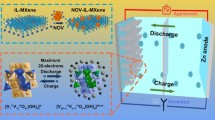
Abstract
The global trend towards new energy storage systems has stimulated the development of electrochemical energy storage technologies. Among these technologies, rechargeable aqueous zinc-ion batteries (AZIBs) have attracted considerable interest as a potential alternative to lithium-ion batteries (LIBs) due to their affordable cost, environmental compatibility and high safety standards. In this study, a high-quality electrode for AZIBs has been successfully developed using a dehydrated mixed-valence polyoxometalate-based three-dimensional (3D) inorganic framework material known as [H6Mn3VIV15VV4O46(H2O)12] (3D-MnVO). This innovative 3D-MnVO material is built from the alternate connections of {V19O46} "sphere-shaped" clusters and μ2-{Mn(H2O)4} bridges, where each {V19O46} cluster is surrounded by three pairs of vertically distributed {Mn(H2O)4} units, thus resulting in the 3D interpenetrating grid-like network from the infinite [-{V19O46}-µ2-Mn(H2O)4-{V19O46}]∞ chains in three mutually perpendicular directions. The 3D framework structure of 3D-MnVO possesses abundant oxygen vacancies, spacious and multi-level interconnected channels for ion transport, which facilitates the efficient intercalation/deintercalation of hydrated Zn2+ into the pores of the primary structure via the intercalation capacitance mechanism. As a result, the 3D-MnVO electrode exhibits excellent diffusion rates and minimal interfacial resistance. At a current density of 0.1 A·g−1, the 3D-MnVO cathode delivers a commendable discharge capacity of 170.5 mAh·g−1 with 81.6% capacity retention after 100 charge/discharge cycles. Furthermore, even at a high current density of 1.0 A·g−1, the 3D-MnVO electrode delivers a remarkable reversible capacity of 198.9 mAh·g−1. Our research results provide valuable insights into the development of advanced polyoxometalate-based 3D inorganic framework electrode materials for high-performance rechargeable AZIBs.
Graphical abstract

摘要
全球对新型能源体系的迫切需求和持续探索推动了电化学储能技术的快速发展。在这些技术中, 可充电水系锌离子电池 (AZIBs) 由于其成本低廉、环境兼容性和高安全标准等特点而引起了广泛关注, 被视为锂离子电池 (LIBs) 的潜在替代品之一。在这项研究中, 我们利用脱去结晶水的混合价多金属氧簇基三维无机框架材料[H6Mn3VIV15VV4O46(H2O)12] (3D-MnVO)作为正极材料, 开发了一种高性能水系锌离子电池电极。这种新颖的3D-MnVO材料由球形簇{V19O46}和μ2-{Mn(H2O)4}桥交替连接而成, 每个{V19O46}簇周围都有三对垂直分布的{Mn(H2O)4}单元, 从而在三个互相垂直的方向上形成了无限的{V19O46}-µ2-Mn(H2O)4-{V19O46}]∞链的三维网格结构。3D-MnVO材料的三维结构中具有丰富的氧空位、宽敞的多级互连通道, 有利于通过插层电容机制将水合锌离子高效地插入到3D-MnVO主体结构的孔隙中进行Zn2+插入/脱出反应。因此, 3D-MnVO电极具有出色的扩散速率和较小的界面电阻, 在0.1 A‧g−1的电流密度下, 3D-MnVO正极具有可观的170.5 mAh‧g−1的放电容量, 并在100次充放电循环后保持81.6%的容量保留率。此外, 即使在较高电流密度1.0 A‧g−1下, 3D-MnVO电极仍具有出色的可逆容量198.9 mAh‧g−1。我们的研究结果为开发高性能可充电水系锌电池的先进多金属氧簇基电极材料提供了宝贵的见解。





Similar content being viewed by others
References
Li M, Lu J, Chen ZW, Amine K. 30 years of lithium-ion batteries. Adv Mater. 2018;30(33):1800561. https://doi.org/10.1002/adma.201800561.
Wu FX, Maier J, Yu Y. Guidelines and trends for next-generation rechargeable lithium and lithium-ion batteries. Chem Soc Rev. 2020;49(5):1569. https://doi.org/10.1039/C7CS00863E.
