Abstract
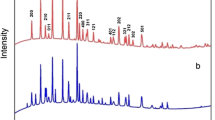
In this study, the LiFePO4 cathode was synthesized by the ionic thermal method using the deep eutectic mixture of tetramethyl ammonium chloride and urea. The synthetic conditions were systematically investigated by orthogonal experiments, which indicate that the optimal reaction time, reaction temperature, molar ratio of Li to DES and rotate speed are 96 h, 220 °C, 1:14 and 20 r·min −1, respectively. X-ray diffraction (XRD), scanning electron microscope (SEM) and transmission electron microscope (TEM) were characterized to investigate the crystalline structure and morphology of the obtained materials, indicating well-crystallized LiFePO4 with olivine structure. And the physical properties of LiFePO4 were explored through Fourier transform infrared spectroscopy (FTIR), 57Fe Mössbauer absorption spectra and Raman spectra. An initial discharge capacity can reach 151 mAh·g−1 at 0.1C rate for LiFePO4 following by calcining at 600 °C under the optimal conditions, and it retains 125.1 mAh·g−1 after 100 cycles. These results demonstrated that the addition of ionic liquids can improve the rate performance, cycle performance and ion diffusion rate of LiFePO4.
Graphical abstract

摘要
本文以四甲基氯化铵和尿素为原料, 采用离子热法合成了LiFePO4正极材料。通过正交实验对合成条件进行了系统的研究, 结果表明最佳反应时间、反应温度、Li与DES的摩尔比、转速分别为96h、220℃、1:14和20。通过X射线衍射(XRD)、扫描电子显微镜(SEM)和透射电子显微镜(TEM)对所得材料的晶体结构和形貌进行了表征, 表明LiFePO4结晶良好, 具有橄榄石结构。通过傅里叶变换红外光谱(FTIR)、57Fe穆谱吸收光谱和拉曼光谱探究了LiFePO4的物理性质。在最佳条件下, LiFePO4在600℃下煅烧后, 以0.1C的速率放电, 初始放电容量可达151 mAh·g−1, 循环100次后仍可保持125.1 mAh·g−1。这些结果表明, 离子液体的加入可以提高LiFePO4的速率性能、循环性能和离子扩散速率。








Similar content being viewed by others
References
Li Y, Guo C, Yue L, Qu W, Chen N, Dai Y, Chen R, Wu F. A scalable synthesis of silicon nanoparticles as high-performance anode material for lithium-ion batteries. Rare Met. 2019;38(3):199.
Zheng ZM, Wu HH, Liu HD, Zhang QB, He X, Yu SC, Petrova V, Feng J, Kostecki R, Liu P, Peng DL, Liu ML, Wang MS. Achieving fast and durable lithium storage through amorphous FeP nanoparticles encapsulated in ultrathin 3D P-doped porous carbon nanosheets. ACS Nano. 2020;14(8):9545.
Pang GY, Zhuang WD, Bai XT, Ban LQ, Zhao CR, Sun XY. Research advances of co-free and Ni-rich LiNixMn1-xO2(0.5<x<1) cathode materials. Chin J Rare Met. 2020;44(9):996.
Li T, Shi P, Zhang Q. Recent progress in carbon/lithium metal composite anode for safe lithium metal batteries. Rare Met. 2018;37(6):449.
Liang Y, Zhao JT, Han ZJ, Yu HJ. Application of lithium rare metal in rechargeable batteries. Chin J Rare Met. 2019;43(11):1187.
Mai L, Tian X, Xu X, Chang L, Xu L. Nanowire electrodes for electrochemical energy storage devices. Chem Rev. 2014;114(23):11828.
Zhang F, Shen F, Fan ZY, Ji X, Zhao B, Sun ZT, Xuan YY, Han XG. Ultrathin Al2O3-coated reduced graphene oxide membrane for stable lithium metal anode. Rare Met. 2018;37(6):510.
Wu MG, Liao JQ, Yu LX, Lv RT, Li P, Sun WP, Tan R, Duan XC, Zhang L, Li F, Kim J, Shin KH, Park HS, Zhang WC, Guo ZP, Wang HT, Tang YB, Gorgolis G, Galiotis C, Ma JM. 2020 Roadmap on carbon materials for energy storage and conversion. Chem-Asian J. 2020;15(7):995.
Zhong S, Wu L, Liu J. Sol–gel synthesis and electrochemical properties of 9LiFePO4·Li3V2(PO4)3/C composite cathode material for lithium ion batteries. Electrochim Acta. 2012;74:8.
Luo S, Tang Z, Lu J, Zhang Z. Electrochemical properties of carbon-mixed LiFePO4 cathode material synthesized by the ceramic granulation method. Ceram Int. 2008;34(5):1349.
