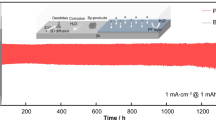
Abstract
Aqueous zinc-ion batteries (AZIBs) have emerged as a promising high-efficiency energy storage system due to the high energy density, low-cost and environmental friendliness. However, the practical application of AZIBs is severely restricted by the challenges faced by the Zn anode, which include uncontrollable dendrite growth, corrosion and hydrogen evolution reaction. Herein, a simple and convenient physical vapor deposition (PVD) method is reported for fabricating uniform graphite as a protection layer on the surface of Zn anode. The high conductivity graphite layer on Zn anode (denoted as Zn@C) not only benefits the uniform distribution of the electric field, but also provides numerous Zn nucleation sites to regulate and navigate Zn-ion stripping/plating behaviors. Additionally, the graphite layer with a poor catalytic activity endows the Zn@C anode with a highly suppressed hydrogen evolution. Consequently, a hydrogen and dendrite free anode is achieved with artificial anticatalytic carbon layer on Zn anode, exhibiting a high reversibility and excellent cycling stability over 2600 h at the current density of 5 mA·cm−2 with a capacity of 2.5 mAh·cm−2 and longtime cycling stability for assembled full cells. This work strategically designs the properties of the artificial interface layer to effectively address various challenges simultaneously, which presents insights for the future development of high-performance rechargeable AZIBs.
Graphical abstract

摘要
抑制析氢的抗催化碳层助力高度可逆的锌负极.
张文多, 孙闯, 朱俞宣*, 高峰*, 赖超*
江苏师范大学.
摘要 (Chinese Abstract): 水系锌离子电池 (AZIBs) 具有能量密度高、成本低和环境友好性的特点被视为最有前景储能系统之一。然而, 锌负极面临的枝晶不可控生长、腐蚀和析氢问题严重限制了锌离子电池在实际应用中的发展。本文通过简单的物理气相沉积法 (PVD) 在锌负极构筑了一层均匀的石墨保护层。石墨界面层所具备的高导电性不仅有利于电场的均匀分布, 且均匀分布的石墨提供了大量的锌成核位点, 有利于调控和诱导锌离子的沉积-剥离行为。此外, 石墨的抗催化活性对析氢反应具有良好的抑制作用, 这赋予石墨界面修饰的锌负极 (Zn@C) 在循环过程中可有效抑制氢气产生。因此, 通过在锌负极构筑具有抗催化析氢活性的碳层有助于解决锌负极析氢枝晶问题所带来的挑战。基于石墨界面修饰对称电池可以在5 mA·cm−2的电流密度和2.5 mAh·cm−2面容量的条件下, 稳定循环2600小时, 且所组装的全电池同样展现了优异的电化学性能。本工作通过设计具有抗催化活性的功能性人工界面层, 可以协同解决锌负极所面临的不同挑战, 为推进AZIBs的实用化进程和未来发展提供了新见解。




Similar content being viewed by others
References
Qin RZ, Wang YT, Yao L, Yang LY, Zhao QH, Ding SX, Liu LL, Pan F. Progress in interface structure and modification of zinc anode for aqueous batteries. Nano Energy. 2022;98: 107333. https://doi.org/10.1016/j.nanoen.2022.107333.
Chen M, Zhang SC, Zou ZG, Zhong SL, Ling WQ, Geng J, Liang FA, Peng XX, Gao Y, Yu FG. Review of vanadium-based oxide cathodes as aqueous zinc-ion batteries. Rare Met. 2023;42(9):2868. https://doi.org/10.1007/s12598-023-02303-2.
Zheng XH, Liu ZC, Sun JF, Luo RH, Xu K, Si MY, Kang J, Yuan Y, Liu S, Ahmad T, Jiang TL, Chen N, Wang MM, Xu Y, Chuai MY, Zhu ZX, Peng Q, Meng YH, Zhang K, Wang WP, Chen W. Constructing robust heterostructured interface for anode-free zinc batteries with ultrahigh capacities. Nat Commun. 2023;14(1):76. https://doi.org/10.1038/s41467-022-35630-6.
Zong Y, He HW, Wang YZ, Wu MH, Ren XC, Bai ZC, Wang NN, Ning X, Dou SX. Functionalized separator strategies toward advanced aqueous zinc-ion batteries. Adv Energy Mater. 2023;13(20):2300403. https://doi.org/10.1002/aenm.202300403.
Li L, Jia SF, Cao MH, Ji YQ, Qiu HW, Zhang D. Progress in research on metal-based materials in stabilized Zn anodes. Rare Met. 2023. https://doi.org/10.1007/s12598-023-02441-7.
