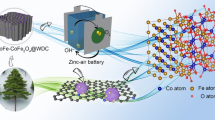
Abstract
Ingenious establishment of transition metal–nitrogen–carbon electrocatalysts with diverse catalytic active sites and hierarchically porous frameworks is highly significant to boost the oxygen reduction reaction (ORR) in Zn–air batteries (ZABs). In this study, Fe/Co co-doped zeolitic imidazolium frameworks (ZIFs) and graphitic carbon nitride (g-C3N4) were integrated and pyrolyzed to construct carbon-based electrocatalysts containing Fe, Co and N elements (labelled as Co–CoFe@NRPC), in which atomic-scale FeNx and CoNx, and nanoscale metallic Co and CoFe alloy moieties were aligned with hierarchically porous N-doped carbon frameworks constructed by interconnected micropolyhedrons, nanotubes and nanosheets. The diverse active moieties guaranteed excellent intrinsic catalytic activity, while the hierarchically porous N-doped carbon frameworks ensured admirable accessibility of the catalytic active sites, excellent electrical conductivity, satisfactory mass transport and good durability. Expectedly, the optimized Co–CoFe@NRPC-90 (with 90 mg g-C3N4 added) electrocatalyst exhibited excellent ORR performance with a high half-wave potential of 885 mV (vs. reversible hydrogen electrode (RHE)), diffusion-limiting current density of 6.15 mA·cm−2, desirable durability and methanol tolerance. Simultaneously, the liquid ZAB established with Co–CoFe@NRPC-90 as an air–cathode electrocatalyst manifested an outstanding power density (281 mW·cm−2) and specific capacity (820.9 mAh·gZn−1), transcending the liquid ZAB based on a commercial Pt/C electrocatalyst.
Graphical abstract

摘要
设计具有多种催化活性位点和分级多孔结构的过渡金属-氮-碳电催化剂,对促进锌空气电池(ZABs)的氧还原反应(ORR)具有重要意义。本文将Fe/Co共掺杂沸石咪唑骨架(ZIFs)和石墨氮化碳(g-C3N4)集成并热解制备Co-CoFe@NRPC电催化剂,其中原子尺度的FeNx和CoNx,纳米尺度的金属Co和CoFe合金与相互连接的微多面体、纳米管和纳米片构建的分级多孔N掺杂碳骨架相结合。多样化的活性中心保证了良好的内在催化活性,而分级多孔N掺杂碳框架确保了催化活性位点的可及性、导电性、传质和稳定性。结果表明,最优样品Co-CoFe@NRPC-90电催化剂具有良好的ORR性能,半波电位为885 mV(相对于可逆氢电极),极限扩散电流密度为6.15 mA·cm‒2,并具有良好的稳定性和抗甲醇毒性。同时,以Co-CoFe@NRPC-90作为空气阴极电催化剂组装的液态ZAB表现出优异的功率密度(281 mW·cm‒2)和比容量(820.9 mAh·gZn ‒1),超越了基于商用Pt/C电催化剂的液态ZAB。







Similar content being viewed by others
References
Li YG, Dai HJ. Recent advances in zinc–air batteries. Chem Soc Rev. 2014;43(15):5257. https://doi.org/10.1039/C4CS00015C.
Kulkarni A, Siahrostami S, Patel A, Nørskov JK. Understanding catalytic activity trends in the oxygen reduction reaction. Chem Rev. 2018;118(5):2302. https://doi.org/10.1021/acs.chemrev.7b00488.
Peng JH, Tao P, Song CY, Shang W, Deng T, Wu JB. Structural evolution of Pt-based oxygen reduction reaction electrocatalysts. Chin J Catal. 2022;43(1):47. https://doi.org/10.1016/S1872-2067(21)63896-2
Li CL, Tan HB, Lin JJ, Luo XL, Wang SP, You J, Kang YM, Bando Y, Yamauchi Y, Kim J. Emerging Pt-based electrocatalysts with highly open nanoarchitectures for boosting oxygen reduction reaction. Nano Today. 2018;21:91. https://doi.org/10.1016/j.nantod.2018.06.005
Liu MY, Xiao XD, Li Q, Luo LY, Ding MH, Zhang B, Li YX, Zou JL, Jiang BJ. Recent progress of electrocatalysts for oxygen reduction in fuel cells. J Colloid Interf Sci. 2022;607:791. https://doi.org/10.1016/j.jcis.2021.09.008
Dai LM, Xue YH, Qu LT, Choi HJ, Baek JB. Metal-free catalysts for oxygen reduction reaction. Chem Rev. 2015;115(11):4823. https://doi.org/10.1021/cr5003563.
