Abstract
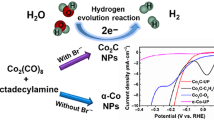
Cobalt carbide nanoparticles (NPs), as a typical carbide material, have attracted extensive attention in the fields of magnetism and electrochemistry. Herein, we adopted a modified solution route by pyrolysis long-chain amines at high temperatures to obtain Co2C NPs, in which different forms of Co NPs were used as precursors. The results reveal that no matter what the structure of the precursor and the type of long-chain amine, single-phase Co2C NPs with good crystallinity are obtained. At the same time, carbonization of hexagonal close packed (hcp) cobalt as the precursor gives the materials high magnetic anisotropy, exhibiting a large coercivity (∼ 1,300 Oe) on the nanoscale. In terms of catalytic properties, benefiting from intrinsically high activity of Co2C NPs, the material demonstrates superior hydrogen evolution reaction (HER) performance, with optimal overpotential as low as 73 mV at the current density of 10 mA·cm−2. This provides new ideas for the further development of transition metal carbides (TMCs) and the improvement of their magnetic and electrocatalytic properties.

Similar content being viewed by others
References
Xiao, Y.; Sun, P. P.; Cao, M. H. Core-shell bimetallic carbide nanoparticles confined in a three-dimensional N-doped carbon conductive network for efficient lithium storage. ACS Nano 2014, 8, 7846–7857.
Yang, W. L.; Rehman, S.; Chu, X.; Hou, Y. L.; Gao, S. Transition metal (Fe, Co and Ni) carbide and nitride nanomaterials: Structure, chemical synthesis and applications. ChemNanoMat 2015, 1, 376–398.
Harris, V. G.; Chen, Y.; Yang, A.; Yoon, S.; Chen, Z.; Geiler, A. L.; Gao, J.; Chinnasamy, C. N.; Lewis, L. H.; Vittoria, C. et al. High coercivity cobalt carbide nanoparticles processed via polyol reaction: A new permanent magnet material. J. Phys. D: Appl. Phys. 2010, 606, 598–604.
Zhao, Y. H.; Su, H. Y.; Sun, K. J.; Liu, J. X.; Li, W. X. Structural and electronic properties of cobalt carbide Co2C and its surface stability: Density functional theory study. Surf. Sci. 2012, 606, 598–604.
Claeys, M.; Dry, M. E.; Van Steen, E.; du Plessis, E.; van Berge, P. J.; Saib, A. M.; Moodley, D. J. In situ magnetometer study on the formation and stability of cobalt carbide in Fischer-Tropsch synthesis. J. Catal. 2014, 318, 193–202.
Lin, T. J.; Yu, F.; An, Y. L.; Qin, T. T.; Li, L. S.; Gong, K.; Zhong, L. S.; Sun, Y. H. Cobalt carbide nanocatalysts for efficient syngas conversion to value-added chemicals with high selectivity. Acc. Chem. Res. 2021, 54, 1961–1971.
Guo, Q.; Xia, S. G.; Li, X. B.; Wang, Y.; Liang, F.; Lin, Z. S.; Tung, C. H.; Wu, L. Z. Flower-like cobalt carbide for efficient carbon dioxide conversion. Chem. Commun. 2020, 56, 7849–7852.
Shen, X. F.; Zhang, T. F.; Suo, H. Y.; Yan, L.; Huang, L. C.; Ma, C. W.; Li, L. G.; Wen, X. D.; Li, Y. W.; Yang, Y. A facile one-pot method for synthesis of single phase Co2C with magnetic properties. Mater. Lett. 2020, 271, 127783.
El-Gendy, A. A.; Almugaiteeb, T.; Carpenter, E. E. CoxC nanorod magnets: Highly magnetocrystalline anisotropy with lower curie temperature for potential applications. J. Magn. Magn. Mater. 2013, 348, 136–139.
Zamanpour, M.; Bennett, S.; Taheri, P.; Chen, Y. J.; Harris, V. G. Magnetic properties and scale-up of nanostructured cobalt carbide permanent magnetic powders. J. Appl. Phys. 2014, 115, 17A747.
