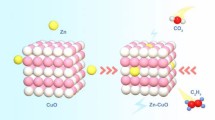
Abstract
Designing catalysts with capable dual-active sites to drive catalytic hydrogen generation is necessary for the future hydrogen economy. Herein, the interfacial active sites consisting of Co and Co-C on Co-Co2C@carbon heterostructure are designed through annealing and high-pressure carbonization. The operating temperature during the high-pressure carbonization under a CO-reducing environment is responsible for the construction and regulation of Co-Co2C@C heterostructure. The optimal catalyst has a high turnover frequency (TOF) of 33.1 min−1 and low activation energy (Ea) of 27.3 kJ·mol–1 during the hydrolysis of NH3BH3. The catalytic stability of Co-Co2C@C has no dramatic deterioration even after 5 cyclic usages. The interfacial active sites and the carbon on the catalyst surface enhance hydrogen generation kinetics and catalytic stability. The construction of interfacial active sites in Co-Co2C@C prompts the dissociation of reactants (NH3BH3 and H2O molecules), leading to an enhanced catalytic hydrogen generation from NH3BH3 hydrolysis (Co activates NH3BH3 and Co-C activates H2O). The construction of hetero-structural catalysts provides theoretical direction for the rational design of advanced transition metal carbide materials in the field of energy catalysis and conversion.
Graphical abstract

摘要
设计具有双活性位点的催化剂驱动催化制氢对未来的“氢经济”发展至关重要。基于此,通过煅烧和高压碳化的策略设计了具有Co和Co–C界面活性位点的Co-Co2C@Carbon异质结构催化剂。高压碳化是在CO还原气体存在的情况下进行的,其中不同的高压碳化温度在构建Co-Co2C@carbon异质结构的过程中有着重要的作用。设计的最佳催化剂在NH3BH3水解制氢过程中表现出优异的催化活性 (TOF = 33.1 min−1) 和较低的活化能 (Ea = 27.3 kJ mol−1)。而且Co-Co2C@C的催化活性在经过5圈循环之后没有剧烈的下降。催化剂中界面活性位点和碳的存在增强了催化产氢动力学和稳定性。Co-Co2C@C中界面活性位点的构建加快了NH3BH3和H2O分子的吸附和解离,进而促进NH3BH3催化制氢活性,其中Co活化NH3BH3,Co–C活化H2O。该异质结构的构建为能源催化转化领域合理设计高效的过渡金属碳化物催化剂提供了理论指导。






Similar content being viewed by others
References
Ding Q-W, Luo Q, Lin L, Fu XP, Wang LS, Yue GH, Lin J, Xie QS, Peng DL. Facile synthesis of PdCu nanocluster-assembled granular films as highly efficient electrocatalysts for formic acid oxidation. Rare Met. 2022;41(8):2595. https://doi.org/10.1007/s12598-022-01997-0.
Guang HL, Zhu SL, Liang YQ, Wu SL, Li ZY, Luo SY, Cui ZD, Inoue A. Highly efficient nanoporous CoBP electrocatalyst for hydrogen evolution reaction. Rare Met. 2021;40(5):1031. https://doi.org/10.1007/s12598-020-01697-7.
Qin PL, Zeng K, Lan ZQ, Huang XT, Liu HZ, Guo J. Enhanced dydrogen storage properties of Mg-Al alloy catalyzed with reduced graphene oxide supported with LaClO. Chin J Rare Met. 2020;44(5):499. https://doi.org/10.13373/j.cnki.cjrm.XY20030007.html.
Shen R, Liu Y, Wen H, Liu T, Peng Z, Wu X, Ge X, Mehdi S, Cao H, Liang E, Jiang J, Li B. Engineering VO–Ti ensemble to boost the activity of Ru towards water dissociation for catalytic hydrogen generation. Appl Catal B Environ. 2022;306:121100. https://doi.org/10.1016/j.apcatb.2022.121100.
Zhao DL, Han ZG, Huo TT, Yuan ZM, Qi Y, Zhang YH. Advances in activation property of hydrogen storage for TiFe-based alloy. Chin J Rare Met. 2020;44(4):337. https://doi.org/10.13373/j.cnki.cjrm.xy19120004.