Wu YF, Ma JW, Huang YH. Enhancing oxygen reduction reaction of Pt–Co/C nanocatalysts via synergetic effect between Pt and Co prepared by one-pot synthesis. Rare Met. 2023;42(1):146. https://doi.org/10.1007/s12598-022-02119-6.
Cui H, Liao HX, Wang ZL, Xie JP, Tan PF, Chu DW, Jun P. Synergistic electronic interaction between ruthenium and nickel–iron hydroxide for enhanced oxygen evolution reaction. Rare Met. 2022;41(8):2606. https://doi.org/10.1007/s12598-022-02003-3.
Adabi H, Shakouri A, Ul Hassan N, Varcoe JR, Zulevi B, Serov A, Regalbuto JR, Mustain WE. High-performing commercial Fe–N–C cathode electrocatalyst for anion-exchange membrane fuel cells. Nat Energy. 2021;6(8):834. https://doi.org/10.1038/s41560-021-00878-7.
Singh SK, Takeyasu K, Nakamura J. Active sites and mechanism of oxygen reduction reaction electrocatalysis on nitrogen-doped carbon materials. Adv Mater. 2019;31(13):1804297. https://doi.org/10.1002/adma.201804297.
Zhang J, Zhang JJ, He F, Chen YJ, Zhu JW, Wang DL, Mu SC, Yang HY. Defect and doping co-engineered non-metal nanocarbon ORR electrocatalyst. Nano-Micro Lett. 2021;13(1):65. https://doi.org/10.1007/s40820-020-00579-y.
Huang Z, Liao ZW, Yang WJ, Zhou HH, Fu CP, Gong Y, Chen L, Kuang YF. Different types of nitrogen species in nitrogen-doped carbon material: the formation mechanism and catalytic role on oxygen reduction reaction. Electrochim Acta. 2017;245:957. https://doi.org/10.1016/j.electacta.2017.06.026
Zhao CX, Li BQ, Liu JN, Zhang Q. Intrinsic electrocatalytic activity regulation of M-N–C single-atom catalysts for the oxygen reduction reaction. Angew Chem Int Ed. 2021;60(9):4448. https://doi.org/10.1002/anie.202003917.
Wan CZ, Duan XF, Huang Y. Molecular design of single-atom catalysts for oxygen reduction reaction. Adv Energy Mater. 2020;10(14):1903815. https://doi.org/10.1002/aenm.201903815.
Qiao MF, Wang Y, Li L, Hu GZ, Zou GA, Mamat X, Dong YM, Hu X. Self-templated nitrogen-doped mesoporous carbon decorated with double transition-metal active sites for enhanced oxygen electrode catalysis. Rare Met. 2020;39(7):824. https://doi.org/10.1007/s12598-019-01345-9.
Hu CX, Jin HH, Liu BS, Liang LH, Wang Z, Chen D, He DP, Mu SC. Propagating Fe–N4 active sites with vitamin C to efficiently drive oxygen electrocatalysis. Nano Energy. 2021;82: 105714.
Zhu JW, Mu SC. Active site engineering of atomically dispersed transition metal–heteroatom–carbon catalysts for oxygen reduction. Chem Comm. 2021;57(64):7869. https://doi.org/10.1039/D1CC03076K.
Liu Y, He SQ, Huang B, Kong ZY, Guan LH. Influence of different fe doping strategies on modulating active sites and oxygen reduction reaction performance of Fe, N-doped carbonaceous catalysts. J Energy Chem. 2022;70:511. https://doi.org/10.1016/j.jechem.2022.03.005
Zhang YP, Wang N, Jia N, Wang J, Sun J, Shi F, Liu ZH, Jiang RB. A low-cost and facile method for the preparation of Fe–N/C-based hybrids with superior catalytic performance toward oxygen reduction reaction. Adv Mater Interfaces. 2019;6(8):1900273. https://doi.org/10.1002/admi.201900273.