Zamanpour, M.; Bennett, S. P.; Majidi, L.; Chen, Y. J.; Harris, V. G. Process optimization and properties of magnetically hard cobalt carbide nanoparticles via modified polyol method. J. Alloys Compd. 2011, 625, 138–143.
Yang, C.; Zhao, H. B.; Hou, Y. L.; Ma, D. Fe5C2 nanoparticles: A facile bromide-induced synthesis and as an active phase for fischer-tropsch synthesis. J. Am. Chem. Soc. 2012, 134, 15814–15821.
Ge, W. Y.; Gao, W. X.; Zhu, J. F.; Li, Y. X. In situ synthesis of hägg iron carbide (Fe5C2) nanoparticles with a high coercivity and saturation magnetization. J. Alloys Compd. 2019, 781, 1069–1073.
Mohammed-Ibrahim, J.; Sun, X. M. Recent progress on earth abundant electrocatalysts for hydrogen evolution reaction (HER) in alkaline medium to achieve efficient water splitting—A review. J. Energ. Chem. 2019, 34, 111–160.
Chen, W. F.; Muckerman, J. T.; Fujita, E. Recent developments in transition metal carbides and nitrides as hydrogen evolution electrocatalysts. Chem. Commun. 2013, 49, 8896–8909.
Han, C.; Li, W. J.; Liu, H. K.; Dou, S. X.; Wang, J. Z. Design strategies for developing non-precious metal based bi-functional catalysts for alkaline electrolyte based zinc-air batteries. Mater. Horiz. 2019, 6, 1812–1827.
Li, S. W.; Yang, C.; Yin, Z.; Yang, H. J.; Chen, Y. F.; Lin, L. L.; Li, M. Z.; Li, W. Z.; Hu, G.; Ma, D. Wet-chemistry synthesis of cobalt carbide nanoparticles as highly active and stable electrocatalyst for hydrogen evolution reaction. Nano Res. 2017, 10, 1322–1328.
Kawashima, K.; Shin, K.; Wygant, B. R.; Kim, J. H.; Cao, C. L.; Lin, J.; Son, Y. J.; Liu, Y.; Henkelman, G.; Mullins, C. B. Cobalt metal-cobalt carbide composite microspheres for water reduction electrocatalysis. ACS Appl. Energy Mater. 2020, 3, 3909–3918.
Wang, P. Y.; Zhu, J. W.; Pu, Z. H.; Qin, R.; Zhang, C. T.; Chen, D.; Liu, Q.; Wu, D. L.; Li, W. Q.; Liu, S. L. et al. Interfacial engineering of Co nanoparticles/Co2C nanowires boosts overall water splitting kinetics. Appl. Catal. B: Environ. 2021, 296, 120334.
Gu, W. L.; Hu, L. Y.; Shang, C. S.; Li, J.; Wang, E. K. Enhancement of the hydrogen evolution performance by finely tuning the morphology of co-based catalyst without changing chemical composition. Nano Res. 2019, 12, 191–196.
Xia, S. G.; Zhang, Z.; Wu, J. N.; Wang, Y.; Sun, M. J.; Cui, Y.; Zhao, C. L.; Zhong, J. Y.; Cao, W.; Wang, H. P. et al. Cobalt carbide nanosheets as effective catalysts toward photothermal degradation of mustard-gas simulants under solar light. Appl. Catal. B: Environ. 2021, 284, 119703.
Carroll, K. J.; Huba, Z. J.; Spurgeon, S. R.; Qian, M. C.; Khanna, S. N.; Hudgins, D. M.; Taheri, M. L.; Carpenter, E. E. Magnetic properties of Co2C and Co3C nanoparticles and their assemblies. Appl. Phys. Lett. 2012, 101, 012409.
Zhang, Y. J.; Zhu, Y.; Wang, K. J.; Li, D.; Wang, D. P.; Ding, F.; Meng, D.; Wang, X. L.; Choi, C.; Zhang, Z. D. Controlled synthesis of Co2C nanochains using cobalt laurate as precursor: Structure, growth mechanism and magnetic properties. J. Magn. Magn. Mater. 2018, 456, 71–77.
Roy, N.; Ali, M. A.; Sen, A.; Adroja, D. T.; Sen, P.; Banerjee, S. S. Exploring a low temperature glassy state, exchange bias effect, and high magnetic anisotropy in Co2C nanoparticles. J. Phys.:Condens. Matter 2021, 33, 375804.