Zhu Y, Hoh HY, Qian S, Sun C, Wu Z, Huang Z, Wang L, Batmunkh M, Lai C, Zhang S, Zhong YL. Ultrastable zinc anode enabled by CO2-induced interface layer. ACS Nano. 2022;16(9):14600. https://doi.org/10.1021/acsnano.2c05124.
Zhu QN, Wang ZY, Wang JW, Liu XY, Yang D, Cheng LW, Tang MY, Qin Y, Wang H. Challenges and strategies for ultrafast aqueous zinc-ion batteries. Rare Met. 2020;40:309. https://doi.org/10.1007/s12598-020-01588-x.
Shin J, Lee J, Park Y, Choi JWJCS. Aqueous zinc ion batteries: focus on zinc metal anodes. Chem Sci. 2020;11(8):2028. https://doi.org/10.1039/d0sc00022a.
Ruan P, Liang S, Lu B, Fan HJ, Zhou J. Design strategies for high energy density aqueous zinc batteries. Angew Chem. 2022;61(17): e202200598. https://doi.org/10.1002/anie.202200598.
Yang Q, Li Q, Liu ZX, Wang DH, Guo Y, Li XL, Tang YC, Li HF, Dong BB, Zhi C. Dendrites in Zn-based batteries. Adv Mater. 2020;32(48):2001854. https://doi.org/10.1002/adma.202001854.
Wang LY, Huang WW, Guo WB, Guo ZH, Chang CY, Gao L, Pu X. Sn alloying to inhibit hydrogen evolution of Zn metal anode in rechargeable aqueous batteries. Adv Func Mater. 2021;32(1):2108533. https://doi.org/10.1002/adfm.202108533.
Hong L, Wang LY, Wang YL, Wu XM, Huang W, Zhou YF, Wang KX, Chen JS. Toward hydrogen-free and dendrite-free aqueous zinc batteries: formation of zincophilic protective layer on Zn anodes. Adv Sci. 2022;9(6):202104866. https://doi.org/10.1002/advs.202104866.
Liu SL, Mao JF, Pang WK, Vongsvivut J, Zeng XH, Thomsen L, Wang YY, Liu JW, Li D, Guo ZP. Tuning the electrolyte solvation structure to suppress cathode dissolution, water reactivity, and Zn dendrite growth in zinc-ion batteries. Adv Func Mater. 2021;31(38):202104281. https://doi.org/10.1002/adfm.202104281.
Yang FH, Yuwono JA, Hao JN, Long J, Yuan LB, Wang YY, Liu SL, Fan YM, Zhao SY, Davey K. Understanding H2 evolution electrochemistry to minimize solvated water impact on zinc anode performance. Adv Mater. 2022;34(45):202104281. https://doi.org/10.1002/adma.202206754.
Zeng XH, Mao JF, Hao JN, Liu JT, Liu SL, Wang ZJ, Wang YY, Zhang SL, Zheng T, Liu JW, Rao PH, Guo ZP. Electrolyte design for in situ construction of highly Zn2+-conductive solid electrolyte interphase to enable high-performance aqueous Zn-ion batteries under practical conditions. Adv Mater. 2021;33(11):2007416. https://doi.org/10.1002/adma.202007416.
Shi MX, Wu M, Xiao SS, Liu SP, Yao RQ, Li YQ, Wang YH, Li YG, Tan HQ. Amorphous NiMo3S13/nickel foam integrated anode for lithium-ion batteries. Tungsten. 2023. https://doi.org/10.1007/s42864-022-00201-1.
Hao JN, Li B, Li XL, Zeng XH, Zhang SL, Yang FH, Liu SL, Li D, Wu C, Guo ZP. An in-depth study of Zn metal surface chemistry for advanced aqueous Zn-ion batteries. Adv Mater. 2020;32(34):2003021. https://doi.org/10.1002/adma.202003021.
Yang Q, Guo Y, Yan BX, Wang CD, Liu ZX, Huang ZD, Wang YK, Li YR, Li HF, Song L, Fan J, Zhi CY. Hydrogen-substituted graphdiyne ion tunnels directing concentration redistribution for commercial-grade dendrite-free zinc anodes. Adv Mater. 2020;32(25):22001755. https://doi.org/10.1002/adma.202001755.
Wu K, Yi J, Liu XY, Sun Y, Cui J, Xie YH, Liu YY, Xia YY, Zhang JJ. Regulating Zn deposition via an artificial solid-electrolyte interface with aligned dipoles for long life Zn anode. Nanomicro Lett. 2021;13(1):79. https://doi.org/10.1007/s40820-021-00599-2.
Zhang Q, Luan JY, Tang YG, Ji XB. Interfacial design of dendrite-free zinc anodes for aqueous zinc-ion batteries. Angew Chem. 2020;59(32):13180. https://doi.org/10.1002/anie.202000162.