Zhu LM, Ding GC, Han Q, Miao YX, Li X, Yang XL, Chen L, Wang GK, Xie LL, Cao XY. Enhancing electrochemical performances of small quinone toward lithium and sodium energy storage. Rare Met. 2022;41(2):425. https://doi.org/10.1007/s12598-021-01813-1.
Wang L, Wang ZH, Xie LL, Zhu LM, Cao XY. ZIF-67-derived N-doped Co/C nanocubes as high-performance anode materials for lithium-ion batteries. ACS Appl Mater Interfaces. 2019;11(18):16619. https://doi.org/10.1021/acsami.9b03365.
Li RT, Du YX, Li YH, He ZX, Dai L, Wang L, Wu XW, Zhang JJ, Yi J. Alloying strategy for high-performance zinc metal anodes. ACS Energy Lett. 2023;8(1):457. https://doi.org/10.1021/acsenergylett.2c01960.
Parker JF, Chervin CN, Pala IR, Machler M, Burz MF, Long JW, Rolison DR. Rechargeable nickel-3D zinc batteries: an energy-dense, safer alternative to lithium-ion. Science. 2017;356(6336):414. https://doi.org/10.1126/science.aak9991.
Li Y, Li X, Duan H, Xie SY, Dai RY, Rong JH, Kang FY, Dong LB. Aerogel-structured MnO2 cathode assembled by defect-rich ultrathin nanosheets for zinc-ion batteries. Chem Eng J. 2022;441:136008. https://doi.org/10.1016/j.cej.2022.136008.
Kundu D, Adams BD, Duffort V, Vajargah SH, Nazar LF. A high capacity and long-life aqueous rechargeable zinc battery using a metal oxide intercalation cathode. Nat Energy. 2016;1(10):16119. https://doi.org/10.1038/nenergy.2016.119.
Wang TT, Wang PJ, Pan L, He ZX, Dai L, Wang L, Liu SD, Jun SC, Lu BA, Liang SQ, Zhou J. Stabling zinc metal anode with polydopamine regulation through dual effects of fast desolvation and ion confinement. Adv Energy Mater. 2022;13(5):2203523. https://doi.org/10.1002/aenm.202203523.
Selvakumaran D, Pan AQ, Liang SQ, Cao GZ. A review on recent developments and challenges of cathode materials for rechargeable aqueous Zn-ion batteries. J Mater Chem A. 2019;7(31):18209. https://doi.org/10.1039/C9TA05053A.
Wu K, Cui J, Yi J, Liu XY, Ning FH, Liu YY, Zhang JJ. Biodegradable gel electrolyte suppressing water-induced issues for long-life zinc metal anodes. ACS Appl Mater Interfaces. 2022;14(30):34612. https://doi.org/10.1021/acsami.2c05887.
Xie YH, Huang JH, Kong TY, Zhou X, Wu K, Liu XY, Yi J, Xing LD, Xia YY. Moisture-activated deep eutectic electrolyte enabling stable metal Zn anode. Energy Storage Mater. 2023;56:218. https://doi.org/10.1016/j.ensm.2023.01.013.
Song Y, Ruan PC, Mao CW, Chang YX, Wang L, Dai L, Zhou P, Lu BA, Zhou J, He ZX. Metal–organic frameworks functionalized separators for robust aqueous zinc-ion batteries. Nano-Micro Lett. 2022;14(1):218. https://doi.org/10.1007/s40820-022-00960-z.
Wu K, Zhan SK, Liu W, Liu XY, Ning FH, Liu YY, Zhang JJ, Yi J. Targeted delivery of zinc ion derived by pseudopolyrotaxane gel polymer electrolyte for long-life Zn anode. ACS Appl Mater Interfaces. 2023;15(5):6839. https://doi.org/10.1021/acsami.2c20194.
Xu CJ, Li BH, Du HD, Kang FY. Energetic zinc ion chemistry: the rechargeable zinc ion battery. Angew Chem Int Ed. 2012;51(4):933. https://doi.org/10.1002/anie.201106307.