Huo TT, Nie N, Liu YY, Zhang JL, Yu F, Li W. Naphthalene-modulated microporous carbon layers of LiFePO4 improve the high-rate electrochemical performance. J Energy Chem. 2019;30(3):84.
Larcher D, Tarascon J-M. Towards greener and more sustainable batteries for electrical energy storage. Nat Chem. 2015;7(1):19.
Andersson AS, Thomas JO. The source of first-cycle capacity loss in LiFePO4. J Power Sources. 2001;97–98:498.
Ji BF, Yao WJ, Zheng YP, Kidkhunthod P, Zhou XL, Tunmee S, Sattayaporn S, Cheng HM, He HY, Tang YB. A fluoroxalate cathode material for potassium-ion batteries with ultra-long cyclability. Nat Comm. 2020;11(1):1225.
Kim HS, Kam DW, Kim WS, Koo HJ. Synthesis of the LiFePO4 by a solid-state reaction using organic acid as a reducing agent. Ionics. 2011;17(4):293.
Deng ZL, Wu F, Gao XG, Wu WP. Development of a LiFePO4-based high power lithium secondary battery for HEVs applications. Rare Met. 2020;39(12):1457.
Hsu KF, Tsay SY, Hwang BJ. Synthesis and characterization of nano-sized LiFePO4 cathode materials prepared by a citric acid-based sol–gel route. J Mater Chem. 2004;14(17):2690.
Choi DW, Kumta PN. Surfactant based sol–gel approach to nanostructured LiFePO4 for high rate Li-ion batteries. J Power Sources. 2007;163(2):1064.
Jin B, Gu HB. Preparation and characterization of LiFePO4 cathode materials by hydrothermal method. Solid State Ionics. 2008;178(37):1907.
Chen JJ, Wang SJ, Whittingham MS. Hydrothermal synthesis of cathode materials. J Power Sources. 2007;174(2):442.
Wang C, Li S, Han Y, Lu Z. Assembly of LiMnPO4 nanoplates into microclusters as a high-performance cathode in lithium-ion batteries, ACS Appl. Mater Interfaces. 2017;9(33):27618.
Jiang T, Pan W, Wang J, Bie X, Du F, Wei Y. Carbon coated Li3V2(PO4)3 cathode material prepared by a PVA assisted sol-gel method. Electrochim Acta. 2010;55(12):3864.
Yang SF, Zavalij PY, Whittingham MS. Hydrothermal synthesis of lithium iron phosphate cathodes. Electrochem Commun. 2001;3(9):505.
Clarke CJ, Tu WC, Levers O, Brohl A, Hallett JP. Green and sustainable solvents in chemical processes. Chem Rev. 2018;118(2):747.
Zeng XL, Li JH. Implications for the carrying capacity of lithium reserve in China. Resour Conserv Recycl. 2013;80(1):58.
Abbott AP, Capper G, Davies DL, Rasheed RK, Tambyrajah V. Novel solvent properties of choline chloride/urea mixtures. Chem Commun. 2003;9(1):70.
Chaabene N, Ngo K, Turmine M, Vivier V. New hydrophobic deep eutectic solvent for electrochemical applications. J Mol Liq. 2020;319:114198.
Zhang QH, De Oliveira VK, Royer S, Jerome F. Deep eutectic solvents: syntheses, properties and applications. Chem Soc Rev. 2012;41(21):7108.
Wagle DV, Deakyne CA, Baker GA. Quantum chemical insight into the interactions and thermodynamics present in choline chloride based deep eutectic solvents. J Phys Chem B. 2016;120(27):6739.
Figueiredo M, Gomes C, Costa R, Martins A, Pereira CM, Silva F. Differential capacity of a deep eutectic solvent based on choline chloride and glycerol on solid electrodes. Electrochim Acta. 2009;54(9):2630.
Jhong HR, Wong DS, Wan CC, Wang YY, Wei TC. A novel deep eutectic solvent-based ionic liquid used as electrolyte for dye-sensitized solar cells. Electrochem Commun. 2009;11(1):209.
Cooper ER, Andrews CD, Wheatley PS, Webb PB, Wormald P, Morris RE. Ionic liquids and eutectic mixtures as solvent and template in synthesis of zeolite analogues. Nature. 2004;430:1012.
Thai TA, Tuyen TK, Linh TM, Tuyen TT, Oanh H. Deep eutectic solvent based on lithium bis[(trifluoromethyl)-sulfonyl] Imide (LiTFSI) and 2,2,2-trifluoroacetamide (TFA) as a promising electrolyte for a high voltage lithium-ion battery with a LiMn2O4 cathode. ACS Omega. 2020;5(2):23843.
Sheu CY, Lee SF, Lii KH. Ionic liquid of choline chloride/malonic acid as a solvent in the synthesis of open-framework iron oxalatophosphates. Inorg Chem. 2006;45(5):1891.