Lin HJ, Lu YS, Zhang LT, Liu HZ, Edalati K, Révész A. Recent advances in metastable alloys for hydrogen storage: a review. Rare Met. 2022;41(6):1797. https://doi.org/10.1007/s12598-021-01917-8.
Zhang H, Zhang K, Ashraf S, Fan Y, Guan S, Wu X, Liu Y, Liu B, Li B. Polar O–Co–P surface for bimolecular activation in catalytic hydrogen generation. Energy Environ Mater. 2022. https://doi.org/10.1002/eem2.12273.
Zhang H, Fan Y, Liu B, Liu Y, Ashraf S, Wu X, Han G, Gao J, Li B. Birdcage-type CoOx-carbon catalyst derived from metal–organic frameworks for enhanced hydrogen generation. ACS Sustain Chem Eng. 2019;7(11):9782. https://doi.org/10.1021/acssuschemeng.8b06660.
Liu Y, Chen N, Li W, Sun M, Wu T, Huang B, Yong X, Zhang Q, Gu L, Song H, Bauer R, Tse JS, Zang S, Yang B, Lu S. Engineering the synergistic effect of carbon dots-stabilized atomic and subnanometric ruthenium as highly efficient electrocatalysts for robust hydrogen evolution. SmartMat. 2022;3:249. https://doi.org/10.1002/smm2.1067.
Cai J, Ding J, Wei D, Xie X, Li B, Lu S, Zhang J, Liu Y, Cai Q, Zang S. Coupling of Ru and O-vacancy on 2D Mo-based electrocatalyst via a solid-phase interface reaction strategy for hydrogen evolution reaction. Adv Energy Mater. 2021;11(26):2100141. https://doi.org/10.1002/aenm.202100141.
Wang L, Li H, Zhang W, Zhao X, Qiu J, Li A, Zheng X, Hu Z, Si R, Zeng J. Supported rhodium catalysts for ammonia–borane hydrolysis: dependence of the catalytic activity on the highest occupied state of the single rhodium atoms. Angew Chem Int Ed. 2017;56(17):4712. https://doi.org/10.1002/anie.201701089.
Xu H, Cheng D. First-principles-aided thermodynamic modeling of transition-metal heterogeneous catalysts: a review. Green Energy Environ. 2020;5(3):286. https://doi.org/10.1016/j.gee.2020.07.006.
Yoo JM, Shin H, Chung DY, Sung YE. Carbon shell on active nanocatalyst for stable electrocatalysis. Acc Chem Res. 2022;55(9):1278. https://doi.org/10.1021/acs.accounts.1c00727.
Wang H. Nanostructure@ metal–organic frameworks (MOFs) for catalytic carbon dioxide (CO2) conversion in photocatalysis, electrocatalysis, and thermal catalysis. Nano Res. 2022;15(4):2834. https://doi.org/10.1007/s12274-021-3984-9.
Zang D, Gao XJ, Li L, Wei Y, Wang H. Confined interface engineering of self-supported Cu@ N-doped graphene for electrocatalytic CO2 reduction with enhanced selectivity towards ethanol. Nano Res. 2022. https://doi.org/10.1007/s12274-022-4698-3.
Zhang C, Xie C, Gao Y, Tao X, Ding C, Fan F, Jiang HL. Charge separation by creating band bending in metal–organic frameworks for improved photocatalytic hydrogen evolution. Angew Chem Int Ed. 2022;61(28):e202204108. https://doi.org/10.1002/anie.202204108.
Xu W, Li W, Wen H, Ding J, Liu Y, Li W, Li B. Metal/metal–organic framework interfacial ensemble-induced dual site catalysis towards hydrogen generation. Appl Catal B Environ. 2021;286:119946. https://doi.org/10.1016/j.apcatb.2021.119946.
Liu Y, Han G, Zhang X, Xing C, Du C, Cao H, Li B. Co–Co3O4@carbon core–shells derived from metal–organic framework nanocrystals as efficient hydrogen evolution catalysts. Nano Res. 2017;10(9):3035. https://doi.org/10.1007/s12274-017-1519-1.