Ye, Z. T.; Qie, Y. Q.; Fan, Z. P.; Liu, Y. X.; Shi, Z.; Yang, H. Soft magnetic Fe5C2-Fe3C@C as an electrocatalyst for the hydrogen evolution reaction. Dalton Trans. 2019, 48, 4636–4642.
Li, S. S.; Hao, X. G.; Abudula, A.; Guan, G. Q. Nanostructured co-based bifunctional electrocatalysts for energy conversion and storage: Current status and perspectives. J. Mater. Chem. A 2019, 7, 18674–18707.
Fan, X. J.; Peng, Z. W.; Ye, R. Q.; Zhou, H. Q.; Guo, X. M3C (M: Fe, Co, Ni) nanocrystals encased in graphene nanoribbons: An active and stable bifunctional electrocatalyst for oxygen reduction and hydrogen evolution reactions. ACS Nano 2015, 9, 7407–7418.
Zhang, X.; Zhang, X.; Xu, H. M.; Wu, Z. S.; Wang, H. L.; Liang, Y. Y. Iron-doped cobalt monophosphide nanosheet/carbon nanotube hybrids as active and stable electrocatalysts for water splitting. Adv. Funct. Mater. 2017, 27, 1606635.
Ma, X. Z.; Li, K. Y.; Zhang, X.; Wei, B.; Yang, H.; Liu, L. N.; Zhang, M. Y.; Zhang, X. T.; Chen, Y. J. The surface engineering of cobalt carbide spheres through N, B Co-doping achieved by room-temperature in situ anchoring effects for active and durable multifunctional electrocatalysts. J. Mater. Chem. A 2019, 7, 14904–14915.
Zhang, S. G.; Gao, G. H.; Hao, J. C.; Wang, M. M.; Zhu, H.; Lu, S. L.; Duan, F.; Dong, W. F.; Du, M. L.; Zhao, Y. L. Low-electronegativity vanadium substitution in cobalt carbide induced enhanced electron transfer for efficient overall water splitting. ACS Appl. Mater. Interfaces 2019, 11, 43261–43269.
Guo, P.; Wu, Y. X.; Lau, W. M.; Liu, H.; Liu, L. M. Porous CoP nanosheet arrays grown on nickel foam as an excellent and stable catalyst for hydrogen evolution reaction. Int. J. Hydrog. Energy 2017, 42, 26995–27003.
Zheng, X. Z.; Chen, Y. Z.; Bao, X. B.; Mao, S. J.; Fan, R. X.; Wang, Y. In situ formed bimetallic carbide Ni6Mo6C nanodots and NiMoOx nanosheet array hybrids anchored on carbon cloth: Efficient and flexible self-supported catalysts for hydrogen evolution. ACS Catal. 2020, 10, 11634–11642.
Wang, S. P.; Wang, J.; Zhu, M. L.; Bao, X. B.; Xiao, B. Y.; Su, D. F.; Li, H. R.; Wang, Y. Molybdenum-carbide-modified nitrogen-doped carbon vesicle encapsulating nickel nanoparticles: A highly efficient, low-cost catalyst for hydrogen evolution reaction. J. Am. Chem. Soc. 2015, 137, 15753–15759.
Chen, J. D.; Chen, C. H.; Chen, Y. Z.; Wang, H. Y.; Mao, S. J.; Wang, Y. Improving alkaline hydrogen evolution reaction kinetics on molybdenum carbide: Introducing Ru dopant. J. Catal. 2020, 392, 313–321.
Acknowledgements
This work was supported by the National Natural Science Foundation of China (No. 51872111) and the Natural Science Foundation of Jilin Province (No. 20190201253JC).
Author information
Authors and Affiliations
Corresponding author
Electronic Supplementary Material
Rights and permissions
About this article
Cite this article
Qie, Y., Liu, Y., Kong, F. et al. High coercivity cobalt carbide nanoparticles as electrocatalysts for hydrogen evolution reaction. Nano Res. 15, 3901–3906 (2022). https://doi.org/10.1007/s12274-021-4036-1
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12274-021-4036-1