Zhang XL, Ruan QD, Liu LL, Li D, Xu Y, Wang YC, Liu JY, Huang C, Xiong FY, Wang B, Chu PK. Stable zinc metal anode with an ultrathin carbon coating for zinc-ion batteries. J Electroanal Chem. 2023;936:117357. https://doi.org/10.1016/j.jelechem.2023.117357.
Zhang NN, Huang S, Yuan ZS, Zhu JC, Zhao ZF, Niu ZQ. Direct self-assembly of MXene on Zn anodes for dendrite-free aqueous zinc-ion batteries. Angew Chem. 2021;133(6):2861. https://doi.org/10.1002/anie.202012322.
Wang MM, Ma JL, Meng YH, Sun JF, Yuan Y, Chuai MY, Chen N, Xu Y, Zheng XH, Li ZY, Chen W. High-capacity zinc anode with 96% utilization rate enabled by solvation structure design. Angew Chem. 2022;62(3):e202214966. https://doi.org/10.1002/anie.202214966.
Wang DD, Lv D, Liu HX, Zhang SJ, Wang C, Wang CT, Yang J, Qian YT. In situ formation of nitrogen-rich solid electrolyte interphase and simultaneous regulating solvation structures for advanced Zn metal batteries. Angew Chem. 2022;134(52):e202212839. https://doi.org/10.1002/ange.202212839.
Cao J, Zhang DD, Zhang XY, Zeng ZY, Qin JQ, Huang YH. Strategies of regulating Zn2+ solvation structures for dendrite-free and side reaction-suppressed zinc-ion batteries. Energy Environ Sci. 2022;15(2):499. https://doi.org/10.1039/d1ee03377h.
Cao LS, Li D, Hu EY, Xu JJ, Deng T, Ma L, Wang Y, Yang XQ, Wang CS. Solvation structure design for aqueous Zn metal batteries. J Am Chem Soc. 2020;142(51):21404. https://doi.org/10.1021/jacs.0c09794.
Yu HM, Chen YJ, Wei WF, Ji XB, Chen LB. A functional organic zinc-chelate formation with nanoscaled granular structure enabling long-term and dendrite-free Zn anodes. ACS Nano. 2022;16:9736. https://doi.org/10.1021/acsnano.2c03398.
Su YW, Yang XZ, Zhang QH, Sun JY, Liu ZF. Carbon nanomaterials for highly stable Zn anode: recent progress and future outlook. J Electroanal Chem. 2022;904: 115883. https://doi.org/10.1016/j.jelechem.2021.115883.
Mao CW, Chang YX, Zhao XT, Dong XY, Geng YF, Zhang N, Dai L, Wu XW, Wang L, He ZX. Functional carbon materials for high-performance Zn metal anodes. J Energy Chem. 2022;75:135. https://doi.org/10.1016/j.jechem.2022.07.034.
Hong L, Wu XM, Wang LY, Zhong M, Zhang PY, Jiang LS, Huang W, Wang YL, Wang KX, Chen JS. Highly reversible zinc anode enabled by a cation-exchange coating with Zn-ion selective channels. ACS Nano. 2022;16(4):6906. https://doi.org/10.1021/acsnano.2c02370.
Sun C, Wu CP, Gu XX, Wang C, Wang QH. Interface engineering via Ti3C2Tx MXene electrolyte additive toward dendrite-free zinc deposition. Nanomicro Lett. 2021;13(1):89. https://doi.org/10.1007/s40820-021-00612-8.
Liu HY, Wang JG, Hua W, Sun HH, Huyan Y, Tian S, Hou ZD, Yang JC, Wei CG, Kang FY. Building ohmic contact interfaces toward ultrastable Zn metal anodes. Adv Sci. 2021;8(23):2102612. https://doi.org/10.1002/advs.202102612.
Wang J, Xu F, Jin HY, Chen YQ, Wang Y. Non-noble metal-based carbon composites in hydrogen evolution reaction: fundamentals to applications. Adv Mater. 2017;29(14):1605838. https://doi.org/10.1002/adma.201605838.
Capon JF, Gloaguen F, Schollhammer P, Talarmin J. Catalysis of the electrochemical H evolution by di-iron sub-site models. Coord Chem Rev. 2005;249(15–16):1664. https://doi.org/10.1016/j.ccr.2004.11.018.
Zhang Q, Wang YL, Yan JY, Fan SW, Wang CL, Liu X. Facile synthesis of Ag/Co3O4 nanocubes for on-demand H2 evolution upon dimethylamine borane hydrolysis. Fuel. 2023;351:128983. https://doi.org/10.1016/j.fuel.2023.128983.
Patnaik S, Sahoo DP, Parida K. Photo-catalytic H2 evolution over Au modified mesoporous g-C3N4. Mater Today: Proc. 2021;35:247. https://doi.org/10.1016/j.matpr.2020.05.346.