Luo SH, Li JZ, Bao S, Liu YY, Wang ZY. Na3V2(PO4)3/C composite prepared by sol-gel method as cathode for sodium ion batteries. J Electrochem Soc. 2018;165(7):A1460.
Sui YL, Wu L, Hong W, Liu JQ, Zhang XP, Li W, Zhong SK. Synthesis and electrochemical properties of spherically shaped LiVPO4F/C cathode material by a spray drying–roasting method. Rare Met. 2020;40(1):72.
Li X, Jiang YZ, Li XK, Jiang HX, Liu JL, Feng J, Lin SB, Guan X. Electrochemical property of LiFePO4/C composite cathode with different carbon sources. Rare Met. 2018;37(9):743.
Belharouak I, Johnson C, Amine K. Synthesis and electrochemical analysis of vapor-deposited carbon-coated LiFePO4. Electrochem Commun. 2005;7(10):983.
Hong J, Wang CS, Dudney NJ, Lance MJ. Characterization and performance of LiFePO4 thin-film cathodes prepared with radio-frequency magnetron-sputter deposition. J Electrochem Soc. 2007;154(8):A805.
Doeff MM, Hu YQ, McLarnon F, Kostecki R. Effect of surface carbon structure on the electrochemical performance of LiFePO4. Electrochem Solid ST. 2003;6(10):A207.
Faques-Ledent MT, Tarte P. Vibrational studies of olivine-type compounds-II orthophosphates, arsenates and vanadates AIBIIXVO4. Spectrochim Acta, Part A. 1974;30(5):673.
Prince AAM, Mylswamy S, Chan TS, Liu RS, Hannoyer B, Jean M, Shen CH, Huang SM, Lee JF, Wang GX. Investigation of Fe valence in LiFePO4 by Mössbauer and XANES spectroscopic techniques. Solid State Commun. 2004;132(7):455.
Yamada A, Chung SC, Hinokuma K. Optimized LiFePO4 for lithium battery cathodes. J Electrochem Soc. 2001;148:A224.
Andersson AS, Kalska B, Haggstrom L, Thomas JO. Lithium extraction/insertion in LiFePO4: an X-ray diffraction and Mössbauer spectroscopy study. Solid State Ionics. 2000;130(1–2):41.
Burba CM, Frech R. Raman and FTIR Spectroscopic study of Li[1-x]FePO4 (0≤x≤1). J Electrochem Soc. 2004;151(3):A1032.
Ait-Salah A, Dodd J, Mauger A, Yazami R, Julien CM. Structural and magnetic properties of LiFePO4 and lithium extraction effect. S Z Anorg Allg Chem. 2006;632(8–9):1598.
Shahid R, Murugavel S. Synthesis and characterization of olivine phosphate cathode material with different particle sizes for rechargeable lithium-ion batteries. Mater Chem Phys. 2013;140(2–3):659.
Salah AA, Mauger A, Julien CM, Gendron F. Nano-sized impurity phases in relation to the mode of preparation of LiFePO4. Mater Sci Eng B. 2006;129(1–3):232.
Hou PQ, Yang L, Wang YF, Li SN, Luo SH. Optimize hydrothermal synthesis and electrochemical performance of Li2FeTiO4 composite cathode materials by using orthogonal experimental design method. Ionics. 2019;26(4):1657.
Bi YJ, Yang WC, Yang BC, Wang CY, Wang D, Shi SQ. Influence of Li3V2(PO4)3 complexing on the performance of LiMnPO4 based materials utilized in lithium-ion battery. Ceram Int. 2014;40(5):7637.
Lin Y, Wu JB, Huang XH, Cao YQ, Guo RQ. Effect of synthesis temperature on the structure and electrochemical performances of LiFePO4/C. Ionics. 2019;25(4):5697.
Acknowledgements
This study was financially supported by the National Natural Science Foundation of China (Nos. 51674068, 51874079, 51804035 and 11775226), the Natural Science Foundation of Hebei Province (No. E2018501091), Hebei Province Key Research and Development Plan Project (No. 19211302D), the Fundamental Research Funds for the Central Universities (Nos. N172302001, N182306001, N182312007, N182304018 and N2023040) and Research Project on Distribution of Heavy Metals in Soil and Comprehensive Utilization Technology of Tailings in Typical Iron Tailing Reservoir Areas of Hebei Province (No. 802060671901).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Li, SN., Luo, SH., Yang, L. et al. Synthesis and electrochemical properties of LiFePO4 cathode material by ionic thermal method using eutectic mixture of tetramethyl ammonium chloride–urea. Rare Met. 40, 3477–3484 (2021). https://doi.org/10.1007/s12598-021-01783-4
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12598-021-01783-4