Abstract
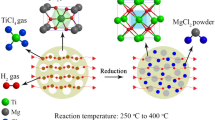
The novel method for the preparation of titanium powder by multi-stage reduction was proposed. The primary reduction adopted self-propagating high-temperature synthesis (SHS) mode. This paper focuses on the primary reduction process of Mg–TiO2 system under the condition of off-balance reaction. The effects of different material ratios, material arrangement methods and reaction initiation modes on the SHS reaction process of Mg–TiO2 system and its reaction mechanism were systematically studied. SHS mode was used to Mg–TiO2 system, and non-stoichiometric low-valent titanium oxide intermediate including α-Ti (Ti2O type) and TiO was directly obtained (with oxygen content of 13.93 wt%). SHS reaction initiated by local ignition is more sufficient than by overall heating method. Compared with the loose setting materials, the compacts can increase the effective contact interface of the reactants, and SHS reaction proceeds more sufficiently, which is favorable for obtaining lower oxygen content product. The adiabatic temperatures of the Mg–TiO2 system at different initial conditions were calculated according to the improved calculation method. When the initial temperature is 298 K, the adiabatic temperature of Mg–TiO2 system is between 1363 and 2067 K at different material ratios. Therefore, unreacted or partially excess Mg at the reaction front will diffuse into the unreacted region in gas or liquid form, thereby preheating the material and initiating further SHS reaction.











Similar content being viewed by others
References
Kroll WJ. The production of ductile titanium. J Electrochem Soc. 1940;78(1):34.
Chen GZ, Fray DJ, Farthing TW. Direct electrochemical reduction of titanium dioxide to titanium in molten calcium chloride. Nature. 2000;407(6802):361.
Ono K, Suzuki RO. A new concept for producing Ti sponge: calciothermie reduction. JOM. 2002;54(2):59.
Park I, Abiko T, Okabe TH. Production of titanium powder directly from TiO2 in CaCl2 through an electronically mediated reaction (EMR). J Phys Chem Solids. 2005;66(2–4):410.
Zhao ZG, Lu XG, Ding WZ. A new technology using SOM to produce titanium sponge. Shanghai Met. 2005;27(2):40.
Pal Uday B, Powell Adam C. The use of solid-oxide-membrane technology for electrometallurgy. JOM. 2007;59(5):44.
Chen GZ, Fray DJ, Farthing TW. Cathodic deoxygenation of the alpha-case on titanium and alloys in molten calcium chloride. Metall Mater Trans B. 2001;32(6):1041.
Chen GZ, Fray DJ. Electro-Deoxidation of Metal Oxides. Light Metals. TMS: Warrendale; 2001. 1147.
Fray DJ. Emerging molten salt technologies for metals production. JOM. 2001;53(10):26.
Zhu HM, Jiao SQ, Gu XF. A production method of pure titanium by electrolysis from titanium oxide/titanium carbide soluble anode: China Patent: CN 1712571A, 2005.
Jiao SQ, Zhu HM. Electrolysis of Ti–C–O solid solution prepared by TiC and TiO2. J Alloys Compd. 2007;438(1–2):243.
Choi K, Choi H, Sohn I. Understanding the magnesiothermic reduction mechanism of TiO2 to produce Ti. Metall Mater Trans B. 2017;48(2):922.
Zhang Y, Fang ZZ, Xia Y. Hydrogen assisted magnesiothermic reduction of TiO2. Chem Eng J. 2017;308:299.
Nersisyan HH, Lee JH, Won CW. Combustion of TiO2–Mg and TiO2–Mg–C systems in the presence of NaCl to synthesize nanocrystalline Ti and TiC powders. Mater Res Bull. 2003;38(7):1135.
Okabe TH, Oda T, Mitsuda Y. Titanium powder production by preform reduction process (PRP). J Alloys Compd. 2004;364(1–2):156.
Wan HL, Xu BQ, Dai YN. Preparation of titanium powders by calciothermic reduction of titanium dioxide. J Cent South Univ. 2012;19(9):2434.
Fan SG, Dou ZH, Zhang TA, Liu Y, Niu LP. Deoxidation mechanism in reduced titanium powder prepared by multistage deep reduction of TiO2. Metall Mater Trans B. 2019;50B:282.
Zhang TA, Dou ZH. Research growth mechanism of TiB2 powder prepared by SHS-metallurgy. J Inorg Mater. 2006;21(3):583.
Weimin W, Zhengyi F, Hao W. Chemistry reaction processes during combustion synthesis of B2O3–TiO2–Mg system. J Mater Process Technol. 2002;128(1–3):162.
Merzhanov AG. Combustion and Plasma Synthesis of High-Temperature Materials. New York: Material Neurk VCH Publisher; 1990. 1.
Mossino P. Some aspects in self-propagating high-temperature synthesis. Ceram Int. 2004;30(3):311.
Cheng C, Dou ZH, Zhang TA, Su JM, Zhang HJ, Liu Y, Niu LP. Oxygen content of high ferrotitanium prepared by thermite method with different melt separation temperatures. Rare Met. 2019;38(9):892.
Yeh CL, Shen YG. Formation of TiAl–Ti2AlC in situ composites by combustion synthesis. Intermetallics. 2009;17(3):169.
Atong D, Clark DE. Ignition behavior and characteristics of microwave-combustion synthesized Al2O3–TiC powders. Ceram Int. 2004;30(7):1909.
Zhan L, Shen P, Yang Y. Self-propagating high-temperature synthesis of TiCxNy–TiB ceramics from a Ti–BC–BN system. Int J Refract Met Hard Mater. 2009;27(5):829.
Yeh CL, Kuo CW, Wu FS. Formation of Ti2AlC0.5N0.5, solid solutions by combustion synthesis of Al4C3-containing samples in nitrogen. J Alloys Compd. 2010;508(2):324.
Sharifitabar M, Khaki JV, Sabzevar MH. Effects of Fe additions on self-propagating high temperature synthesis characteristics of TiO2–Al–C system. Int J Refract Met Hard Mater. 2014;47:93.
Su X, Fu F, Yan Y. Self-propagating high-temperature synthesis for compound thermoelectrics and new criterion for combustion processing. Nat Commun. 2014;5(1):4908.
Ying GB, He XD, Du SY, Zhu CC, Zheng YT, Wu YP, Wang C. Formation of Mn+1AXn phases in Ti–Cr–Al–C systems by self-propagating high-temperature synthesis. Rare Met. 2013;33(4):419.
Ying G, He X, Du SY, Hu CC, Wu YP, Wang C. Kinetics and numerical simulation of self-propagating high-temperature synthesis in Ti–Cr–Al–C systems. Rare Met. 2014;33(5):527.
Acknowledgements
This research was financially supported by the National Natural Science Foundation of China (Nos. U1908225, U1702253 and 51774078) and the Fundamental Research Funds for the Central Universities (Nos. N172506009, N170908001 and N182515007).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Fan, SG., Dou, ZH., Zhang, TA. et al. Self-propagating reaction mechanism of Mg–TiO2 system in preparation process of titanium powder by multi-stage reduction. Rare Met. 40, 2645–2656 (2021). https://doi.org/10.1007/s12598-020-01554-7
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12598-020-01554-7