Xing C, Liu Y, Su Y, Chen Y, Hao S, Wu X, Wang X, Cao H, Li B. Structural evolution of Co-based metal organic frameworks in pyrolysis for synthesis of core–shells on nanosheets: Co@CoOx@Carbon-rGO composites for enhanced hydrogen generation activity. ACS Appl Mater Interfaces. 2016;8(24):15430. https://doi.org/10.1021/acsami.6b04058.
Dong W, Liu J, Zhu H, Ding Y, Pei Y, Liu J, Du H, Jiang M, Liu T, Su H, Li W. Co–Co2C and Co–Co2C/AC catalysts for hydroformylation of 1-hexene under low pressure: experimental and theoretical studies. J Phys Chem C. 2014;118(33):19114. https://doi.org/10.1021/jp504215y.
Guo S, Liu G, Zhang Y, Liu Y. Oxygen vacancies boosted Co-Co2C catalysts for higher alcohols synthesis from syngas. Appl Surf Sci. 2022;576:151846. https://doi.org/10.1016/j.apsusc.2021.151846.
Lin T, Yu F, An Y, Qin T, Li L, Gong K, Zhong L, Sun Y. Cobalt carbide nanocatalysts for efficient syngas conversion to value-added chemicals with high selectivity. Acc Chem Res. 2021;54(8):1961. https://doi.org/10.1021/acs.accounts.0c00883.
Zhang S, Liu X, Shao Z, Wang H, Sun Y. Direct CO2 hydrogenation to ethanol over supported Co2C catalysts: studies on support effects and mechanism. J Catal. 2020;382:86. https://doi.org/10.1016/j.jcat.2019.11.038.
Song XZ, Wang H, Li Z, Meng YL, Tan Z, Zhu M. Double-shelled carbon nanocages grafted with carbon nanotubes embedding Co nanoparticles for enhanced hydrogen evolution electrocatalysis. Chem Commun. 2021;57:3022. https://doi.org/10.1039/D0CC08416F.
Chen D, Yang W, Jiao L, Li L, Yu S, Jiang H. Boosting catalysis of Pd nanoparticles in MOFs by pore wall engineering: the roles of electron transfer and adsorption energy. Adv Mater. 2020;32(30):2000041. https://doi.org/10.1002/adma.202000041.
Xu M, Li D, Sun K, Jiao L, Xie C, Ding C, Jiang H. Interfacial microenvironment modulation boosting electron transfer between metal nanoparticles and MOFs for enhanced photocatalysis. Angew Chem Int Ed. 2021;60(30):16372. https://doi.org/10.1002/anie.202104219.
Xu K, Ma C, Yan H, Gu H, Wang WW, Li SQ, Meng QL, Shao WP, Ding GH, Wang FR, Jia CJ. Catalytically efficient Ni–NiOx–Y2O3 interface for medium temperature water–gas shift reaction. Nat Commun. 2022;13:2443. https://doi.org/10.1038/s41467-022-30138-5.
Su L, Gong D, Yao N, Li Y, Li Z, Luo W. Modification of the intermediate binding energies on Ni/Ni3N heterostructure for enhanced alkaline hydrogen oxidation reaction. Adv Funct Mater. 2021;31:2106156. https://doi.org/10.1002/adfm.202106156.
Wang B, Liu F, Guan W, Wang A, Zhang T. Promoting the effect of Au on the selective hydrogenolysis of glycerol to 1,3-propanediol over the Pt/WOx/Al2O3 catalyst. ACS Sustain Chem Eng. 2021;9(16):5705. https://doi.org/10.1021/acssuschemeng.1c00880.
Lin Y, Yang L, Jiang H, Zhang Y, Cao D, Wu C, Zhang G, Jiang J, Song L. Boosted reactivity of ammonia borane dehydrogenation over Ni/Ni2P heterostructure. J Phys Chem Lett. 2019;10(5):1048. https://doi.org/10.1021/acs.jpclett.9b00122.