Li KK, Zhang YT, Wang P, Long XY, Zheng LS, Liu GY, He XF, Qiu JS. Core–shell ZIF-67@ZIF-8-derived multi-dimensional cobalt–nitrogen doped hierarchical carbon nanomaterial for efficient oxygen reduction reaction. J Alloys Compd. 2022;903:163701. https://doi.org/10.1016/j.jallcom.2022.163701
Zhu YG, Shang CQ, Wang ZY, Zhang JQ, Yang MY, Cheng H, Lu ZG. Co and N co-modified carbon nanotubes as efficient electrocatalyst for oxygen reduction reaction. Rare Met. 2021;40(1):90. https://doi.org/10.1007/s12598-019-01270-x.
Luo Y, Zhang J, Chen JW, Chen YH, Zhang CY, Luo YJ, Wang G, Wang RL. Bi-functional electrocatalysis through synergetic coupling strategy of atomically dispersed Fe and Co active sites anchored on 3D nitrogen-doped carbon sheets for Zn–air battery. J Catal. 2021;397:223. https://doi.org/10.1016/j.jcat.2021.03.030
Duan XD, Ren SS, Pan N, Zhang MD, Zheng HG. MOF-derived Fe, Co@N–C bifunctional oxygen electrocatalysts for Zn–air batteries. J Mater Chem A. 2020;8(18):9355. https://doi.org/10.1039/D0TA02825H.
Wang ZZ, Zhou XZ, Jin HH, Chen D, Zhu JW, Hempelmann R, Chen L, Mu SC. Ionic liquid-derived feco alloys encapsulated in nitrogen-doped carbon framework as advanced bifunctional catalysts for rechargeable Zn–air batteries. J Alloys Compd. 2022;908:164565. https://doi.org/10.1016/j.jallcom.2022.164565
Zhu YG, Ning SL, Yu XL, Niu XJ, Chen MZ, Zhou W, Zhao DK, Li ZL, Wang N, Li NW, Li LG. Cobalt nanoparticles encapsulated in iron and nitrogen co-doped urchin-like porous carbons as an efficient bifunctional oxygen reversible catalyst for Zn–air batteries. Chem Eng J. 2022;436:135191. https://doi.org/10.1016/j.cej.2022.135191
Rui T, Lu G-P, Zhao X, Cao X, Chen Z. The synergistic catalysis on Co nanoparticles and CoNx sites of aniline-modified ZIF derived Co@NCs for oxidative esterification of HMF. Chinese Chem Lett. 2021;32(2):685. https://doi.org/10.1016/j.cclet.2020.06.027
Cheng XY, Yang J, Yan W, Han Y, Qu XM, Yin SH, Chen C, Ji RY, Li YR, Li G, Li G, Jiang YX, Sun SG. Nano-geometric deformation and synergistic Co nanoparticles–Co–N4 composite sites for proton exchange membrane fuel cells. Energ Environ Sci. 2021;14(11):5958. https://doi.org/10.1039/D1EE01715B.
Lei Z, Tan YY, Zhang ZY, Wu W, Cheng NC, Chen RZ, Mu SC, Sun XL. Defects enriched hollow porous Co–N-doped carbons embedded with ultrafine CoFe/Co nanoparticles as bifunctional oxygen electrocatalyst for rechargeable flexible solid zinc–air batteries. Nano Res. 2021;14(3):868. https://doi.org/10.1007/s12274-020-3127-8.
Lu XF, Xia BY, Zang S-Q, Lou XW. Metal–organic frameworks based electrocatalysts for the oxygen reduction reaction. Angew Chem Int Ed. 2020;59(12):4634. https://doi.org/10.1002/anie.201910309.
Meng ZH, Chen N, Cai SC, Wang R, Wu JW, Tang HL. Recent advances of hierarchically porous bifunctional oxygen electrocatalysts derived from metal–organic frameworks for Zn–air batteries. Mater Chem Front. 2021;5(6):2649. https://doi.org/10.1039/D0QM00878H.
Jafari M, Parnian MJ, Gharibi H, Liang ZB, Zou RQ. Carbon-based nonprecious metal electrocatalysts derived from MOFs for oxygen-reduction reaction. Int J Energy Res. 2021;45(11):15676. https://doi.org/10.1002/er.6834.
Hu WH, Zheng MB, Xu BY, Wei Y, Zhu W, Li Q, Pang H. Design of hollow carbon-based materials derived from metal–organic frameworks for electrocatalysis and electrochemical energy storage. J Mater Chem A. 2021;9(7):3880. https://doi.org/10.1039/D0TA10666F.
