, Hu YM, Xin C, Xie XX, Zhang LP, Gao J, Hao SM, Zhou WD, Zhang LQ. The influences of DMF content in composite polymer electrolytes on Li+-conductivity and interfacial stability with Li-metal. Adv Funct Mater. 2023. https://doi.org/10.1002/adfm.202301165.
Liu W, Lee SW, Lin DC, Shi FF, Wang S, Sendek AD, Cui Y. Enhancing ionic conductivity in composite polymer electrolytes with well-aligned ceramic nanowires. Nat Energy. 2017;2(5):17035. https://doi.org/10.1038/nenergy.2017.35.
Shen FY, Jonson RA, Tucker MC. The impact of residual solvent on catholyte performance in solid-state batteries. J Mater Chem A. 2022;10(47):25159. https://doi.org/10.1039/d2ta04847g.
Yao PC, Zhu B, Zhai HW, Liao XB, Zhu YX, Xu WH, Cheng Q, Jayyosi C, Li Z, Zhu J, Myers KM, Chen X, Yang Y. PVDF/palygorskite nanowire composite electrolyte for 4 V rechargeable lithium batteries with high energy density. Nano Lett. 2018;18(10):6113. https://doi.org/10.1021/acs.nanolett.8b01421.
Callegari D, Bonizzoni S, Berbenni V, Quartarone E, Mustarelli P. Is it possible to obtain solvent-free, Li+-conducting solid electrolytes based on pure PVDF? comment on “self-suppression of lithium dendrite in all-solid-state lithium metal batteries with poly(vinylidene difluoride) based solid electrolytes”. Adv Mater. 2020;32(14):1907375. https://doi.org/10.1002/adma.201907375.
Zhang X, Han J, Niu XF, Xin CZ, Xue CJ, Wang S, Shen Y, Zhang L, Li LL, Nan CW. High cycling stability for solid-state Li metal batteries via regulating solvation effect in poly(vinylidene fluoride)-based electrolytes. Batteries Supercaps. 2020;3(9):876. https://doi.org/10.1002/batt.202000081.
Bao KP, Pang YP, Yang JH, Sun DL, Fang F, Zheng SY. Modulating composite polymer electrolyte by lithium closo-borohydride achieves highly stable solid-state battery at 25 degrees C. Sci Chin-Mater. 2022;65(1):95. https://doi.org/10.1007/s40843-021-1740-7.
Guo ZM, Pang YP, Xia SX, Xu F, Yang JH, Sun LX, Zheng SY. Uniform and anisotropic solid electrolyte membrane enables superior solid-state Li metal batteries. Adv Sci. 2021;8(16):2100899. https://doi.org/10.1002/advs.202100899.
Lu FQ, Pang YP, Zhu MF, Han FD, Yang JH, Fang F, Sun DL, Zheng SY, Wang CS. A high-performance Li-B-H electrolyte for all-solid-state Li batteries. Adv Func Mater. 2019;29(15):1809219. https://doi.org/10.1002/adfm.201809219.
Zhao Q, Wang JK, Ai XH, Duan YJ, Pan ZH, Xie SR, Wang J, Gao YF. Three-dimensional knotting of W17O47@PEDOT:PSS nanowires enables high-performance flexible cathode for dual-functional electrochromic and electrochemical device. Infomat. 2022;4(4):e12298. https://doi.org/10.1002/inf2.12298.
Su Y, Rong XH, Gao A, Liu Y, Li JW, Mao ML, Qi XG, Chai GL, Zhang QH, Suo LM, Gu L, Li H, Huang XJ, Chen LQ, Liu BY, Hu YS. Rational design of a topological polymeric solid electrolyte for high-performance all-solid-state alkali metal batteries. Nat Commun. 2022;13(1):4181. https://doi.org/10.1038/s41467-022-31792-5.
Zhou Q, Ma J, Dong SM, Li XF, Cui GL. Intermolecular chemistry in solid polymer electrolytes for high-energy-density lithium batteries. Adv Mater. 2019;31(50):1902029. https://doi.org/10.1002/adma.201902029.
Yao YH, Zhang ZJ, Wang K, Fu Q. Effects of plasticizing on mechanical and viscous characteristics of poly(vinyl alcohol) a comparative study between glycerol and diethanolamine. Macromol Mater Eng. 2023. https://doi.org/10.1002/mame.202300090.
Wu CF. Cooperative behavior of poly(vinyl alcohol) and water as revealed by molecular dynamics simulations. Polymer. 2010;51(19):4452. https://doi.org/10.1016/j.polymer.2010.07.019.
Li Z, Fu JL, Zhou XY, Gui SW, Wei L, Yang H, Li H, Guo X. Ionic conduction in polymer-based solid electrolytes. Adv Sci. 2023;10(10):2201718. https://doi.org/10.1002/advs.202201718.
Lin Y, Gao MX, Zhu D, Liu YF, Pan HG. Effects of carbon coating and iron phosphides on the electrochemical properties of LiFePO4/C. J Power Sources. 2008;184(2):444. https://doi.org/10.1016/j.jpowsour.2008.03.026.
Acknowledgements
This study was financially supported by the National Natural Science Foundation of China (Nos. 51971146, 51971147, 52171218 and 52271222), Shanghai Municipal Science and Technology Commission (No. 21010503100), the Major Program for the Scientific Research Innovation Plan of Shanghai Education Commission (No. 2019-01-07-00-07-E00015), Shanghai Outstanding Academic Leaders Plan, Guangxi Key Laboratory of Information Materials (Guilin University of Electronic Technology, 201017-K), Shanghai Rising-Star Program (No. 20QA1407100) and General Program of Natural Science Foundation of Shanghai (No. 20ZR1438400).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Ye, XY., Bao, KP., Luo, SN. et al. Evolution and function of residual solvent in polymer-Li2B12H12 composite solid electrolyte. Rare Met. (2024). https://doi.org/10.1007/s12598-024-02710-z
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s12598-024-02710-z