Li W, Liu J, Guo P, Li H, Fei B, Guo Y, Pan H, Sun D, Fang F, Wu R. Co/CoP heterojunction on hierarchically ordered porous carbon as a highly efficient electrocatalyst for hydrogen and oxygen evolution. Adv Energy Mater. 2021;11(42):2102134. https://doi.org/10.1002/aenm.202102134.
Cheng S, Zheng H, Shen C, Jiang B, Liu F, Li A. Hierarchical iron phosphides composite confined in ultrathin carbon layer as effective heterogeneous electro-fenton catalyst with prominent stability and catalytic activity. Adv Funct Mater. 2021;31(48):2106311. https://doi.org/10.1002/adfm.202106311.
Jin S, Shao W, Chen S, Li L, Shang S, Zhao Y, Zhang X, Xie Y. Ultrathin in-plane heterostructures for efficient CO2 chemical fixation. Angew Chem Int Ed. 2022;61(3):e202113411. https://doi.org/10.1002/anie.202113411.
Wang L, Tang G, Liu S, Dong H, Liu Q, Sun J, Tang H. Interfacial active-site-rich 0D Co3O4/1D TiO2 p-n heterojunction for enhanced photocatalytic hydrogen evolution. Chem Eng J. 2022;428:131338. https://doi.org/10.1016/j.cej.2021.131338.
Wang M, Wang Y, Mao SS, Shen S. Transition-metal alloy electrocatalysts with active sites modulated by metal-carbide heterophases for efficient oxygen evolution. Nano Energy. 2021;88:106216. https://doi.org/10.1016/j.nanoen.2021.106216.
Chen L, Song XL, Ren JT, Yuan ZY. Precisely modifying Co2P/black TiO2 S-scheme heterojunction by in situ formed P and C dopants for enhanced photocatalytic H2 production. Appl Catal B Environ. 2022;315:121546. https://doi.org/10.1016/j.apcatb.2022.121546.
Su P, Zhang J, Xiao K, Zhao S, Djellabi R, Li X, Yang B, Zhao X. C3N4 modified with single layer ZIF67 nanoparticles for efficient photocatalytic degradation of organic pollutants under visible light. Chin J Catal. 2020;41:1894. https://doi.org/10.1016/S1872-2067(20)63620-8.
Yang J, Zhang F, Lu H, Hong X, Jiang H, Wu Y, Li Y. Hollow Zn/Co ZIF particles derived from core–shell ZIF-67@ZIF-8 as selective catalyst for the semi-hydrogenation of acetylene. Angew Chem Int Ed. 2015;127(37):11039. https://doi.org/10.1002/ange.201504242.
Zheng J, Cai J, Jiang F, Xu Y, Liu X. Investigation of the highly tunable selectivity to linear α-olefins in Fischer–Tropsch synthesis over silica supported Co and CoMn catalysts by carburization–reduction pretreatment. Catal Sci Technol. 2017;7:4736. https://doi.org/10.1039/C7CY01764B.
Guan S, Guo Y, Zhang H, Liu X, Fan Y, Liu B. The alloy-oxide interfacial ensemble effect of a multilayer core–shell nanomotor for hydrogen generation from ammonia borane. Sustain Energy Fuels. 2022;6:1753. https://doi.org/10.1039/d1se01994e.
Yang X, Li Q, Li L, Lin J, Yang X, Yu C, Liu Z, Fang Y, Huang Y, Tang C. CuCo binary metal nanoparticles supported on boron nitride nanofibers as highly efficient catalysts for hydrogen generation from hydrolysis of ammonia borane. J Power Sources. 2019;431:135. https://doi.org/10.1016/j.jpowsour.2019.05.038.
Ren X, Wei S, Wang Q, Shi L, Wang XS, Wei Y, Yang G, Philo D, Ichihara F, Ye J. Rational construction of dual cobalt active species encapsulated by ultrathin carbon matrix from MOF for boosting photocatalytic H2 generation. Appl Catal B Environ. 2021;286:119924. https://doi.org/10.1016/j.apcatb.2021.119924.
Zhang S, Gao G, Hao J, Wang M, Zhu H, Lu S, Duan F, Dong W, Du M, Zhao Y. Low-electronegativity vanadium substitution in cobalt carbide induced enhanced electron transfer for efficient overall water splitting. ACS Appl Mater Interfaces. 2019;11(46):43261. https://doi.org/10.1021/acsami.9b16390.