Song M, Tan H, Chao DL, Fan HJ. Recent advances in Zn-ion batteries. Adv Funct Mater. 2018;28(41):1802564. https://doi.org/10.1002/adfm.201802564.
Zhou T, Zhu LM, Xie LL, Han Q, Yang XL, Chen L, Wang GK, Cao XY. Cathode materials for aqueous zinc-ion batteries: a mini review. J Colloid Interface Sci. 2022;605:828. https://doi.org/10.1016/j.jcis.2021.07.138.
Tang BY, Shan LT, Liang SQ, Zhou J. Issues and opportunities facing aqueous zinc ion batteries. Energy Environ Sci. 2019;12(11):3288. https://doi.org/10.1039/C9EE02526J.
Gan Y, Wang C, Li JY, Zheng JJ, Wan HZ, Wang H. Stability optimization strategy of aqueous zinc ion batteries. Chin J Rare Met. 2022;46(6):753. https://doi.org/10.13373/j.cnki.cjrm.XY21100036.
Du WC, Ang EH, Yang Y, Zhang YF, Ye MH, Li CC. Challenges in the material and structural design of zinc anode towards high-performance aqueous zinc-ion batteries. Energy Environ Sci. 2020;13(10):3330. https://doi.org/10.1039/D0EE02079F.
Xu XM, Xiong FY, Meng JS, Wang XP, Niu CJ, An QY, Mai LQ. Vanadium-based nanomaterials: a promising family for emerging metal-ion batteries. Adv Funct Mater. 2020;30(10):1904398. https://doi.org/10.1002/adfm.201904398.
Zhou T, Xie LL, Niu Y, Xiao HR, Li YJ, Han Q, Qiu XJ, Yang XL, Wu XY, Zhu LM, Pang H, Cao XY. New insights on (V10O28)6–based electrode materials for energy storage: a brief review. Rare Met. 2023;42(5):1431. https://doi.org/10.1007/s12598-022-02207-7.
Lv TT, Peng Y, Zhang GX, Jiang S, Yang ZL, Yang SY, Pang H. How about vanadium-based compounds as cathode materials for aqueous zinc ion batteries? Adv Sci. 2023;10(12):2206907. https://doi.org/10.1002/advs.202206907.
Chen M, Zhang SC, Zou ZG, Zhong SL, Ling WQ, Geng J, Liang FA, Peng XX, Gao Y, Yu FG. Review of vanadium-based oxide cathodes as aqueous zinc-ion batteries. Rare Met. 2023;42(9):2868. https://doi.org/10.1007/s12598-023-02303-2.
Zhang N, Wang JC, Guo YF, Wang PF, Zhu YR, Yi TF. Insights on rational design and energy storage mechanism of Mn-based cathode materials towards high performance aqueous zinc-ion batteries. Coord Chem Rev. 2023;479:215009.
Mathew V, Sambandam B, Kim S, Kim S, Park S, Lee S, Alfaruqi MH, Soundharrajan V, Islam S, Putro DY, Hwang JY, Sun YK, Kim J. Manganese and vanadium oxide cathodes for aqueous rechargeable zinc-ion batteries: a focused view on performance, mechanism and developments. ACS Energy Lett. 2020;5(7):2376. https://doi.org/10.1021/acsenergylett.0c00740.
Zhang H, Peng J, Li L, Zhao YN, Gao Y, Wang JZ, Cao YL, Dou SX, Chou SL. Low-cost zinc substitution of iron-based Prussian blue analogs as long lifespan cathode materials for fast charging sodium-ion batteries. Adv Funct Mater. 2023;33(2):2210725. https://doi.org/10.1002/adfm.202210725.
Yu F, Wang Y, Liu Y, Hui HY, Wang FX, Li JF, Wang Q. An aqueous rechargeable zinc-ion battery on basis of an organic pigment. Rare Met. 2022;41(7):2230. https://doi.org/10.1007/s12598-021-01941-8.
Ye F, Liu Q, Dong HL, Guan KL, Chen ZY, Ju N, Hu LF. Organic zinc-ion battery: planar, π-conjugated quinone-based polymer endows ultrafast ion diffusion kinetics. Angew Chem Int Ed. 2022;61(51):202214244. https://doi.org/10.1002/anie.202214244.
