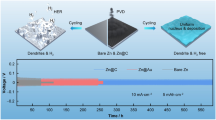
Abstract
Aqueous zinc-ion batteries have attracted much attention due to their high theoretical capacity, low cost, high safety, and eco-friendly. However, challenges such as dendrite growth and side reactions severely hinder the electrochemical performance of the Zn anode, leading to low Coulombic efficiency (CE) or even short circuits. Herein, phenolic resin (PF) is used as a protective coating on the surface of the zinc metal electrode. The PF layer can greatly improve the corrosion resistance of the Zn electrode in the electrolyte. Importantly, this artificial electrode/electrolyte interphase also elevates the nucleation barrier and restrains Zn2+ 2D diffusion, regulating the zinc deposition/dissolution behavior. As a result, PF@Zn symmetric cells exhibit superior performance than that of bare Zn symmetric cells, achieving a stable and dendrite-free cycle of 1400 h. Furthermore, the carbon nanotube/MnO2||PF@Zn (CNT/MnO2||PF@Zn) full cell also delivers a long cycle life with a high-capacity retention of about 180.0 mAh·g−1 after 1400 cycles, far exceeding that of bare Zn anode. This work provides a facile strategy for achieving a dendrite-free and rechargeable zinc anode.
Graphical abstract

摘要
水系锌离子电池因其理论容量高, 成本低, 安全性高和生态友好而备受关注。然而,锌枝晶生长和副反应等问题严重影响了锌金属负极的电化学性能,导致电池的库伦效率低甚至短路。本文利用酚醛树脂(PF)作为锌金属负极表面的保护涂层,其可以有效提高锌金属负极在电解液中的耐腐蚀性。更重要的是此电极/电解液界面层可提高锌离子的成核势垒并抑制其二维扩散,从而有效调节锌的沉积/溶解行为。结果表明,制备的PF@Zn对称电池的稳定性明显优于纯锌对称电池,可稳定循环1400 h。此外,CNT/MnO2||PF@Zn 全电池在1400 次循环后仍能保持约 180.0 mAh·g−1 的高比容量,远超过纯锌负极。这项研究为实现无枝晶, 可充电锌金属负极提供了一种简便有效的策略。






Similar content being viewed by others
References
Zheng J, Huang Z, Ming F, Zeng Y, Wei B, Jiang Q, Qi Z, Wang Z, Liang H. Surface and interface engineering of Zn anodes in aqueous rechargeable Zn-ion batteries. Small. 2022;18(21):e2200006. https://doi.org/10.1002/smll.202200006.
Liu Y, Li L, Ji X, Cheng S. Scientific challenges and improvement strategies of Zn-based anodes for aqueous Zn-ion batteries. Chem Rec. 2022;22(10):e202200114. https://doi.org/10.1002/tcr.202200114.
Chen M, Zhang S, Zou Z, Zhong S, Ling W, Geng J, Liang F, Peng X, Gao Y, Yu F. Review of vanadium-based oxide cathodes as aqueous zinc-ion batteries. Rare Met. 2023;42(9):2868. https://doi.org/10.1007/s12598-023-023032.
Yi Z, Chen G, Hou F, Wang L, Liang J. Strategies for the stabilization of Zn metal anodes for Zn-ion batteries. Adv Energy Mater. 2020;11(1):2003065. https://doi.org/10.1002/aenm.202003065.
Jia X, Liu C, Neale Z, Yang J, Cao G. Active materials for aqueous zinc ion batteries: synthesis crystal structure morphology and electrochemistry. Chem Rev. 2020;120(15):7795. https://doi.org/10.1021/acs.chemrev.9b00628.
Gan Y, Wang C, Li J, Zheng J, Wan H, Wang H. Stability optimization strategy of aqueous zinc ion batteries. Chin J Rare Met. 2022;46(6):753. https://doi.org/10.13373/j.cnki.cjrm.XY21100036.
Du W, Ang E, Yang Y, Zhang Y, Ye M, Li C. Challenges in the material and structural design of zinc anode towards high-performance aqueous zinc-ion batteries. Energy Environ Mater. 2020;13(10):3330. https://doi.org/10.1039/d0ee02079f.
Lu W, Xie C, Zhang H, Li X. Inhibition of zinc dendrite growth in zinc-based batteries. Chem Sus Chem. 2018;11(23):3996. https://doi.org/10.1002/cssc.201801657.
Cui B, Han X, Hu W. Micronanostructured design of dendrite-free zinc anodes and their applications in aqueous zinc-based rechargeable batteries. Small Struct. 2021;2(6):2000128. https://doi.org/10.1002/sstr.202000128.
He W, Zuo S, Xu X, Zeng L, Liu L, Zhao W, Liu J. Challenges and strategies of zinc anode for aqueous zinc-ion batteries. Mater Chem Front. 2021;5(5):2201. https://doi.org/10.1039/d0qm00693a.