Lobe S, Bauer A, Uhlenbruck S, Fattakhova-Rohlfing D. Physical vapor deposition in solid-state battery development: from materials to devices. Adv Sci. 2021;8(11):2002044. https://doi.org/10.1002/advs.202002044.
Cui MW, Xiao Y, Kang LT, Du W, Gao YF, Sun XQ, Zhou YL, Li XM, Li HF, Jiang FY, Zhi CY. Quasi-isolated Au particles as heterogeneous seeds guide uniform Zn deposition for aqueous zinc-ion batteries. ACS Appl Energy Mater. 2019;2(9):6490. https://doi.org/10.1021/acsaem.9b01063.
Krishnamurthy S, Esterle A, Sharma NC, Sahi SV. Yucca-derived synthesis of gold nanomaterial and their catalytic potential. Nanoscale Res Lett. 2014;9(1):627. https://doi.org/10.1186/1556-276X-9-627.
Nzulu G, Eklund P, Magnuson M. Characterization and identification of Au pathfinder minerals from an artisanal mine site using X-ray diffraction. J Mater Sci. 2021;56(12):7659. https://doi.org/10.1007/s10853-020-05681-5.
Zhong YL, Swager TM. Enhanced electrochemical expansion of graphite for in situ electrochemical functionalization. J Am Chem Soc. 2012;134(43):17896. https://doi.org/10.1021/ja309023f.
Lee DW, Santos VL, Seo JW, Felix LL, Bustamante DA, Cole JM, Barnes CHW. The structure of graphite oxide: investigation of its surface chemical groups. J Phys Chem B. 2010;114(17):5723. https://doi.org/10.1021/jp1002275.
Hong L, Wang LY, Wang YL, Wu XM, Huang W, Zhou YF, Wang KX, Chen JS. Toward hydrogen-free and dendrite-free aqueous zinc batteries: formation of zincophilic protective layer on Zn anodes. Adv Sci. 2022;9(6):2104866. https://doi.org/10.1002/advs.202104866.
Rashid RT, Chen YQ, Liu XD, Chowdhury FA, Liu MX, Song J, Mi ZT, Zhou BW. Tunable green syngas generation from CO2 and H2O with sunlight as the only energy input. Proc Natl Acad Sci. 2022;119(26):e2121174119. https://doi.org/10.1073/pnas.2121174119.
Zhao ZM, Zhao JW, Hu ZL, Li JD, Li JJ, Zhang YJ, Wang C, Cui GL. Long-life and deeply rechargeable aqueous Zn anodes enabled by a multifunctional brightener-inspired interphase. Energy Environ Sci. 2019;12(6):1938. https://doi.org/10.1039/c9ee00596j.
Yang Y, Chen T, Zhu MK, Gao G, Wang YM, Nie QQ, Jiang YP, Xiong T, Lee WSV, Xue JM. Regulating dendrite-free Zn deposition by a self-assembled OH-terminated SiO2 nanosphere layer toward a Zn metal anode. ACS Appl Mater Interfaces. 2022;14(33):37759. https://doi.org/10.1021/acsami.2c09144.
Yan Y, Shu CZ, Zeng T, Wen XJ, Liu S, Deng DH, Zeng Y. Surface-preferred crystal plane growth enabled by underpotential deposited monolayer toward dendrite-free zinc anode. ACS Nano. 2022;16(6):9150. https://doi.org/10.1021/acsnano.2c01380.
Wu CP, Sun C, Ren KX, Yang FL, Du YX, Gu XX, Wang QH, Lai C. 2-methyl imidazole electrolyte additive enabling ultra-stable Zn anode. Chem Eng J. 2023;452:139465. https://doi.org/10.1016/j.cej.2022.139465.
Jia H, Wang ZQ, Dirican M, Qiu S, Chan CY, Fu SH, Fei B, Zhang XW. A liquid metal assisted dendrite-free anode for high-performance Zn-ion batteries. J Mater Chem A. 2021;9(9):5597. https://doi.org/10.1039/d0ta11828a.
Wang SB, Ran Q, Yao RQ, Shi H, Wen Z, Zhao M, Lang XY, Jiang Q. Lamella-nanostructured eutectic zinc-aluminum alloys as reversible and dendrite-free anodes for aqueous rechargeable batteries. Nat Commun. 2020;11(1):1634. https://doi.org/10.1038/s41467-020-15478-4.
Acknowledgements
The study was financially supported by the National Natural Science Foundation of China (No. 22222902) and Natural Science Foundation of Jiangsu Province (No. BK20200047).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interests
The authors declare that they have no conflict of interest.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Zhang, WD., Sun, C., Zhu, YX. et al. Highly reversible Zn anode enabled by anticatalytic carbon layer with suppressed hydrogen evolution. Rare Met. (2024). https://doi.org/10.1007/s12598-024-02673-1
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s12598-024-02673-1