Horn MR, Singh A, Alomari S, Goberna-Ferrón S, Benages-Vilau R, Chodankar N, Motta N, Ostrikov K, MacLeod J, Sonar P, Gomez-Romero P, Dubal D. Polyoxometalates (POMs): from electroactive clusters to energy materials. Energy Environ Sci. 2021;14(4):1652. https://doi.org/10.1039/D0EE03407J.
Gumerova NI, Rompel A. Synthesis, structures and applications of electron-rich polyoxometalates. Nat Rev Chem. 2018;2:0112. https://doi.org/10.1038/s41570-018-0112.
Cherevan AS, Nandan SP, Roger I, Liu RJ, Streb C, Eder D. Polyoxometalates on functional substrates: concepts, synergies, and future perspectives. Adv Sci. 2020;7(8):1903511. https://doi.org/10.1002/advs.201903511.
Liu JC, Wang JF, Han Q, Shangguan P, Liu LL, Chen LJ, Zhao JW, Streb C, Song YF. Multicomponent self-assembly of a giant heterometallic polyoxotungstate supercluster with antitumor activity. Angew Chem Int Ed. 2021;60(20):11153. https://doi.org/10.1002/anie.202017318.
Mialane P, Mellot-Draznieks C, Gairola P, Duguet M, Benseghir Y, Oms O, Dolbecq A. Heterogenisation of polyoxometalates and other metal-based complexes in metal–organic frameworks: from synthesis to characterisation and applications in catalysis. Chem Soc Rev. 2021;50(10):6152. https://doi.org/10.1039/D0CS00323A.
Monakhov KY, Bensch W, Kögerler P. Semimetal-functionalised polyoxovanadates. Chem Soc Rev. 2015;44(23):8443. https://doi.org/10.1039/C5CS00531K.
Proe KR, Schreiber E, Matson EM. Proton-coupled electron transfer at the surface of polyoxovanadate-alkoxide clusters. Acc Chem Res. 2023;56(12):1602. https://doi.org/10.1021/acs.accounts.3c00166.
Chen JJ, Ye JC, Zhang XG, Symes MD, Fan SC, Long DL, Zheng MS, Wu DY, Cronin L, Dong QF. Design and performance of rechargeable sodium ion batteries, and symmetrical Li-ion batteries with supercapacitor-like power density based upon polyoxovanadates. Adv Energy Mater. 2018;8(6):1701021. https://doi.org/10.1002/aenm.201701021.
Chen JJ, Symes MD, Fan SC, Zheng MS, Miras HN, Dong QF, Cronin L. High-performance polyoxometalate-based cathode materials for rechargeable lithium-ion batteries. Adv Mater. 2015;27(31):4649. https://doi.org/10.1002/adma.201501088.
Vangelder LE, Kosswattaarachchi AM, Forrestel PL, Cook TR, Matson EM. Polyoxovanadate-alkoxide clusters as multi-electron charge carriers for symmetric non-aqueous redox flow batteries. Chem Sci. 2018;9(6):1692. https://doi.org/10.1039/C7SC05295B.
Li XL, Lin ZF, Jin N, Sun L, Yang XJ, Liu Y. Electrochemically induced crystalline-to-amorphous transition of dinuclear polyoxovanadate for high-rate lithium-ion batteries. Adv Funct Mater. 2023;33(20):2214667. https://doi.org/10.1002/adfm.202214667.
Lin CC, Lin WH, Huang SC, Hu CW, Chen TY, Hsu CT, Yang H, Haider A, Lin ZG, Kortz U, Stimming U, Chen HY. Mechanism of sodium ion storage in Na7[H2PV14O42] anode for sodium-ion batteries. Adv Mater. 2018;5(15):1800491. https://doi.org/10.1002/admi.201800491.
Yang K, Hu YY, Li LY, Cui LL, He L, Wang SJ, Zhao JW, Song YF. First high-nuclearity mixed-valence polyoxometalate with hierarchical interconnected Zn2+ migration channels as an advanced cathode material in aqueous zinc-ion battery. Nano Energy. 2020;74:104851. https://doi.org/10.1016/j.nanoen.2020.104851.