Song M, Zhong C. Achieving both high reversible and stable Zn anode by a practical glucose electrolyte additive toward high-performance Zn-ion batteries. Rare Met. 2022;41(2):356. https://doi.org/10.1007/s12598-021-01858-2.
Li C, Shi X, Liang S, Ma X, Han M, Wu X, Zhou J. Spatially homogeneous copper foam as surface dendrite-free host for zinc metal anode. Chem Eng J. 2020;379:122248. https://doi.org/10.1016/j.cej.2019.122248.
Li L, Jia S, Cheng Z, Zhang C. Improved strategies for separators in zinc-ion batteries. Chem Sus Chem. 2023;16(8):e202202330. https://doi.org/10.1002/cssc.202202330.
Yan Z, Xin W, Zhu Z. Artificial interphase engineering to stabilize aqueous zinc metal anodes. Nanoscale. 2021;12(47):19828. https://doi.org/10.1039/d1nr06058a.
Liu Y, Liu Y, Wu X. Toward long-life aqueous zinc ion batteries by constructing stable zinc anodes. Chem Rec. 2022;22(10):e202200088. https://doi.org/10.1002/tcr.202200088.
Tao F, Liu Y, Ren X, Wang J, Zhou Y, Miao Y, Ren F, Wei S, Ma J. Different surface modification methods and coating materials of zinc metal anode. J Energy Chem. 2022;66:397. https://doi.org/10.1016/j.jechem.2021.08.022.
Wang Y, Sun H, Li N, Yang X, Liu H, Zheng G, Liu J, Wu Z, Zhai L, Mi L. Highly reversible and stable zinc anode enabled by a fully conjugated porous organic polymer protective layer. ACS Appl Energy Mater. 2022;5(2):2375. https://doi.org/10.1021/acsaem.1c03864.
Hao J, Li X, Zhang S, Yang F, Zeng X, Zhang S, Bo G, Wang C, Guo Z. Designing dendrite-free zinc anodes for advanced aqueous zinc batteries. Adv Funct Mater. 2020;30(30):2001263. https://doi.org/10.1002/adfm.202001263.
Zhao Z, Zhao J, Hu Z, Li J, Li J, Zhang Y, Wang C, Cui G. Long-life and deeply rechargeable aqueous Zn anodes enabled by a multifunctional brightener-inspired interphase. Energy Environ Sci. 2019;12(6):1938. https://doi.org/10.1039/c9ee00596j.
Wang Y, Chen Y, Liu W, Ni X, Qing P, Zhao Q, Wei W, Ji X, Ma J, Chen L. Uniform and dendrite-free zinc deposition enabled by in situ formed AgZn3 for the zinc metal anode. J Mater Chem A. 2021;9(13):8452. https://doi.org/10.1039/d0ta12177k.
Li L, Jia S, Cao M, Ji Y, Qiu H, Zhang D. Progress in research on metal-based materials in stabilized Zn anodes. Rare Met. 2023. https://doi.org/10.1007/s12598-023-02441-7.
Wang A, Zhou W, Huang A, Chen M, Chen J, Tian Q, Xu J. Modifying the Zn anode with carbon black coating and nanofibrillated cellulose binder: a strategy to realize dendrite-free Zn–MnO2 batteries. J Colloid Interface Sci. 2020;577:256. https://doi.org/10.1016/j.jcis.2020.05.102.
Zheng J, Zhao Q, Tang T, Yin J, Quilty C, Renderos G, Liu X, Deng Y, Wang L, Bock D, Jaye C, Zhang D, Takeuchi E, Takeuchi K, Marschilok A, Archer L. Reversible epitaxial electrodeposition of metals in battery anodes. Science. 2019;366:645. https://doi.org/10.1126/science.aax6873.
Zhai S, Wang N, Tan X, Jiang K, Quan Z, Li Y, Li Z. Interface-engineered dendrite-free anode and ultraconductive cathode for durable and high-rate fiber Zn dual-ion microbattery. Adv Funct Mater. 2021;31(13):2008894. https://doi.org/10.1002/adfm.202008894.
Kang L, Cui M, Jiang F, Gao Y, Luo H, Liu J, Liang W, Zhi C. Nanoporous CaCO3 coatings enabled uniform Zn stripping/plating for long-life zinc rechargeable aqueous batteries. Adv Energy Mater. 2018;8(25):1801090. https://doi.org/10.1002/aenm.201801090.
Liang P, Yi J, Liu X, Wu K, Wang Z, Cui Y, Wang Y, Xia Y, Zhang J. Highly reversible Zn anode enabled by controllable formation of nucleation sites for Zn-based batteries. Adv Funct Mater. 2020;30(13):1908528. https://doi.org/10.1002/adfm.201908528.