Li C, Zhao DH, Long HL, Li M. Recent advances in carbonized non-noble metal–organic frameworks for electrochemical catalyst of oxygen reduction reaction. Rare Met. 2021;40(10):2657. https://doi.org/10.1007/s12598-020-01694-w.
Radwan A, Jin HH, He DP, Mu SC. Design engineering, synthesis protocols, and energy applications of MOF-derived electrocatalysts. Nano-Micro Lett. 2021;13(1):132. https://doi.org/10.1007/s40820-021-00656-w.
Xia BY, Yan Y, Li N, Wu HB, Lou XW, Wang X. A metal–organic framework-derived bifunctional oxygen electrocatalyst. Nat Energy. 2016;1(1):15006. https://doi.org/10.1038/nenergy.2015.6.
Jiang Y, Deng Y-P, Liang RL, Chen N, King G, Yu AP, Chen ZW. Linker-compensated metal–organic framework with electron delocalized metal sites for bifunctional oxygen electrocatalysis. J Am Chem Soc. 2022;144(11):4783. https://doi.org/10.1021/jacs.1c10295.
Imran M, Ikram M, Dilpazir S, Naseem B, Lin YJ, Pan JQ. Functionality and design of Co-MOFs: unique opportunities in electrocatalysts for oxygen reduction reaction. Cat Sci Technol. 2022;12(6):1723. https://doi.org/10.1039/D2CY00153E.
Zhou Y, Yu YN, Ma DS, Foucher AC, Xiong L, Zhang JH, Stach EA, Yue Q, Kang YJ. Atomic Fe dispersed hierarchical mesoporous Fe–N–C nanostructures for an efficient oxygen reduction reaction. ACS Catal. 2021;11(1):74. https://doi.org/10.1021/acscatal.0c03496.
Li YY, Zhang PW, Wan LY, Zheng YP, Qu XM, Zhang HK, Wang YS, Zaghib K, Yuan JY, Sun SH, Wang YC, Zhou ZY, Sun SG. A general carboxylate-assisted approach to boost the ORR performance of ZIF-derived Fe/N/C catalysts for proton exchange membrane fuel cells. Adv Funct Mater. 2021;31(15):2009645. https://doi.org/10.1002/adfm.202009645.
Zhang WD, Liu XM, Gao M, Shang H, Liu XH. Co–Zn–MOFs derived N-doped carbon nanotubes with crystalline Co nanoparticles embedded as effective oxygen electrocatalysts. Nanomater. 2021;11(2):261. https://doi.org/10.3390/nano11020261
Wan XJ, Wu R, Deng JH, Nie Y, Chen SG, Ding W, Huang X, Wei ZD. A metal–organic framework derived 3D hierarchical Co/N-doped carbon nanotube/nanoparticle composite as an active electrocatalyst for oxygen reduction in alkaline electrolyte. J Mater Chem A. 2018;6(8):3386. https://doi.org/10.1039/C7TA10022A.
Bagchi D, Phukan N, Sarkar S, Das R, Ray B, Bellare P, Ravishankar N, Peter SC. Ultralow non-noble metal loaded MOF derived bi-functional electrocatalysts for the oxygen evolution and reduction reactions. J Mater Chem A. 2021;9(14):9319. https://doi.org/10.1039/D0TA12439G.
Zhou WS, Liu YY, Liu H, Wu DC, Zhang GY, Jiang JC. Co/N-doped hierarchical porous carbon as an efficient oxygen electrocatalyst for rechargeable Zn–air battery. RSC Adv. 2021;11(26):15753. https://doi.org/10.1039/D1RA01639C.
Zhou YZ, Chen GB, Wang Q, Wang D, Tao XF, Zhang TR, Feng XL, Müllen K. Fe–N–C electrocatalysts with densely accessible Fe–N4 sites for efficient oxygen reduction reaction. Adv Funct Mater. 2021;31(34):2102420. https://doi.org/10.1002/adfm.202102420.
Chen LL, Zhang YL, Dong LL, Yang WX, Liu XJ, Long L, Liu CY, Dong SJ, Jia JB. Synergistic effect between atomically dispersed Fe and Co metal sites for enhanced oxygen reduction reaction. J Mater Chem A. 2020;8(8):4369. https://doi.org/10.1039/C9TA12516G.
Zhang J, Chen Y, Liu Y, Liu XP, Gao SY. Self-catalyzed growth of Zn/Co–N–C carbon nanotubes derived from metal-organic frameworks as efficient oxygen reduction catalysts for Zn–air battery. Sci China Mater. 2022;65(3):653. https://doi.org/10.1007/s40843-021-1775-2.