Yang K, Hu YY, Zhang TS, Wang BY, Qin JX, Li NX, Zhao ZW, Zhao JW, Chao DL. Triple-functional polyoxovanadate cluster in regulating cathode, anode, and electrolyte for tough aqueous zinc-ion battery. Adv Energy Mater. 2022;12(42):2202671. https://doi.org/10.1002/aenm.202202671.
Zhou T, Zhu LM, Xie LL, Han Q, Yang XL, Cao XY, Ma JM. New insight on K2Zn2V10O28 as an advanced cathode for rechargeable aqueous zinc-ion batteries. Small. 2022;18(12):2107102. https://doi.org/10.1002/smll.202107102.
Zhou T, Xie LL, Han Q, Yang XL, Zhu LM, Cao XY. Investigation of Na6V10O28 as a promising rechargeable aqueous zinc-ion batteries cathode. Chem Eng J. 2022;445:136789. https://doi.org/10.1016/j.cej.2022.136789.
Zhou T, Xiao HR, Xie LL, Han Q, Qiu XJ, Xiao YM, Yang XL, Zhu LM, Cao XY. Research on the electrochemical performance of polyoxovanadate material as a novel aqueous zinc-ion batteries cathode. Electrochim Acta. 2022;424:140621. https://doi.org/10.1016/j.electacta.2022.140621.
Huang R, Wang WW, Zhang C, He P, Han YY, Chen N, Yan J. A bi-component polyoxometalate-derivative cathode material showed impressive electrochemical performance for the aqueous zinc-ion batteries. Chin Chem Lett. 2022;33(8):3955. https://doi.org/10.1016/j.cclet.2021.11.094.
Zou QX, He HS, Xie J, Han SB, Lin WJ, Mondal AK, Huang F. Study on the mechanism of acid modified H-Beta zeolite acidic sites on the catalytic pyrolysis of Kraft lignin. Chem Eng J. 2023;462:142029. https://doi.org/10.1016/j.cej.2023.142029.
Kim HJ, Yeon JS, Park HR, Lee SJ, Kim WI, Jang G, Park HS. Intercalation pseudocapacitance of cation-exchanged molybdenum-based polyoxometalate for the fast and stable zinc ion storage. ACS Appl Mater Interfaces. 2023;15(7):9350. https://doi.org/10.1021/acsami.2c21034.
Tian Y, Chang ZH, Zhang YC, Wang XL, Chen YZ, Liu QQ, Yu L. Two Anderson-type POM-based metal-organic complexes as multifunctional materials for electrocatalytic sensing and zinc-ion batteries. Polyhedron. 2021;204:115245. https://doi.org/10.1016/j.poly.2021.115245.
Yang K, Ying YX, Cui LL, Sun JC, Luo H, Hu YY, Zhao JW. Stable aqueous Zn−Ag and Zn−polyoxometalate hybrid battery driven by successive Ag+ cation and polyoxoanion redox reactions. Energy Storage Mater. 2021;34:203. https://doi.org/10.1016/j.ensm.2020.09.011.
Shen FC, Wang YR, Li SL, Liu J, Dong LZ, Wei T, Cui YC, Wu XL, Xu Y, Lan YQ. Self-assembly of polyoxometalate/reduced graphene oxide composites induced by ionic liquids as a high-rate cathode for batteries: “killing two birds with one stone”. J Mater Chem A. 2018;6(4):1743. https://doi.org/10.1039/C7TA09810C.
Khan MI, Yohannes E, Powell D. Vanadium oxide clusters as building blocks for the synthesis of metal oxide surfaces and framework materials: synthesis and X-ray crystal structure of [H6Mn3VIV15VV4O46(H2O)12]·30H2O. Inorg Chem. 1999;38(2):212. https://doi.org/10.1021/ic981077f.
Peng Z, Li YH, Ruan PC, He ZX, Dai L, Liu SD, Wang L, Jun SC, Lu BA, Zhou J. Metal-organic frameworks and beyond: the road toward zinc-based batteries. Coord Chem Rev. 2023;488:215190. https://doi.org/10.1016/j.ccr.2023.215190.