Zhang F, Wang C, Pan J, Tian F, Zeng S, Yang J, Qian Y. Polypyrrole-controlled plating/stripping for advanced zinc metal anodes. Mater Today Energy. 2020;17:100443. https://doi.org/10.1016/j.mtener.2020.100443.
Xu Y, Guo L, Zhang H, Zhai H, Ren H. Research status industrial application demand and prospects of phenolic resin. RSC Adv. 2019;9:28924. https://doi.org/10.1039/C9RA06487G.
Asim M, Saba N, Jawaid M, Nasir M, Pervaiz M, Alothman O. A review on phenolic resin and its composites. Curr Anal Chem. 2018;14(3):185. https://doi.org/10.2174/1573411013666171003154410.
Wang J, Yang Y, Zhang Y, Li Y, Sun R, Wang Z, Wang H. Strategies towards the challenges of zinc metal anode in rechargeable aqueous zinc ion batteries. Energy Storage Mater. 2021;35:19. https://doi.org/10.1016/j.ensm.2020.10.027.
Carotenuto G, Nicolais L. Kinetic study of phenolic resin cure by IR spectroscopy. J Appl Polym Sci. 1999;74:2703. https://doi.org/10.1002/(sici)1097-4628(19991209)74:11%3c2703::aid-app18%3e3.0.co;2-m.
Abdallah M. Ethoxylated fatty alcohols as corrosion inhibitors for dissolution of zinc in hydrochloric acid. Corros Sci. 2003;45(12):2705. https://doi.org/10.1016/s0010-938x(03)00107-0.
Stupnisek-Lisac E, Kasunic D, Vorkapic-Furac J. Imidazole derivatives as corrosion inhibitors for zinc in hydrochloric acid. Corros Sci. 1995;51(10):767. https://doi.org/10.5006/1.3293554.
Oxtoby D, Kashchiev D. A general relation between the nucleation work and the size of the nucleus in multicomponent nucleation. J Chern Phys. 1994;100(10):7665. https://doi.org/10.1063/1.466859.
Zhang Q, Hua Y. Effects of 1-butyl-3-methylimidazolium hydrogen sulfate-[BMIM]HSO4 on zinc electrodeposition from acidic sulfate electrolyte. J Appl Electrochem. 2008;39(2):261. https://doi.org/10.1007/s10800-008-9665-5.
Ballesteros J, Díaz-Arista P, Meas Y, Ortega R, Trejo G. Zinc electrodeposition in the presence of polyethylene glycol 20000. Electrochim Acta. 2007;52(11):3686. https://doi.org/10.1016/j.electacta.2006.10.042.
Trejo G, Ruiz H, Borges R, Meas Y. Influence of polyethoxylated additives on zinc electrodeposition from acidic solutions. J Appl Electrochem. 2001;31:685. https://doi.org/10.1023/A:1017580025961.
Briggs D. XPS studies of polymer surface modifications and adhesion mechanisms. J Adhes. 1982;13(3–4):287. https://doi.org/10.1080/00218468208073192.
Pan H, Shao Y, Yan P, Cheng Y, Han K, Nie Z, Wang C, Yang J, Li X, Bhattacharya P, Mueller K, Liu J. Reversible aqueous zinc/manganese oxide energy storage from conversion reactions. Nat Energy. 2016;1(5):16039. https://doi.org/10.1038/nenergy.2016.39.
Xie S, Li X, Li Y, Liang Q, Dong L. Material design and energy storage mechanism of Mn-based cathodes for aqueous zinc-ion batteries. Chem Rec. 2022;22(10):e202200201. https://doi.org/10.1002/tcr.202200201.
Kim S, Oh S. Degradation mechanism of layered MnO2 cathodes in Zn/ZnSO4/MnO2 rechargeable cells. J Power Sources. 1998;72(2):150. https://doi.org/10.1016/S0378-7753(97)02703-1.
Lin S, Zhang T. Review on recent developments, challenges, and perspectives of Mn-based oxide cathode materials for aqueous zinc-ion batteries and the status of Mn resources in China. Energy Fuels. 2023;37(6):4198. https://doi.org/10.1021/acs.energyfuels.2c03997.
Acknowledgements
This study was financially supported by the National Natural Science Foundation of China (No. 52201248) and the Science and Technology Rising Star Project of Hebei University of Technology (No. JBKYXX2201).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interests
The authors declare that they have no conflict of interest.
Supplementary Information
Below is the link to the electronic supplementary material.
Supplementary file2 (MP4 39163 KB)
Supplementary file3 (MP4 28336 KB)
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Wang, YP., Yang, YD., Duan, SQ. et al. Anti-corrosive and highly reversible zinc metal anode enabled by the phenolic resin coating. Rare Met. 43, 2115–2124 (2024). https://doi.org/10.1007/s12598-023-02571-y
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12598-023-02571-y