Song H, Yu J, Tang Z, Yang B, Lu S. Halogen-doped carbon dots on amorphous cobalt phosphide as robust electrocatalysts for overall water splitting. Adv Energy Mater. 2022;12(14):2102573. https://doi.org/10.1002/aenm.202102573.
Ocal C, Ferrer S. The strong metal-support interaction (SMSI) in Pt–TiO2 model catalysts. A new CO adsorption state on Pt–Ti atoms. J Chem Phys. 1986;84:6474. https://doi.org/10.1063/1.450743.
Qie Y, Liu Y, Kong F, Yang Z, Yang H. High coercivity cobalt carbide nanoparticles as electrocatalysts for hydrogen evolution reaction. Nano Res. 2022;15:3901. https://doi.org/10.1007/s12274-021-4036-1.
Kawashima K, Shin K, Wygant BR, Kim JH, Cao CL, Lin J, Lin J, Son YJ, Liu Y, Henkelman G, Mullins CB. Cobalt metal–cobalt carbide composite microspheres for water reduction electrocatalysis. ACS Appl Energy Mater. 2020;3(4):3909. https://doi.org/10.1021/acsaem.0c00321.
Li W, Zhao Y, Liu Y, Sun M, Waterhouse GIN, Huang B, Zhang K, Zhang T, Lu S. Exploiting Ru-induced lattice strain in CoRu nanoalloys for robust bifunctional hydrogen production. Angew Chem Int Ed. 2021;60(6):3290. https://doi.org/10.1002/anie.202013985.
Liu Y, Wen H, Zhou D, Huang X, Wu X, Jiang J, Guo X, Li B. Tuning surface d charge of Ni-Ru alloys for unprecedented catalytic activity towards hydrogen generation from ammonia borane hydrolysis. Appl Catal B Environ. 2021;291:120094. https://doi.org/10.1016/j.apcatb.2021.120094.
Mo B, Li S, Wen H, Zhang H, Zhang H, Wu J, Li B, Hou H. Functional group regulated Ni/Ti3C2Tx (Tx= F, –OH) holding bimolecular activation tunnel for enhanced ammonia borane hydrolysis. ACS Appl Mater Interfaces. 2022;14(14):16320. https://doi.org/10.1021/acsami.2c02594.
Guan S, An L, Ashraf S, Zhang L, Liu B, Fan Y, Li B. Oxygen vacancy excites Co3O4 nanocrystals embedded into carbon nitride for accelerated hydrogen generation. Appl Catal B Environ. 2020;269:118775. https://doi.org/10.1016/j.apcatb.2020.118775.
Zhang H, Liu Y, Wei H, Wang C, Liu T, Wu X, Ashraf S, Mehdi S, Guan S, Fan Y, Yue X, Liu B, Zhang Y, Cao H, Li B. Atomic-bridge structure in B-Co-P dual-active sites on boron nitride nanosheets for catalytic hydrogen generation. Appl Catal B Environ. 2022;314:121495. https://doi.org/10.1016/j.apcatb.2022.121495.
Guan S, Liu Y, Zhang H, Wei H, Liu T, Wu X, Wen H, Shen R, Mehdi S, Ge X, Wang C, Liu B, Liang E, Fan Y, Li B. Atomic interface-exciting catalysis on cobalt nitride-oxide for accelerating hydrogen generation. Small. 2022;18(22):2107417. https://doi.org/10.1002/smll.202107417.
Acknowledgements
This study was financially supported by the National Natural Science Foundation of China (Nos. 52071135, 51871090 and U1804135), the Fundamental Research Funds for the Universities of Henan Province (Nos. NSFRF220201 and NSFRF200402).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interests
The authors declare that they have no conflict of interest.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Zhang, HH., Ren, YB., Yuan, ZL. et al. Interfacial active sites on Co-Co2C@carbon heterostructure for enhanced catalytic hydrogen generation. Rare Met. 42, 1935–1945 (2023). https://doi.org/10.1007/s12598-022-02224-6
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12598-022-02224-6