Wang NN, Hao BN, Chen H, Zheng RK, Chen BJ, Kuang SH, Chen XD, Cui LF. Highly dispersed Co4N nanoparticles coated by g-C3N4 nanotube: an active bifunctional electrocatalyst for oxygen reduction and oxygen evolution reaction. Chem Eng J. 2021;413:127954. https://doi.org/10.1016/j.cej.2020.127954
Wang Y, Zhong KQ, Huang ZY, Chen LY, Dai Y, Zhang HG, Su MH, Yan J, Yang SR, Li M, Xu T, Tang JF. Novel g-C3N4 assisted metal organic frameworks derived high efficiency oxygen reduction catalyst in microbial fuel cells. J Power Sources. 2020;450: 227681. https://doi.org/10.1016/j.jpowsour.2019.227681
Li ZX, Luo XY, Luo MF, Qin Y, Guo CZ, Liu Y, Luo ZL. Molecule-confined modification of graphitic C3N4 to design mesopore-dominated Fe–N–C hybrid electrocatalyst for oxygen reduction reaction. Int J Hydrog Energy. 2021;46(59):30355.
Liu XJ, Yang WX, Chen LL, Liu ZJ, Long L, Wang SY, Liu CY, Dong SJ, Jia JB. Graphitic carbon nitride (g-C3N4)-derived bamboo-like carbon nanotubes/Co nanoparticles hybrids for highly efficient electrocatalytic oxygen reduction. ACS App Mater Inter. 2020;12(4):4463. https://doi.org/10.1021/acsami.9b18454.
Wei XQ, Song SJ, Wu NN, Luo X, Zheng RL, Jiao L, Wang HJ, Fang Q, Hu LY, Gu WL, Song WY, Zhu CZ. Synergistically enhanced single-atomic site Fe by Fe3C@C for boosted oxygen reduction in neutral electrolyte. Nano Energy. 2021;84: 105840. https://doi.org/10.1016/j.nanoen.2021.105840
Li JC, Meng Y, Zhang LL, Li GZ, Shi ZC, Hou PX, Liu C, Cheng HM, Shao MH. Dual-phasic carbon with Co single atoms and nanoparticles as a bifunctional oxygen electrocatalyst for rechargeable Zn–air batteries. Adv Funct Mater. 2021;31(42):2103360. https://doi.org/10.1002/adfm.202103360.
Liu JL, Zhang YQ, Zhang L, Xie FX, Vasileff A, Qiao SZ. Graphitic carbon nitride (g-C3N4)-derived N-rich graphene with tuneable interlayer distance as a high-rate anode for sodium-ion batteries. Adv Mater. 2019;31(24):1901261. https://doi.org/10.1002/adma.201901261.
Shah SSA, Najam T, Cheng C, Peng LS, Xiang R, Zhang L, Deng JH, Ding W, Wei ZD. Exploring Fe–Nx for peroxide reduction: template-free synthesis of Fe–Nx traumatized mesoporous carbon nanotubes as an ORR catalyst in acidic and alkaline solutions. Chem A Eur J. 2018;24(42):10630. https://doi.org/10.1002/chem.201802453.
Liu YP, Li ZF, Wang LK, Zhang L, Niu XL. Tunable Fe/N co-doped 3D porous graphene with high density Fe–Nx sites as the efficient bifunctional oxygen electrocatalyst for Zn–air batteries. Int J Hydrog Energy. 2021;46(74):36811. https://doi.org/10.1016/j.ijhydene.2021.08.200
Chen C, Cheng D, Liu SJ, Wang Z, Hu MZ, Zhou KB. Engineering the multiscale structure of bifunctional oxygen electrocatalyst for highly efficient and ultrastable zinc–air battery. Energy Storage Mater. 2020;24:402. https://doi.org/10.1016/j.ensm.2019.07.028
Zhang YT, Wang P, Yang J, Li KK, Long XY, Li M, Zhang KB, Qiu JS. Fabrication of core-shell nanohybrid derived from iron-based metal–organic framework grappled on nitrogen-doped graphene for oxygen reduction reaction. Chem Eng J. 2020;401: 126001. https://doi.org/10.1016/j.cej.2020.126001
Wang SF, Sun P, Li N, Wang JG, Zhang L, Duan WJ, Li ZF. The catalytic performance enhanced via π-electron cloud interaction of polymerized cobalt phthalocyanine/3D-graphene as bifunctional oxygen catalysts for Zn–air battery. J Power Sources. 2023;556: 232471. https://doi.org/10.1016/j.jpowsour.2022.232471
Liu YP, Bao JH, Li ZF, Zhang L, Zhang SZ, Wang LK, Niu XL, Sun P, Xu LP. Large-scale defect-rich iron/nitrogen co-doped graphene-based materials as the excellent bifunctional electrocatalyst for liquid and flexible all-solid-state zinc-air batteries. J Colloid Interface Sci. 2022;607:1201. https://doi.org/10.1016/j.jcis.2021.09.070
Huang Z, Pan HY, Yang WJ, Zhou HH, Gao N, Fu CP, Li SC, Li HX, Kuang YF. In situ self-template synthesis of Fe–N-doped double-shelled hollow carbon microspheres for oxygen reduction reaction. ACS Nano. 2018;12(1):208. https://doi.org/10.1021/acsnano.7b05832.