He H, Pan FC, Liang XW, Hu Q, Liu SD, Hu JS, Jun SC, Lin DM, Yamauchi Y, Huo Y. Unveiling the effect of structural water on Zn-ion storage of polyoxovanadate for high-rate and long-life aqueous zinc ion battery. Chem Eng J. 2023;462:142221. https://doi.org/10.1016/j.cej.2023.142221.
An HY, Zhang J, Chang SZ, Hou YJ, Zhu QS. 2D hybrid architectures constructed from two kinds of polyoxovanadates as efficient heterogeneous catalysts for cyanosilylation and knoevenagel condensation. Inorg Chem. 2020;59(15):10578. https://doi.org/10.1021/acs.inorgchem.0c00999.
Xu X, Meng RR, Lu CT, Mei L, Chen LJ, Zhao JW. Acetate-decorated tri-Ln(III)-containing antimonotungstates with a tetrahedral WO4 group as a structure-directing template and their luminescence properties. Inorg Chem. 2020;59(6):3954. https://doi.org/10.1021/acs.inorgchem.9b03620.
Han Q, Wen Y, Liu JC, Zhang W, Chen LJ, Zhao JW. Rare-earth-incorporated tellurotungstate hybrids functionalized by 2-picolinic acid ligands: syntheses, structures, and properties. Inorg Chem. 2017;56(21):13228. https://doi.org/10.1021/acs.inorgchem.7b02009.
Zhao MM, Wang Y, Wu NN, Zhang J, Liu B. Photo-assisted synthesis of inorganic polyoxovanadate. Dalton Trans. 2020;49(28):9662. https://doi.org/10.1039/D0DT01945C.
Bi WC, Gao GH, Wu GM, Atif M, AlSalhi MS, Cao GZ. Sodium vanadate/PEDOT nanocables rich with oxygen vacancies for high energy conversion efficiency zinc ion batteries. Energy Storage Mater. 2021;40:209. https://doi.org/10.1016/j.ensm.2021.05.003.
Yan MY, He P, Chen Y, Wang SY, Wei QL, Zhao KN, Xu X, An QY, Shuang Y, Shao YY, Mueller KT, Mai LQ, Liu J, Yang JH. Water-lubricated Intercalation in V2O5·nH2O for high-capacity and high-rate aqueous rechargeable zinc batteries. Adv Mater. 2018;30(1):1703725. https://doi.org/10.1002/adma.201703725.
Liao M, Wang JW, Ye L, Sun H, Wen YZ, Wang C, Sun XM, Wang BJ, Peng HS. A deep-cycle aqueous zinc-ion battery containing an oxygen-deficient vanadium oxide cathode. Angew Chem Int Ed. 2020;59(6):2273. https://doi.org/10.1002/anie.201912203.
Feng JJ, Wang Y, Liu SH, Chen SY, Wen N, Zeng XX, Dong YZ, Huang CM, Kuang Q, Zhao YM. Electrochemically induced structural and morphological evolutions in nickel vanadium oxide hydrate nanobelts enabling fast transport kinetics for high-performance zinc storage. ACS Appl Mater Interfaces. 2020;12(12):24726. https://doi.org/10.1021/acsami.0c04199.
Nam KW, Park SS, dos Reis R, Dravid VP, Kim H, Mirkin CA, Stoddart JF. Conductive 2D metal-organic framework for high-performance cathodes in aqueous rechargeable zinc batteries. Nat Commun. 2019;10(1):4948. https://doi.org/10.1038/s41467-019-12857-4.
Ru Y, Zheng SS, Xue HG, Pang H. Layered V-MOF nanorods for rechargeable aqueous zinc-ion batteries. Mater Today Chem. 2021;21:100513. https://doi.org/10.1016/j.mtchem.2021.100513.
He P, Yan MY, Zhang GB, Sun RM, Chen LN, An QY, Mai LQ. Layered VS2 nanosheet-based aqueous Zn ion battery cathode. Adv Energy Mater. 2017;7(11):1601920. https://doi.org/10.1002/aenm.201601920.