Xia DS, Yang X, Xie L, Wei YP, Jiang WL, Dou M, Li XN, Li J, Gan L, Kang FY. Direct growth of carbon nanotubes doped with single atomic Fe–N4 active sites and neighboring graphitic nitrogen for efficient and stable oxygen reduction electrocatalysis. Adv Funct Mater. 2019;29(49):1906174. https://doi.org/10.1002/adfm.201906174.
Shahbazi Farahani F, Rahmanifar MS, Noori A, El-Kady MF, Hassani N, Neek-Amal M, Kaner RB, Mousavi MF. Trilayer metal–organic frameworks as multifunctional electrocatalysts for energy conversion and storage applications. J Am Chem Soc. 2022;144(8):3411. https://doi.org/10.1021/jacs.1c10963.
Xiong JC, Chen XH, Zhang YP, Lu Y, Liu XD, Zheng YF, Zhang YM, Lin J. Fe/Co/N–C/graphene derived from fe/zif-67/graphene oxide three dimensional frameworks as a remarkably efficient and stable catalyst for the oxygen reduction reaction. RSC Adv. 2022;12(4):2425. https://doi.org/10.1039/D1RA08817C.
Shi Q, Liu Q, Ma Y, Fang Z, Liang Z, Shao G, Tang B, Yang WY, Qin L, Fang XS. High-performance trifunctional electrocatalysts based on FeCo/Co2P hybrid nanoparticles for zinc–air battery and self-powered overall water splitting. Adv Energy Mater. 2020;10(10):1903854. https://doi.org/10.1002/aenm.201903854.
Zhong B, Zhang LY, Yu JG, Fan K. Ultrafine iron–cobalt nanoparticles embedded in nitrogen-doped porous carbon matrix for oxygen reduction reaction and zinc–air batteries. J Colloid Interface Sci. 2019;546:113. https://doi.org/10.1016/j.jcis.2019.03.038
Wang C, Liu YP, Li ZF, Wang LK, Niu XL, Sun P. Novel space-confinement synthesis of two-dimensional Fe, N-codoped graphene bifunctional oxygen electrocatalyst for rechargeable air-cathode. Chem Eng J. 2021;411: 128492. https://doi.org/10.1016/j.cej.2021.128492
Wang C, Li ZF, Wang LK, Niu XL, Wang SW. Facile synthesis of 3D Fe/N codoped mesoporous graphene as efficient bifunctional oxygen electrocatalysts for rechargeable Zn–air batteries. ACS Sustain Chem Eng. 2019;7(16):13873. https://doi.org/10.1021/acssuschemeng.9b02052.
Acknowledgements
This study was financially supported by the National Natural Science Foundation of China (No. 51972109), the Natural Science Foundation of Hunan Province (No. 2023JJ30276), the Scientific Research Fund of Hunan Provincial Education Department, China (Nos. 22A0473 and 20A225), and the Postgraduate Research Innovation Fund of Hunan Institute of Science and Technology (No. 2022-38).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interests
The authors declare that they have no conflict of interest.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Yang, HH., Qian, XR., Zhang, N. et al. Alliance of atomic-scale/nanoscale Fe/Co active sites with hierarchically porous N-doped carbon frameworks for efficient electrocatalytic oxygen reduction. Rare Met. 42, 3766–3779 (2023). https://doi.org/10.1007/s12598-023-02397-8
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12598-023-02397-8