Li X, Li Y, Zhao X, Kang FY, Dong LB. Elucidating the charge storage mechanism of high-performance vertical graphene cathodes for zinc-ion hybrid supercapacitors. Energy Storage Mater. 2022;53:505. https://doi.org/10.1016/j.ensm.2022.09.023.
Pan ZH, Yang J, Yang J, Zhang QC, Zhang H, Li X, Kou ZK, Zhang YF, Chen H, Yan CL, Wang J. Stitching of Zn3(OH)2V2O7·2H2O 2D nanosheets by 1D carbon nanotubes boosts ultrahigh rate for wearable quasi-solid-state zinc-ion batteries. ACS Nano. 2020;14(1):842. https://doi.org/10.1021/acsnano.9b07956.
Zhang N, Jia M, Dong Y, Wang YY, Xu JZ, Liu YC, Jiao LF, Cheng FY. Hydrated layered vanadium oxide as a highly reversible cathode for rechargeable aqueous zinc batteries. Adv Funct Mater. 2019;29(10):1807331. https://doi.org/10.1002/adfm.201807331.
Li YK, Huang ZM, Kalambate PK, Zhong Y, Huang ZM, Xie ML, Shen Y, Huang YH. V2O5 nanopaper as a cathode material with high capacity and long cycle life for rechargeable aqueous zinc-ion battery. Nano Energy. 2019;60:752. https://doi.org/10.1016/j.nanoen.2019.04.009.
Liu WB, Dong LB, Jiang BZ, Huang YF, Wang XL, Xu CJ, Kang Z, Mou J, Kang FY. Layered vanadium oxides with proton and zinc ion insertion for zinc ion batteries. Electrochim Acta. 2019;320:134565. https://doi.org/10.1016/j.electacta.2019.134565.
Su ZH, Wang RH, Huang JH, Sun R, Qin ZX, Zhang YF, Fan HS. Silver vanadate (Ag0.33V2O5) nanorods from Ag intercalated vanadium pentoxide for superior cathode of aqueous zinc-ion batteries. Rare Met. 2022;41(8):2844. https://doi.org/10.1007/s12598-022-02026-w.
Zhu LM, Li WX, Xie LL, Yang Q, Cao XY. Rod-like NaV3O8 as cathode materials with high capacity and stability for sodium storage. Chem Eng J. 2019;372:1056. https://doi.org/10.1016/j.cej.2019.05.009.
Sambandam B, Soundharrajan V, Kim S, Alfaruqi MH, Jo J, Kim S, Mathew V, Sun YK, Kim J. Aqueous rechargeable Zn-ion batteries: an imperishable and high-energy Zn2V2O7 nanowire cathode through intercalation regulation. J Mater Chem A. 2018;6(9):3850. https://doi.org/10.1039/C7TA11237H.
Pu XC, Jiang BZ, Wang XL, Liu WB, Dong LB, Kang FY, Xu CJ. High-performance aqueous zinc-ion batteries realized by MOF materials. Nano-Micro Lett. 2020;12(1):152. https://doi.org/10.1007/s40820-020-00487-1.
Acknowledgements
This study was financially supported by the National Natural Science Foundation of China (Nos. 52071132, 52261135632 and U21A20284), Zhongyuan Thousand People Plan-The Zhongyuan Youth Talent Support Program (in Science and Technology), China (No. ZYQR201810139), the Natural Science Foundation of Henan, China (Nos. 232300421080 and 222300420138), the Science and Technology Project of Henan Province, China (Nos. 232102241038 and 232102241004), the Key Scientific Research Programs in Universities of Henan Province, China-Special Projects for Basic Research (No. 23ZX008), the Innovative Funds Plan of Henan University of Technology, China (No. 2020ZKCJ04), the Ph.D. Programs Foundation of Henan University of Technology, China (No. 2021BS0027), and the Doctoral Education Fund of Henan University of Engineering, China (No. DKJ2019004).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Han, Q., Xiao, HR., Zhou, T. et al. A mixed-valence polyoxometalate-based 3D inorganic framework cathode material for high-efficiency rechargeable AZIBs. Rare Met. (2024). https://doi.org/10.1007/s12598-024-02671-3
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s12598-024-02671-3